Abstract
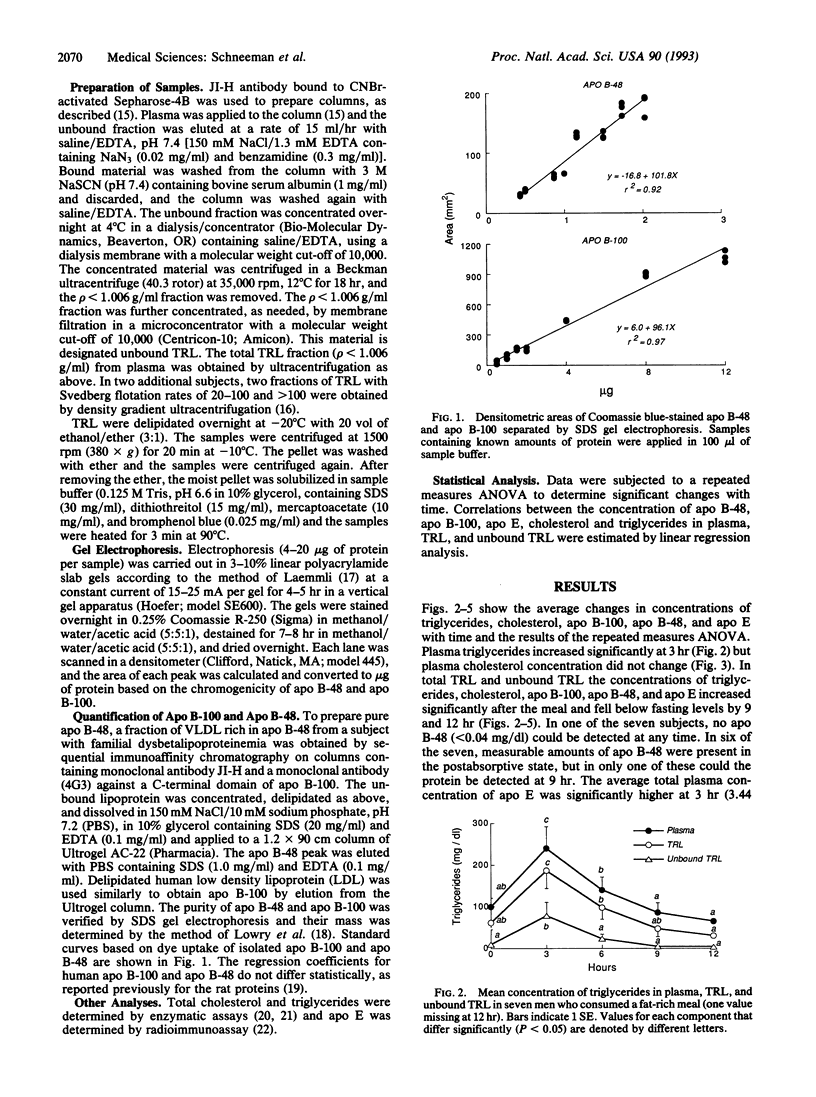
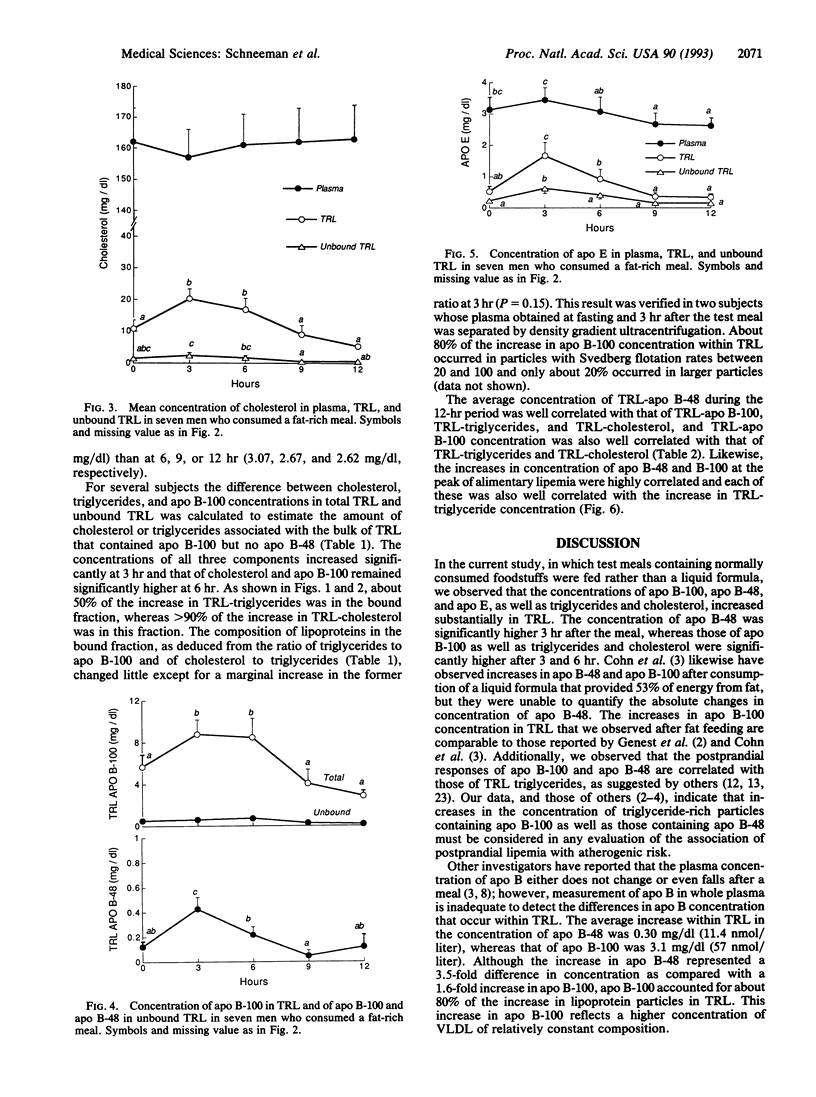
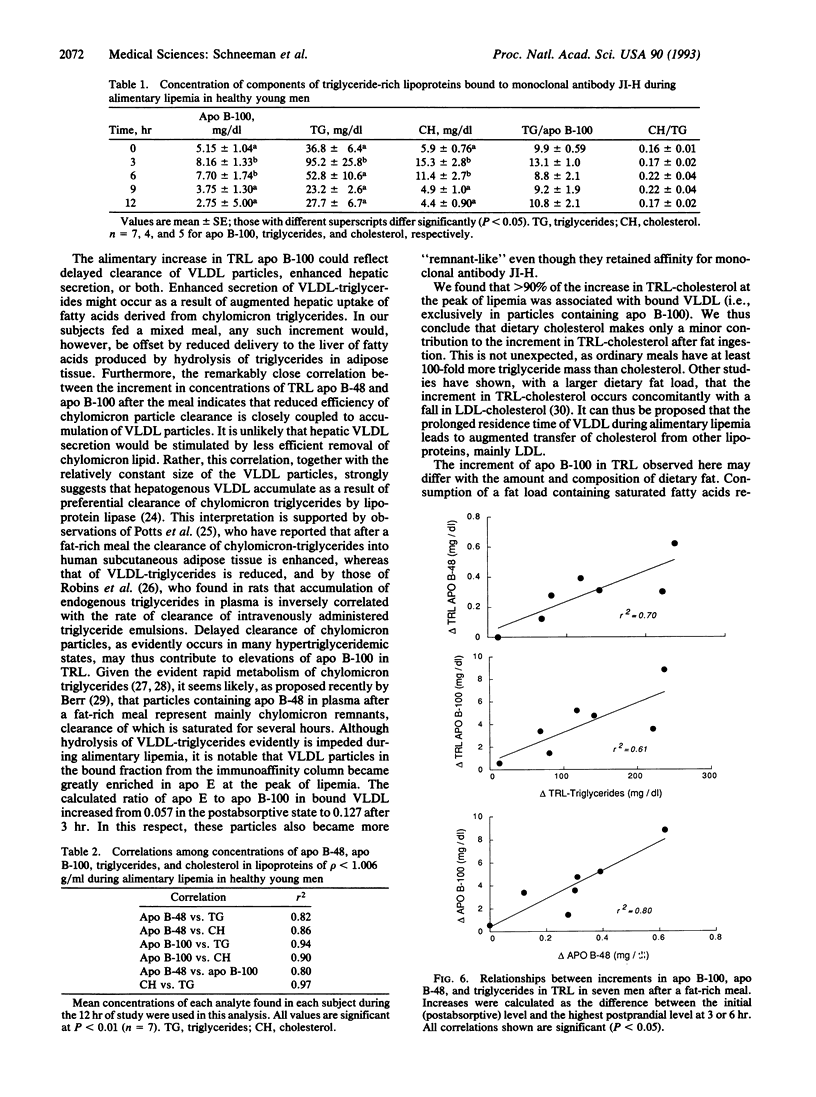
The concentration of triglyceride-rich lipoproteins containing apolipoprotein (apo) B-48 (chylomicrons) and apo B-100 (very low density lipoproteins) was measured in blood plasma of healthy young men after an ordinary meal containing one-third of daily energy and fat. Plasma obtained in the postabsorptive state and at intervals up to 12 hr after the meal was subjected to immunoaffinity chromatography against a monoclonal antibody to apo B-100 that does not bind apo B-48 and a minor fraction of apo B-100 rich in apo E. Measurements of the concentrations of components of the total and unbound triglyceride-rich lipoproteins separated from plasma by ultracentrifugation showed that about 80% of the increase in lipoprotein particle number was in very low density lipoproteins containing apo B-100 and only 20% was in chylomicrons containing apo B-48 that carry dietary fat from the intestine. The maximal increments and the average concentrations of apo B-48 and B-100 during the 12 hr were highly correlated (r2 = 0.80), suggesting that preferential clearance of chylomicron triglycerides by lipoprotein lipase leads to accumulation of hepatogenous very low density lipoproteins during the alimentary period. The composition of the bulk of very low density lipoproteins that were bound to the monoclonal antibody changed little and these particles contained about 90% of the cholesterol and most of the apo E that accumulated in triglyceride-rich lipoproteins. The predominant accumulation of very low density lipoprotein rather than chylomicron particles after ingestion of ordinary meals is relevant to the potential atherogenicity of postprandial lipoproteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Berr F. Characterization of chylomicron remnant clearance by retinyl palmitate label in normal humans. J Lipid Res. 1992 Jun;33(6):915–930. [PubMed] [Google Scholar]
