Abstract
We herein describe a simple, sensitive and specific method for analysis of circulating microRNAs (miRNA), termed S-Poly(T) Plus real-time PCR assay. This new method is based on our previously developed S-Poly(T) method, in which a unique S-Poly(T) primer is used during reverse-transcription to increase sensitivity and specificity. Further increased sensitivity and simplicity of S-Poly(T) Plus, in comparison with the S-Poly(T) method, were achieved by a single-step, multiple-stage reaction, where RNAs were polyadenylated and reverse-transcribed at the same time. The sensitivity of circulating miRNA detection was further improved by a modified method of total RNA isolation from serum/plasma, S/P miRsol, in which glycogen was used to increase the RNA yield. We validated our methods by quantifying miRNA expression profiles in the sera of the patients with pulmonary arterial hypertension associated with congenital heart disease. In conclusion, we developed a simple, sensitive, and specific method for detecting circulating miRNAs that allows the measurement of 266 miRNAs from 100 μl of serum or plasma. This method presents a promising tool for basic miRNA research and clinical diagnosis of human diseases based on miRNA biomarkers.
MicroRNAs (miRNAs) are small noncoding RNAs of ∼22 nucleotide (nt) in length. They exist in almost all eukaryotic organisms and regulate gene expression by binding the 3′-untranslated region (3′-UTR) of target mRNAs1,2,3. Once a miRNA binds to its target mRNA, protein translation is inhibited or the mRNA is degraded via the miRNA-mediated RNA interference1,4. miRNAs are involved in cell differentiation, proliferation, and apoptosis and are implicated in many types of diseases5,6,7,8,9. miRNA expression profiles differ between healthy and diseased tissue10,11,12. Furthermore, miRNAs circulate in blood in a surprisingly high stable form13. Thus, circulating miRNAs have the remarkable potential to be developed as diagnostic, prognostic, and predictive biomarkers14,15,16.
The quantitative real-time PCR (qRT-PCR) has been recognized as one of the most sensitive techniques for miRNA detection17,18,19 although other methods for quantifying miRNA are available, such as northern blotting, microarray or deep sequencing. Several approaches have been reported for detection of miRNAs using qRT-PCR. The most popular stem-loop method requires unique reverse transcription primers and a specific probe for each miRNA assay, and therefore it is fairly costly for screening of a large number of miRNAs17. The poly(A) method relies on adding a poly(A) tail to all types of RNAs, including miRNAs, and using a generic oligo(dT) primer for the reverse transcription (RT)20. This method is capable of assaying miRNA expression in a high-throughput manner. However, it is less specific due to the non-specific reverse transcription. We have previously developed a novel S-Poly(T) method for miRNA qPCR assay, which is based on a uniquely designed S-Poly(T) reverse-transcription (RT) primer that contains six miRNA-specific bases and a oligo(dT) sequence21. Although the sensitivity of the S-Poly(T) method is at least 4 times higher than that of the stem-loop and poly(A) methods, the S-Poly(T) method is still time-consuming and less-efficient since polyadenylation and reverse transcription are performed separately.
Therefore, there is a need for further optimization of miRNA quantitation using qRT-PCR, particularly for high-throughput screening or clinical samples with low miRNA levels, such as human serum/plasma (S/P). Based on our recently developed S-Poly(T) method21, here we describe an improved method for detecting circulating miRNAs, termed S-Poly(T) Plus. The novelty of this approach is the combination of polyadenylation and reverse transcription into one step with retaining the S-Poly(T) primer. We optimized the experimental protocol for the S-Poly(T) Plus method. We also validated this method by quantifying miRNA expression profiles in the sera of pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD-PAH) patients. We finally highlighted the strengths and limitations of this approach for clinical applications.
Results
Design of S-Poly(T) Plus method
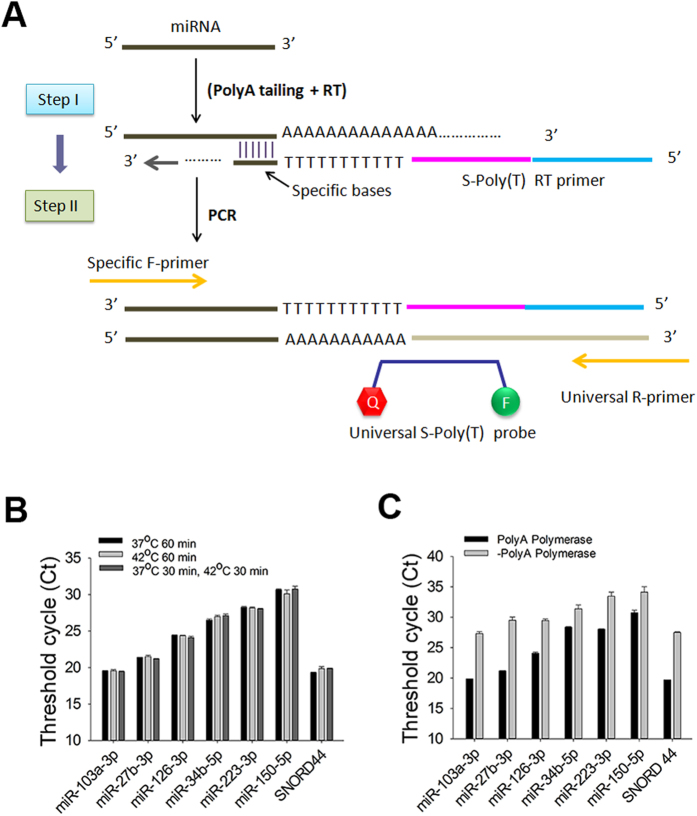
The higher sensitivity and specificity of S-Poly(T) method are achieved by the application of the S-Poly(T) reverse-transcription (RT) primer21. However, polyadenylation and reverse transcription in the S-Poly(T) method are carried out separately, which is time-consuming and costly. In the new version of S-Ploy(T) Plus method, we combined polyadenylation and reverse transcription into a one-step, multiple-stage reaction, which increased efficiency. With the optimized reaction buffer, the reaction time was reduced to 65 min compared to 105 min in the two-step reaction of the S-Poly(T) method; The RNA amount from serum/plasma needed for the assay was decreased to one-fifth (see the Methods). The schematic diagram of the S-Poly(T) Plus protocol was depicted in the Fig. 1A. All the primers and probes used in this study are listed in Supplemental file 1.