- Berr F., Kern F., Jr Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res. 1984 Aug;25(8):805–812. [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Campos E., Nakajima K., Tanaka A., Havel R. J. Properties of an apolipoprotein E-enriched fraction of triglyceride-rich lipoproteins isolated from human blood plasma with a monoclonal antibody to apolipoprotein B-100. J Lipid Res. 1992 Mar;33(3):369–380. [PubMed] [Google Scholar]
- Castro G. R., Fielding C. J. Effects of postprandial lipemia on plasma cholesterol metabolism. J Clin Invest. 1985 Mar;75(3):874–882. doi: 10.1172/JCI111786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn J. S., McNamara J. R., Cohn S. D., Ordovas J. M., Schaefer E. J. Plasma apolipoprotein changes in the triglyceride-rich lipoprotein fraction of human subjects fed a fat-rich meal. J Lipid Res. 1988 Jul;29(7):925–936. [PubMed] [Google Scholar]
- Cohn J. S., McNamara J. R., Krasinski S. D., Russell R. M., Schaefer E. J. Role of triglyceride-rich lipoproteins from the liver and intestine in the etiology of postprandial peaks in plasma triglyceride concentration. Metabolism. 1989 May;38(5):484–490. doi: 10.1016/0026-0495(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Genest J., Sniderman A., Cianflone K., Teng B., Wacholder S., Marcel Y., Kwiterovich P., Jr Hyperapobetalipoproteinemia. Plasma lipoprotein responses to oral fat load. Arteriosclerosis. 1986 May-Jun;6(3):297–304. doi: 10.1161/01.atv.6.3.297. [DOI] [PubMed] [Google Scholar]
- Grundy S. M., Mok H. Y. Chylomicron clearance in normal and hyperlipidemic man. Metabolism. 1976 Nov;25(11):1225–1239. doi: 10.1016/s0026-0495(76)80006-6. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J. Early effects of fat ingestion on lipids and lipoproteins of serum in man. J Clin Invest. 