Figure 1. Optimization of S-Poly(T) Plus method for circulating miRNA assay.
(A) A schematic representation of the S-Poly(T) Plus method for miRNA quantification. miRNAs are polyadenylated and reverse-transcribed into cDNA simultaneously. The S-Poly(T) RT primer consists of four segments, in 5′ to 3′ direction, a universal reverse primer sequence, a universal Taqman probe sequence and 17 bases (11 dT and 6 specific bases) that are complementary to the 3′ end of a particular miRNA with tailed poly(A). The PCR products are generated with a miRNA-specific forward primer and a universal reverse primer and detected by a universal Taqman probe. (B) Effect of reaction temperatures on polyA polymerase and reverse transcriptase. (C) Effects of polyA polymerase on the detection of miRNAs in the one-step polyadenylation/RT procedure. The expression levels of miR-103a-3p, miR-27b-3p, miR-126-3p, miR-34b-5p, miR-223-3p, miR-150-5p and SNORD44 in (B,C) were determined using total RNAs isolated from human HEK293A cells.
Because PolyA polymerase and reverse transcriptase could work at 37 oC or 42 oC, we tested the effects of temperatures on the efficiency of one-step, multiple-stage reaction. As shown in Fig. 1B, we did not observe significant differences in human HEK293A cells for all the miRNAs we tested under the three temperature conditions. We recommend using the condition of 37 oC for 30 min and then 42 oC for 30 min to retain the respective optimal reaction temperature of both PolyA polymerase and reverse transcriptase.
We also used human HEK293A cells to test whether Poly(A) polymerase is required for the one-step, multiple-stage reaction. The Ct values of miRNA qRT-PCR assays decreased by 3.0 ~ 8.3 units when Poly(A) polymerase was neglected in the reaction mixture (Fig. 1C), indicating that the polyadenylation step is critical for the improvement of sensitivity in S-Poly(T) Plus method.
Sensitivity and dynamic range of S-Poly(T) Plus
To demonstrate the improvement of the new method, the sensitivity between S-Poly(T) and S-Poly(T) Plus was compared. We first determined the expression levels of six miRNAs (miR-103a-3p, miR-27b-3p, miR-126-3p, miR-34b-5p, miR-223-3p, and miR-150-5p) and a snoRNA (SNORD44) in human HEK293A cells. The results showed that the Ct values of all the miRNAs as assayed by S-Poly(T) Plus were smaller than those by S-Poly(T) method. miR-223-3p showed the largest reduction in Ct value (3.07) and SNORD44 the smallest reduction (0.52) between two methods. Thus, S-Poly(T) Plus was 1.4 ~ 8.4-fold more sensitive than S-Poly(T) as calculated by 2^-ΔCt method (Fig. 2A).
Figure 2. Comparison of sensitivity in miRNA assay between S-Poly(T) and S-Poly(T) Plus methods.
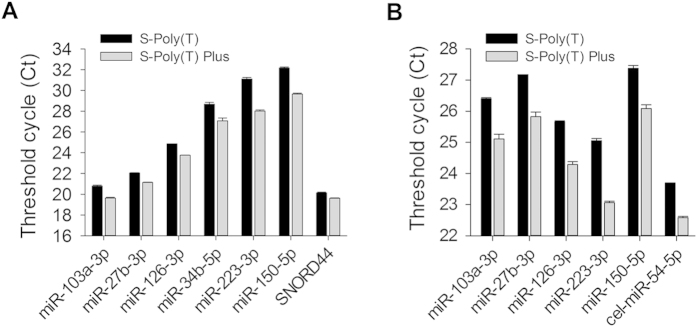
(A) The expression levels of miR-103a-3p, miR-27b-3p, miR-126-3p, miR-34b-5p, miR-223-3p, miR-150-5p and SNORD44 were determined using total RNAs isolated from human HEK293A cells. (B) The expression levels of five human miRNAs of miR-103a-3p, miR-27b-3p, miR-126-3p, miR-223-3p, miR-150-5p and a spiked-in Caenorhabditis elegans miRNA cel-miR-54-5p were measured using total RNAs prepared from 24 healthy human serum mixture.
We also tested the sensitivity of S-Poly(T) Plus using human serum samples. As shown in Fig. 2B, all the Ct values of six human miRNAs (miR-103a-3p, miR-27b-3p, miR-126-3p, miR-223-3p, and miR-150-5p) and a spiked-in Caenorhabditis elegans cel-miR-54-5p were significantly reduced using S-Poly(T) Plus when compared to those using S-Poly(T) method. Similar to HEK293A cells, miR-223-3p displayed the largest difference in Ct value (2.00), and miR-27b-3p the smallest (1.11), with 2.2 ~ 4.0-fold differences in sensitivity between S-Poly(T) and S-Poly(T) Plus methods.
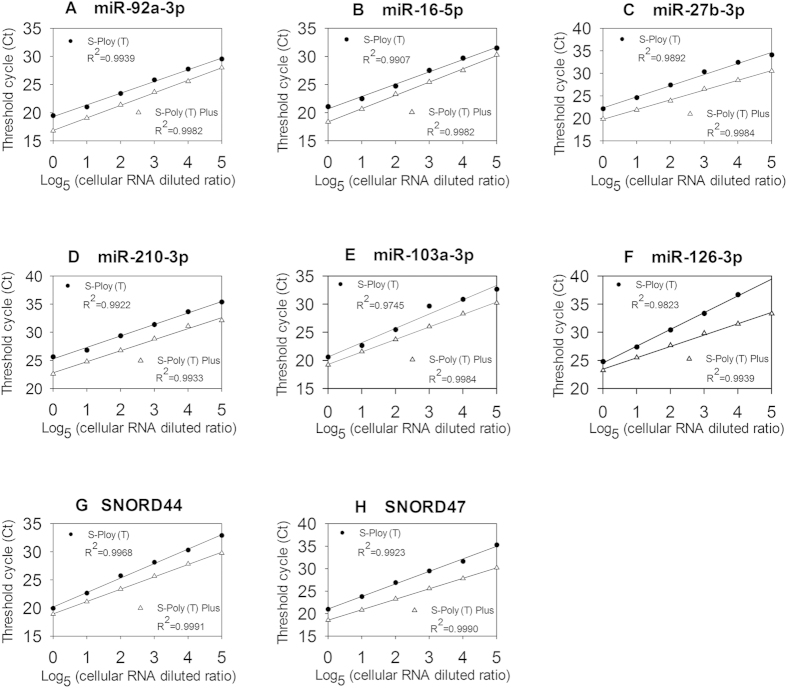
We further evaluated the dynamic range of S-Poly(T) Plus. Six miRNAs (miR-92a-3p, miR-16-5p, miR-27b-3p, miR-210-3p, miR-103a-3p and miR-126-3p) and two snoRNAs (SNORD44 and SNORD47) were selected for this purpose. Total RNAs from HEK293A cells ranged from 2.5 ng (original) to 0.8 pg (diluted 55 times) were used for each miRNA detection. As shown in Fig. 3, the correlation coefficients (R2) produced by S-Poly(T) Plus and S-Poly(T) methods were 0.9933 ~ 0.9991 and 0.9745 ~ 0.9968, respectively. However, the regression lines of S-Poly(T) Plus were below those of S-Poly(T) method and there were 1.0 ~ 5.2 units difference in Ct value between two methods, indicating the increased sensitivity of S-Poly(T) Plus.
Figure 3. The correlation between total RNA inputs and threshold cycle (Ct) values of HEK293A cells as determined by S-Poly (T) and S-Poly (T) Plus methods.
Total RNAs were isolated from human HEK293A cells. The RNA inputs ranged from 2.5 ng (original) to 0.8 pg (diluted 55 times) for each real-time PCR reaction. (A) miR-92a-3p, (B) miR-16-5p, (C) miR-27b-3p, (D) miR-210-3p, (E) miR-103a-3p, (F) miR-126-3p, (G) SNORD44 and (H) SNORD47.
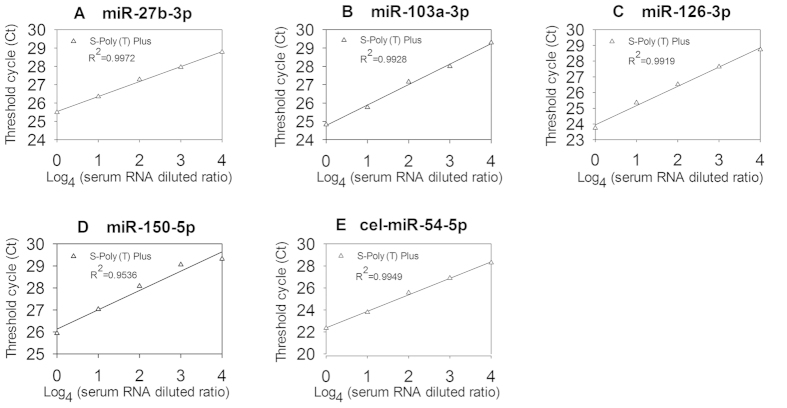
Human serum samples were further used to evaluate the dynamic range of S-Poly(T) Plus. Four miRNAs (miR-27-3p, miR-103a-3p, miR-126-3p and miR-150-5p) and a spiked-in cel-miR-54-5p were tested. We observed an excellent linear relationship between total RNA inputs and Ct values with R2 ranging between 0.9536 and 0.9972. Typically, 0.075 μl (original) ~ 0.3 nl (diluted 44 times) of initial serum RNA, corresponding to 0.38 μl ~ 1.5 nl of human serum sample, was sufficient for detection of certain miRNA using S-Poly(T) Plus (Fig. 4).
Figure 4. The correlation between total RNA inputs and threshold cycle (Ct) values of human sera as determined by S-Poly (T) Plus method.
A 0.075 μl (original) ~ 0.3 nl (diluted 44 times) of initial serum RNA, corresponding to 0.38 μl ~ 1.5 nl of human serum sample were used in each real-time PCR reaction. (A) miR-27-3p, (B) miR-103a-3p, (C) miR-126-3p, (D) miR-150-5p and (E) cel-miR-54-5p.