1957 Jun;36(6 Pt 1):848–854. doi: 10.1172/JCI103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., GORDON R. S., Jr Idiopathic hyperlipemia: metabolic studies in an affected family. J Clin Invest. 1960 Dec;39:1777–1790. doi: 10.1172/JCI104202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Hamilton R. L. Hepatocytic lipoprotein receptors and intracellular lipoprotein catabolism. Hepatology. 1988 Nov-Dec;8(6):1689–1704. doi: 10.1002/hep.1840080637. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Kashyap M. L. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973 Jan;52(1):32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980 Dec;66(6):1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasinski S. D., Cohn J. S., Russell R. M., Schaefer E. J. Postprandial plasma vitamin A metabolism in humans: a reassessment of the use of plasma retinyl esters as markers for intestinally derived chylomicrons and their remnants. Metabolism. 1990 Apr;39(4):357–365. doi: 10.1016/0026-0495(90)90249-c. [DOI] [PubMed] [Google Scholar]
- Krasinski S. D., Cohn J. S., Schaefer E. J., Russell R. M. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest. 1990 Mar;85(3):883–892. doi: 10.1172/JCI114515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis G. F., O'Meara N. M., Soltys P. A., Blackman J. D., Iverius P. H., Druetzler A. F., Getz G. S., Polonsky K. S. Postprandial lipoprotein metabolism in normal and obese subjects: comparison after the vitamin A fat-loading test. J Clin Endocrinol Metab. 1990 Oct;71(4):1041–1050. doi: 10.1210/jcem-71-4-1041. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Billington T., Fidge N. H. Slower removal of intestinal apolipoprotein B-48 than of apolipoprotein B-100 in severely hypertriglyceridemic subjects. Biochim Biophys Acta. 1983 May 16;751(3):422–427. doi: 10.1016/0005-2760(83)90301-6. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Miesenböck G., Hopferwieser T., Mühlberger V., Knapp E., Dunn J. K., Gotto A. M., Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992 Nov;12(11):1336–1345. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- Potts J. L., Fisher R. M., Humphreys S. M., Coppack S. W., Gibbons G. F., Frayn K. N. Peripheral triacylglycerol extraction in the fasting and post-prandial states. Clin Sci (Lond) 1991 Nov;81(5):621–626. doi: 10.1042/cs0810621. [DOI] [PubMed] [Google Scholar]
- Redard C. L., Davis P. A., Schneeman B. O. Dietary fiber and gender: effect on postprandial lipemia. Am J Clin Nutr. 1990 Nov;52(5):837–845. doi: 10.1093/ajcn/52.5.837. [DOI] [PubMed] [Google Scholar]
- Robins S. J., Fasulo J. M., Robins V. F., Patton G. M. Response of serum triglycerides of endogenous origin to the administration of triglyceride-rich lipid particles. Am J Physiol. 1989 Dec;257(6 Pt 1):E860–E865. doi: 10.1152/ajpendo.1989.257.6.E860. [DOI] [PubMed] [Google Scholar]
- Tall A., Sammett D., Granot E. Mechanisms of enhanced cholesteryl ester transfer from high density lipoproteins to apolipoprotein B-containing lipoproteins during alimentary lipemia. J Clin Invest. 1986 Apr;77(4):1163–1172. doi: 10.1172/JCI112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M. S., Zechner R., Brown A., Eisenberg S., Breslow J. L. Dietary polyunsaturated fats of the W-6 and W-3 series reduce postprandial lipoprotein levels. Chronic and acute effects of fat saturation on postprandial lipoprotein metabolism. J Clin Invest. 1988 Dec;82(6):1884–1893. doi: 10.1172/JCI113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilversmit D. B. Atherogenesis: a postprandial phenomenon. Circulation. 1979 Sep;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B., Shea T. M. Quantitation of apoB-48 and apoB-100 by gel scanning or radio-iodination. J Lipid Res. 1989 Oct;30(10):1639–1646. [PubMed] [Google Scholar]