Optimization of RNA isolation
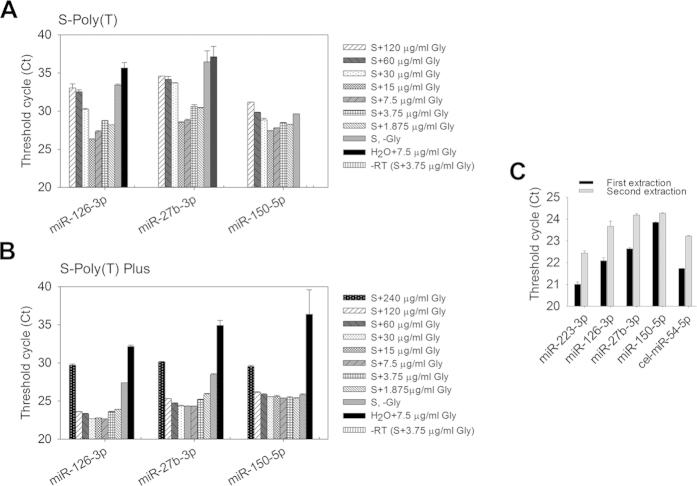
For circulating miRNA assay, total RNAs isolation from serum/plasma samples is very critical. To increase the yield of RNA isolation from serum or plasma samples, various concentrations of glycogen were added during the precipitation step of RNA extraction. We found that the optimized concentrations of glycogen were 1.875 ~ 15 μg/ml for S-Poly(T) method and 1.875 ~ 120 μg/ml for S-Poly(T) Plus (Fig. 5A,B). There was no significant signal detected in the controls without reverse transcriptase (-RT) using both S-Poly(T) and S-Poly(T) Plus methods. The smaller Ct values and a wider concentration range of glycogen suitable for RNA precipitation proved that the S-Poly(T) Plus assay was more sensitive and stable than the S-Poly(T) method. We routinely use 15 μg/ml of glycogen for RNA extraction for both S-Poly(T) and S-Poly(T) Plus methods and we named our RNA extraction method for serum/plasma samples S/P miRsol.
Figure 5. Optimization of serum RNA extraction using the co-precipitant, glycogen.
Various concentrations of glycogen were applied to enhance the precipitation of serum RNA, which were used for detection of miRNAs using S-Poly(T) (A) and S-Poly(T) Plus (B). S: serum, Gly: glycogen and -RT: without reverse transcriptase. The concentrations of glycogen were 240 ~ 1.875 μg/ml. (C) Serum was subjected to the first or second extraction (see Method section for definitions for first/second extraction). The isolated RNA was assayed for miRNA levels using S-Poly(T) Plus method.
A previous study reported that an additional extraction of organic layer of serum samples can increase RNA yield when the amounts of samples are limited22. After the first extraction of RNAs as described above, we added 500 μl of water to the organic phase and collected the aqueous phase as the second extraction of RNAs after mixing and centrifugation. Using S-Poly(T) Plus method, we detected significant signals of the second extracted RNAs in miRNA qRT-PCR assay although the Ct values were 0.42 ~ 1.58 units larger than those of the first-extracted RNAs (Fig. 5C).
We next compared our S/P miRsol with two commercially available RNA isolation kits, miReasy Mini Kit (Qiagen) and mirVana PARIS Kit (Ambion). Due to interference of glycogen on the absorbance of RNA, the concentration of total RNAs cannot be measured correctly by spectrophotometer. Therefore, we evaluated the efficiency of RNA extraction among different methods based on Ct values measured by qPCR using an equal volume of initial serum/plasma. The Ct values using S/P miRsol were decreased by 1.23 ~ 2.92 units compared to the miReasy Mini Kit and mirVana PARIS Kit, indicating that our method was more effective in serum RNA recovery for circulating miRNA assay (Fig. 6).
Figure 6. Comparison of the RNA isolation method, S/P miRsol developed in the current study with two commercial RNA isolation kits, miReasy Mini Kit (Qiagen) and mirVana PARIS Kit (Ambion).
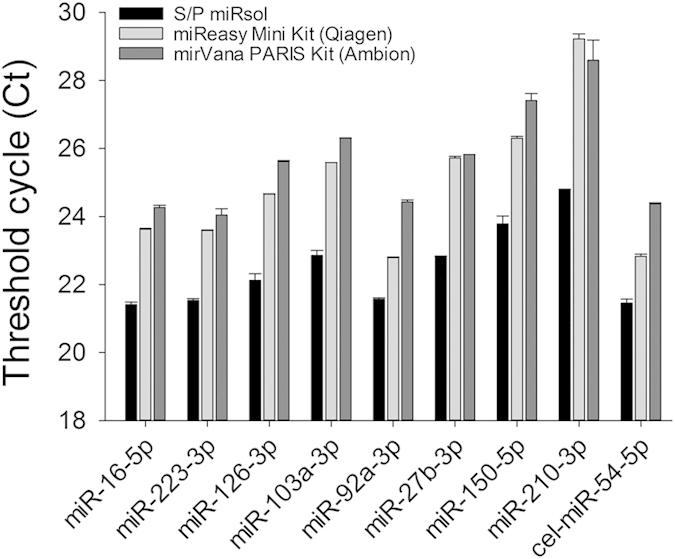
The efficiency of RNA extraction among different methods was evaluated based on Ct values measured by qPCR using an equal volume of initial serum.
microRNA expression profiling in CHD-PAH
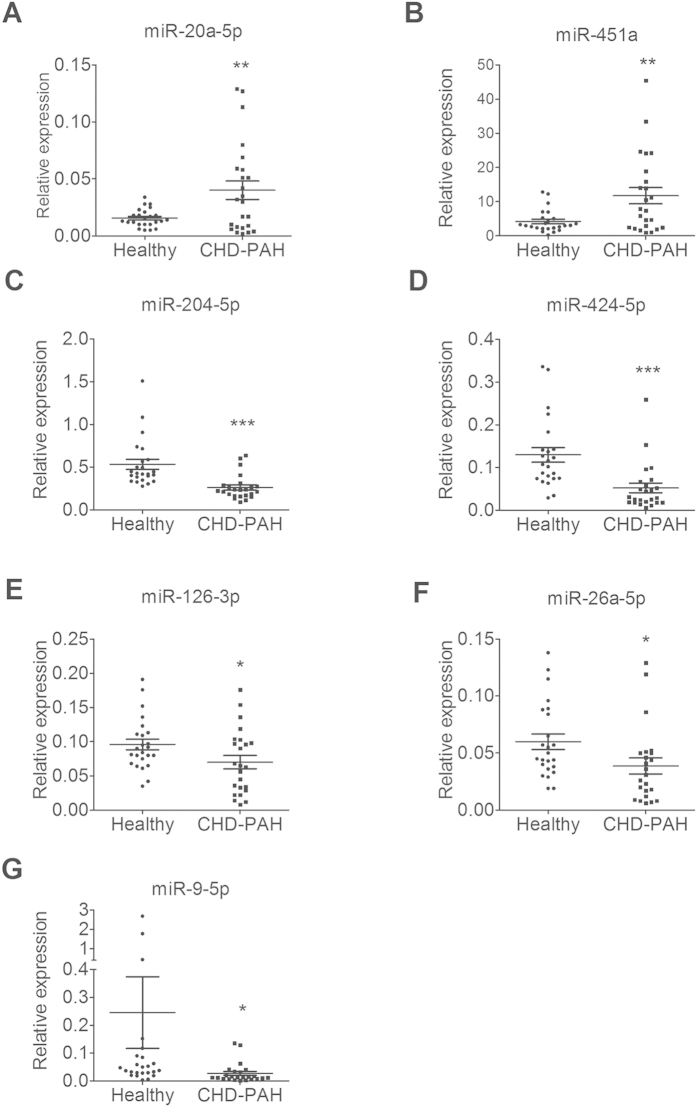
miRNAs can be used as potential biomarkers for many human diseases. We tested the utility of S/P miRsol and S-Poly(T) Plus in clinical applications by determining miRNA profiles in sera. A total of 31 human miRNAs possibly related to pulmonary arterial hypertension (PAH) were selected based on previous reports (Supplemental file 2). The cel-miR-54-5p was used as a spiked-in normalization control. We first performed first screen by pooling serum samples of 24 healthy subjects and 24 patients with CHD-PAH and fourteen miRNAs (miR-451a, miR-9, miR-424, miR-223, miR-204, miR-150, miR-328, miR-21, miR-34a, miR-34b, miR-26a, miR-27b, miR-126 and miR-20a) changed more than 1.5-fold. These miRNAs were further validated in each of the sera from 24 CHD-PAH patients and 24 healthy subjects individually. We found that two miRNAs (miR-20a-5p and miR-451a) were significantly up-regulated and five miRNAs (miR-204-5p, miR-424-5p, miR-126-3p, miR-26a-5p and miR-9-5p) were significantly down-regulated in CHD-PAH sera compared to the healthy controls (Fig. 7).
Figure 7. Differentially expressed miRNAs in the sera of CHD-PAH patients and healthy individuals.
The expression levels of miRNAs were determined by S-Poly(T) Plus in the sera of 24 CHD-PAH patients and 24 healthy controls. miRNA levels were normalized to spiked-in cel-miR-54-5p and represented in scatter plots. Data are shown as means ± SE, *p < 0.1 v.s. healthy control, **p < 0.01 v.s. healthy control, ***p < 0.001 v.s. healthy controls.
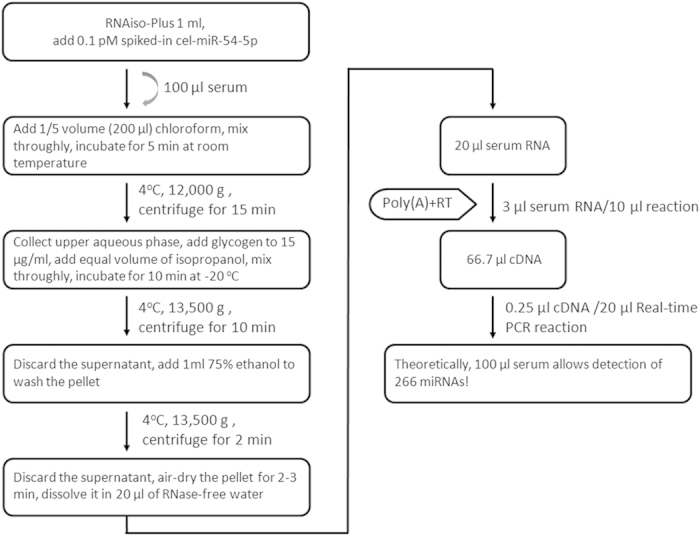
Taken all together, we developed an extremely sensitive and practical protocol for circulating miRNA detection by combining the S/P miRsol RNA extraction and the S-poly(T) Plus miRNA detection methods. If losses during the whole procedure are ignored, 100 μl of serum or plasma can be used to detect 266 miRNAs (Fig. 8).
Figure 8. An optimized protocol for circulating miRNA analysis using S-Poly(T) Plus.
Discussion
The circulating miRNAs have the potential to be used as biomarkers in the diagnosis of human diseases23,24. In our previously reported S-Poly(T) method, a S-Poly(T) primer consisting of an oligo(dT)11 sequence and six miRNA-specific bases is used during reverse transcription, thereby providing higher binding strength and thermodynamic stability between RT primer and miRNA template. The sensitivity of S-Poly(T) assay was at least 4-fold higher than that of the widely used Stem-loop method21.
In the S-Poly(T) Plus assay reported in this study, RNAs were polyadenylated and reverse-transcribed with the S-Poly(T) primer in a single-step reaction. The sensitivity was increased by at least one fold compared to the S-Poly(T) method. The improved sensitivity could be due to the larger RNA substrate concentration in mixture reaction. In S-Poly(T) Plus method, 3 μl serum RNA was used in a 10 μl polyA tailing/RT reaction mixture. In S-Poly(T) method, 7.5 μl serum RNA was first used in a 10 μl of polyA tailing reaction mixture and only 2 μl of the polyadenylation reaction product containing 1.5 μl serum RNA was then used in a 10 μl of RT reaction mixture. Besides, less loss and lower risk of RNA degradation may also contribute to a higher sensitivity of S-Poly(T) Plus.
It is challenging to extract RNAs efficiently from serum/plasma because a tiny amount of RNAs is present in these samples. In this study, we successfully used the co-precipitant, glycogen to significantly improve the recovery of circulating miRNA (Figs 5 and 6). The S-Poly(T) Plus method is also cost-effective since all of the polyA-tailed RNA is used for RT reaction. By contrast, only one-fifth of polyadenylation product is added in the RT reaction in S-Poly(T) method. One hundred microliter of serum or plasma is sufficient for detecting 266 miRNAs using S-Poly(T) Plus method. Thus, the new method provides a powerful tool for miRNA screen when limited blood sample is available. For example, only limited amounts of blood can be drawn from transgenic mice; a droplet of blood can be used for point-of-care diagnosis of diseases.
PAH is characterized by vasculopathy due to increased vasoconstrictor tone and thrombosis. The structural changes are driven by excessive vascular cell growth and inflammation25. PAH occurs in different clinical conditions depending on associated diseases26, and it is commonly associated with congenital heart disease (15–30%)27. In the present study, the expression profiles of miRNAs in the sera of clinical CHD-PAH patients were validated by the S-Poly(T) Plus method. Our results revealed up-regulation of miR-20a-5p and miR-451a, and down-regulation of miR-204-5p, miR-424-5p, miR-126-3p, miR-26a-5p and miR-9-5p in the sera of CHD-PAH patients. These miRNAs may be of potential use as early detection biomarkers for CHD-PAH.
Reduced miR-150 levels have been observed in idiopathic PAH patients23. miR-210 is one of the validated miRNAs that are significantly induced by hypoxia in HPASMC, or other cell lines21,28. However, we did not observe any changes of these two miRNAs in the CHD-PAH patients. The discrepancies are not entirely unexpected and could be the results of the differential pathobiology induced by different diseases, drugs, or hypoxia26.
PAH is also characterized largely by enhanced proliferation and reduced apoptosis of PASMCs29. We have previously reported that miR-20a-5p directly targets the PRKG1 gene, thereby promoting the proliferation and migration of PASMCs but inhibiting cell differentiation30. We have also shown that miR-9-5p increases PAH-PASMC proliferation and resistance to apoptosis by enhancing the activity of NFAT via targeting KPNB1 and DYRK1B31. The down-regulation of miR-204 is found in PAH-PASMCs and pulmonary delivering of miR-204 reduces disease severity in a PAH animal model32. Other miRNAs among the 31 miRNAs selected for miRNA profiling in PAH have also been studied. For instance, the genetic ablation of miR-145 results in a significant decrease in PAH development in mouse models33,34. miR-451 promotes migration of PASMCs, but interestingly, the genetic loss of miR-451 does not attenuate the development of PAH. This is probably because the pathway redundancy compensates for the loss of miR-45135,36,37,38,39,40,41,42,43,44,45. In addition to PAH, cancer or other disease-specific miRNAs should further be explored for their clinical applications as reliable biomarkers.
In summary, the current study provides a simple, sensitive, and inexpensive miRNA profiling tool for detecting circulating miRNAs with the precise quantification. This tool could be used for diagnosis for a variety of human PAH, cancers or other diseases based on miRNA biomarkers.
Methods
Serum collection and cell culture
Human serum samples were collected from 24 healthy donors and 24 patients with CHD-PAH from the Yat-Sen Cardiovascular Hospital (Shenzhen, China). The blood samples were kept at room temperature for 1 h, and then centrifuged at 3,000 g for 10 min at 4 oC. The sera were stored at −80 oC. All procedures were carried out according to the approved guidelines. The Institutional Ethics Committee at the Sun Yat-Sen Cardiovascular Hospital (Shenzhen, China) approved the experiments. All participants were provided with written informed consent.
Human HEK293A cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 oC and 5% CO2 condition.
S/P miRsol RNA extraction
Circulating RNAs were isolated from serum/plasma by S/P miRsol method developed in this study. Briefly, 1 ml of RNAiso-Plus (TaKaRa, Dalian, China) supplemented with 0.1 pM spiked-in Caenorhabditis elegans cel-miR-54-5p for normalization was added to 100 μl of serum or plasma and quickly vortexed thoroughly. Then 200 μl of chloroform was added and mixed by vigorous shaking for 20 s. The samples were incubated for 5 min at room temperature and centrifuged at 12,000 g for 15 min at 4 oC. A 500 μl of aqueous phase was transferred into a fresh tube and mixed with 6 μl of glycogen (AppliChem, Darmstadt, Germany). An equal volume (506 μl) of isopropyl alcohol was added into the mixture and incubated at −20 oC for 10 min followed by centrifugation at 13,500 g for 10 min at 4 oC. The RNA pellet was washed with 1 ml of 75% ethanol and dissolved in 20 μl of RNase-free water. The same procedure was used to isolate total RNAs from cultured cells except that no spiked-in cel-miR-54-5p and glycogen were used.
In certain experiments, second extraction was performed to see whether additional RNAs can be recovered from serum/plasma samples. Briefly, after 500 μl of aqueous phase was removed for the first exaction, an equal volume (500 μl) of RNase-free water was added to the organic phase. A 500 μl aqueous phase was collected as the second extraction of RNAs after mixing and centrifugation
Polyadenylation and reverse transcription
For the S-Poly(T) method, total RNA from cultured cells or serum was polyadenylated with the Poly(A) Polymerase Tailing Kit (Epicentre, Madison, USA) according to manufacturer’s instruction. Briefly, 1 μl of 10 × poly(A) polymerase reaction buffer, 1 μl of 10 mM ATP and 0.8 units of Poly(A) polymerase were added to 500 ng total RNA from cells/tissues or 7.5 μl of 20 μl total RNAs isolated from 100 μl serum/plasma. The reaction mixture was made to a total volume of 10 μl with RNase-free water and incubated at 37 oC for 30 min and then 65 oC for 5 min. The tailed miRNA was reverse-transcribed in a 10 μl reaction containing 2 μl of polyadenylation reaction product, 1 μl of 0.5 μM RT primer, 2.5 μl of 2 mM dNTP, 1 μl of MMLV HP RT 10 × reaction buffer, and 100 units of MMLV High Performance Reverse Transcriptase (Epicentre, Madison, WY, USA). The reaction was incubated at 42 oC for 60 min and terminated at 70 oC for 10 min.
In the S-Poly(T) Plus method, polyadenylation and reverse transcription were performed in a single step with an optimized buffer. In brief, the following components were added to 10 μl of reaction mixture: 100 ng of total RNAs from cells/tissues or 3 μl of 20 μl total RNAs isolated from 100 μl of serum/plasma, 2.5 μl of 4 × reaction buffer mix containing ATP and dNTPs , 1 μl of 0.5 μM RT primer and 1 μl of polyA/RT enzyme mix (with 0.8 units of Poly(A) polymerase and 100 units of MMLV High Performance Reverse Transcriptase). The reaction was incubated at 37 oC for 30 min, 42 oC for 30 min, and then terminated at 75 oC for 5 min.
To compare the sensitivity and dynamic range of two methods, the serially diluted RNAs were used in the polyadenylation and reverse transcription reaction. For RNA samples from cells, the amounts of RNAs for each qPCR reaction ranged from 2.5 ng (original) to 0.8 pg (diluted 55 times). For serum RNA samples, the volumes of RNAs for each qPCR reaction ranged from 0.075 μl (original) to 0.3 nl (diluted 44 times).
Real-time PCR
For comparison, 0.5 μl cDNA for S-Poly(T) and 0.25 μl cDNA for S-Poly(T) Plus were used in real-time PCR reaction for serum/plasma samples to allow an equal amount of RNA inputs. An equal volume of cDNA (0.5 μl) was used in both methods for cell/tissue samples. The reaction mixture (20 μl) consisted of 4 μl 5 × qPCR Reaction Buffer, 0.5 unit hotstar Taq Polymerase (Geneup, Shenzhen, China), 0.4 μl 10 μM forward primer, 0.4 μl 10 μM universal reverse primer, 0.5 μl 10 μM universal Taqman probe, and 0.2 μl 100 × ROX reference dye. Real-time PCR was carried out on ABI StepOnePlus thermal cycler at the following conditions: 95 oC for 3 min, followed by 40 cycles of 95 oC for 10 s and 60 oC for 30 s.
miRNA profiling
A total of 31 human miRNAs possibly related to PAH were selected based on literatures and was listed in Supplemental file 236,37,38,39,40,41,42,43,44,45. For the first screen, we pooled serum samples of 24 healthy subjects and 24 patients with CHD-PAH for miRNA profiling using the S-Poly(T) Plus method. Only those miRNAs with more than a 1.5-fold change in CHD-PAH group compared to healthy group in the first screen were selected for validation in each individual of 24 CHD-PAH patients and 24 healthy controls. Relative quantities of miRNAs were calculated using the 2^-ΔCt method with cel-miR-54-5p spiked-in as a normalization control. Two-tailed Student’s test was used for statistical analysis using the GraphPad Prism 5. Data was shown as means ± SE (standard error).
Additional Information
How to cite this article: Niu, Y. et al. An improved method for detecting circulating microRNAs with S-Poly(T) Plus real-time PCR. Sci. Rep. 5, 15100; doi: 10.1038/srep15100 (2015).
Supplementary Material
Acknowledgments
This study was supported in part by Natural Science Foundation of China 81170047, 81370151 and 81570046 to D.G. and 31571199 to K.K. National Basic Research Program of China 973 Program 2012CB124701, Shenzhen Overseas High-Level Talents Innovation Program YFZZ20111009) to D.G. Shenzhen High-tech Development Project CXZZ20140828163951592 to D.G. Transgenic project from the Ministry of Agriculture 2014ZX08009-051B to D.G., Shenzhen Municipal Basic Research Program JCYJ20130329120507746 to K.K., and Shenzhen University Foundation 201562 to K.K.
Footnotes
Author Contributions Conceived and designed the experiment: Deming Gou and Kang Kang; collected the blood samples: Z.W. and Y.Z.; conducted the experiments: L.Z. andY.W.; performed data analysis: Y.N. and L.Z.; wrote the manuscript: Y.N., H.Q., L.L., J.Q., K.K. and D.G.; All the authors have reviewed the manuscript.
References
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- He L. & Hannon G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 (2004). [DOI] [PubMed] [Google Scholar]
- Lim L. P., Glasner M. E., Yekta S., Burge C. B. & Bartel D. P. Vertebrate microRNA genes. Science 299, 1540–1540 (2003). [DOI] [PubMed] [Google Scholar]
- Bader A. G., Brown D. & Winkler M. The promise of microRNA replacement therapy. Cancer Res. 70, 7027–7030 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N. & Cohen S. M. microRNA functions. Annu. Rev. Cell Dev. Biol. 23, 175–205 (2007). [DOI] [PubMed] [Google Scholar]
- Chen J.-F. et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 38, 228–233 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. & Calin G. A. miRNAs, cancer, and stem cell division. Cell 122, 6–7 (2005). [DOI] [PubMed] [Google Scholar]
- Di Masi A. et al. Characterization of HuH6, Hep3B, HepG2 and HLE liver cancer cell lines by WNT/beta-catenin pathway, microRNA expression and protein expression profile. Cell Mol. Biol. 56, OL1299–1317 (2010). [PubMed] [Google Scholar]
- Diakos C. et al. TEL-AML1 regulation of survivin and apoptosis via miRNA-494 and miRNA-320a. Blood 116, 4885–4893 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. G. et al. Evaluation of two commercial global miRNA expression profiling platforms for detection of less abundant miRNAs. BMC Genomics 12, 435 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick K. E. et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 112, 55–59 (2009). [DOI] [PubMed] [Google Scholar]
- Wang K. et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. P. Nat. A. Sci. 106, 4402–4407 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. P. Nat. A. Sci. 105, 10513–10518 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. K., Bang C. & Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ. Cardiovasc. Gene. 3, 484–488 (2010). [DOI] [PubMed] [Google Scholar]
- Roth C. et al. Circulating microRNAs as blood-based markers for patients with primary and metastatic breast cancer. Breast Cancer Res. 12, R90 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiyama Y. et al. Serum miR-200c is a novel prognostic and metastasis-predictive biomarker in patients with colorectal cancer. Ann. Surg. 259, 735–743 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Ccids Res. 33, e179–e179 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Lee E. J., Gusev Y. & Schmittgen T. D. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Ccids Res. 33, 5394–5403 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenstein I., Aizenberg N., Goshen M., Bentwich Z. & Avni Y. S. A novel qPCR assay for viral encoded microRNAs. J. Virol. methods 163, 323–328 (2010). [DOI] [PubMed] [Google Scholar]
- Design R.-T. P. P. Facile means for quantifying microRNA expression by real-time PCR. Biotechniques 39, 519–525 (2005). [DOI] [PubMed] [Google Scholar]
- Kang K. et al. A novel real-time PCR assay of microRNAs using S-Poly (T), a specific oligo (dT) reverse transcription primer with excellent sensitivity and specificity. PloS one 7, e48536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos K. L. et al. Identification of extracellular miRNA in human cerebrospinal fluid by next-generation sequencing. RNA 19, 712–722 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes C. J. et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am. J. Resp. Crit. Care. 187, 294–302 (2013). [DOI] [PubMed] [Google Scholar]
- Wei C. et al. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One 8, e64396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly R. T., Ghofrani H. A., Wilkins M. R. & Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat. Rev. Cardiol. 8, 443–455 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau G. et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 54, S43–S54 (2009). [DOI] [PubMed] [Google Scholar]
- Landzberg M. J. Congenital heart disease associated pulmonary arterial hypertension. Clin. Chest Med. 28, 243–253 (2007). [DOI] [PubMed] [Google Scholar]
- Camps C. et al. miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin. Cancer Res. 14, 1340–1348 (2008). [DOI] [PubMed] [Google Scholar]
- Mandegar M. et al. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc. Res. 68, 75–103 (2004). [DOI] [PubMed] [Google Scholar]
- Zeng Y. et al. MiR-20a regulates the PRKG1 gene by targeting its coding region in pulmonary arterial smooth muscle cells. FEBS Lett. 588, 4677–4685 (2014). [DOI] [PubMed] [Google Scholar]
- Zeng Y. et al. MicroRNA-9 enhances the transactivation of nuclear factor of activated T cells by targeting KPNB1 and DYRK1B. Am. J. Physiol-Cell Ph. 308, C720–C728 (2015). [DOI] [PubMed] [Google Scholar]
- Courboulin A. et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 208, 535–548 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso P. et al. A role for miR-145 in pulmonary arterial hypertension evidence from mouse models and patient samples. Circ. Res. 111, 290–300 (2012). [DOI] [PubMed] [Google Scholar]
- Caruso P. et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscl. Throm. Vas. 30, 716–723 (2010). [DOI] [PubMed] [Google Scholar]
- Grant J. S. et al. Transient but not genetic loss of miR-451 is protective in the development of pulmonary arterial hypertension. Pulm. Cir. 3, 840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. P. Nat. Acad. Sci. 107, 3240–3244 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J. et al. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am. J. Physiol-Lung C. 299, L861–L871 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanshan L. et al. MicroRNA-138 plays a role in hypoxic pulmonary vascular remodelling by targeting Mst1. Biochem. J. 452, 281–291 (2013). [DOI] [PubMed] [Google Scholar]
- Cheng Y. et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 105, 158–166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno S. et al. p53 Gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am. J. Physiol-Lung C. 300, L753–L761 (2011). [DOI] [PubMed] [Google Scholar]
- Guo L. et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension 59, 1006–1013 (2012). [DOI] [PubMed] [Google Scholar]
- Bockmeyer C. L. et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J. Heart Lung Transpl. 31, 764–772 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat. Med. 19, 74–82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. et al. MicroRNA-124 suppresses the transactivation of nuclear factor of activated T cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J. Biol. Chem. 288, 25414–25427 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck J. et al. MiR-223 Improves Experimental Pulmonary Arterial Hypertension. Circulation 130, A18653–A18653 (2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.