Abstract
Objectives
Non-communicable diseases (NCDs) constitute an increasing slice of the global burden of disease, with the South-East Asia region projected to see the highest increase in NCD-related deaths over the next decade. Mining industry employees may be exposed to various factors potentially elevating their NCD risk. This study aimed to assess the distribution and 5-year longitudinal trends of key metabolic NCD risk factors in a cohort of copper–gold mining company workers in Papua, Indonesia.
Methods
Metabolic indicators of NCD risk were assessed among employees (15 580 at baseline, 6496 prospectively) of a large copper–gold mining operation in Papua, Indonesia, using routinely collected 5-year medical surveillance data. The study cohort comprised individuals aged 18–68 years employed for ≥1 year during 2008–2013. Assessed risk factors were based on repeat measures of cholesterol, blood glucose, blood pressure and body weight, using WHO criteria.
Results
Metabolic risk indicator rates were markedly high and increased significantly from baseline through 5-year follow-up (p<0.001). Adjusting for gender and age, longer duration of employment (≥10 years) predicted raised cholesterol (adjusted OR (AOR)=1.13, p=0.003), raised blood pressure (AOR=1.16, p=0.009) and overweight/obesity (AOR=1.14, p=0.001) at baseline; and persistent raised cholesterol (AOR=1.26, p=0.003), and both incident (AOR=1.33, p=0.014) and persistent raised blood glucose (AOR=1.62, p=0.044) at 3-year follow-up.
Conclusions
Individuals employed for longer periods in a mining operations setting in Papua, Indonesia, may face elevated NCD risk through various routes. Workplace health promotion interventions and policies targeting modifiable lifestyle patterns and environmental exposures present an important opportunity to reduce such susceptibilities and mitigate associated health risks.
What this paper adds.
Non-communicable diseases (NCDs) constitute a large and growing segment of the global disease burden, with the South-East Asia region projected to see the highest increases in NCD-related deaths over the next decade.
Though previous studies have presented snapshots of NCD risk, this is the first to prospectively assess patterns and potential associations arising out of employment with a mining operation in a South-East Asian, developing country context.
Rates of measured metabolic risk indicators (raised cholesterol, blood pressure, blood glucose, and overweight/obesity) were markedly high, varied significantly by gender, and increased appreciably from baseline through 5-year follow-up.
Those employed for longer periods in the copper-gold mining operation at baseline were generally more likely to exhibit and prospectively maintain and/or develop the measured metabolic indicators of NCD risk, independent of gender or age effects.
Workplace health promotion interventions and policies targeting modifiable lifestyle patterns and environmental exposures present an important arena for private sector involvement in tackling the burgeoning NCD epidemic.
Introduction
The last millennium saw a worldwide epidemiological transition, where chronic, non-communicable diseases (NCDs)—principally cardiovascular diseases (CVDs), diabetes mellitus type 2, cancers and chronic respiratory diseases—overtook infectious diseases as the leading cause of morbidity and mortality.1 2 In 2011, 36 million (63%) of all premature deaths globally were attributable to NCDs,2 with the vast majority (85% of those between the ages of 30 and 70 years) occurring in low and middle income countries (LMICs).3 Without appropriate action to reduce NCD risk, the numbers will only continue to rise, imposing major human, social and economic costs.4–6
Notably, the highest increase in NCD-related deaths over the next decade is expected in the South-East Asia (SEA) region, which now accounts for 22% of the global NCD burden.4 In this region, between 2006 and 2015, mortality rates from infectious diseases are projected to fall by 16%, while NCD-related mortalities are expected to register a 21% increase.7 Of the approximately 7.9 million NCD deaths occurring in the SEA region in 2008, Indonesia shouldered the second-highest burden (13%).7 NCDs represent the leading causes of mortality within the country, accounting for around 71% of total deaths—more than all other causes combined.8
Effective prevention and management of NCDs hinges on addressing the root causes, which are predominantly based on social and environmental determinants.5 Across developed and developing countries, expanding globalisation, increased life expectancy and urbanisation, among other factors, have facilitated a global increase in key NCD risk factors such as tobacco use, unhealthy diet, physical inactivity and harmful use of alcohol. These behavioural risk factors contribute to the development of biomedical or metabolic risk factors, including raised cholesterol, blood glucose and blood pressure, as well as overweight and obesity, which can manifest through multiple and inter-related effects. For example, in addition to its direct role in diabetes, raised fasting blood glucose also increases the risk of cardiovascular deaths, and has been estimated to directly contribute to 22% of coronary heart disease deaths and 16% of stroke deaths.9 Similarly, overweight and obesity are associated with increased risk for multiple chronic diseases including cancer, type 2 diabetes, asthma and CVDs.10 11 Raised blood pressure, meanwhile, is perhaps the most powerful CVD risk factor after adjusting for tobacco use, accounting for about 54% of stroke and 47% of ischaemic heart disease globally.12
Evidence has demonstrated that NCDs are, to a great extent, preventable and that, by targeting common risk factors, health systems may effectively confront the ongoing epidemic.13–15 These risk factors are measurable and largely modifiable over time, and thus continuing surveillance of their levels is of fundamental importance in NCD control. Yet, while the prevalence and patterns of NCD risk in developed countries have been well established, few data are available in the SEA region or in most developing countries, where economic growth and associated sociodemographic changes frequently translate to increased prevalence of NCDs.7
The mining industry and NCD risk
Mining remains one of the most hazardous occupations in the world, both in terms of short-term injuries and fatalities, and through long-term exposure impacts such as cancers and chronic respiratory conditions. Various mining-associated environmental hazards have been linked to the development of NCDs. For instance, occupational exposure to noise and whole body vibration, as well as particulate air pollution, have been identified as risk factors for cardiovascular and respiratory morbidity and mortality.16–18 Overall, high levels of occupational stress, isolated living conditions, worksite food catering services, nocturnal work shifts and other related factors have all been shown to affect the development of NCD risk factors and consequent disease, reflected as well in increased mortality risk due to multiple related causes in miners compared with reference populations.19–22
Several studies in the past have presented snapshots of NCD risk,23 but few have prospectively assessed the impact of extended exposure to mining conditions over time or in a South-Asian, developing country context. Hence, the present study was undertaken with the objective of measuring the prevalences and prospective trends in major metabolic risk factors for NCDs and their association with duration of employment in a population of copper–gold mine employees residing in the Timika area of Mimika District, Papua Province, Indonesia.
Methods
Study population and setting
This research forms a part of the ongoing Cardiovascular Outcomes in a Papuan Population and Estimation of Risk (COPPER) Study. The present study population was comprised of both surface and underground workers aged 18–68 years who were employed by a multinational mining company and participated in mandatory annual health examinations performed at two health facilities from January 2008 through December 2013. Patient data are routinely collected as part of normal clinical and public health practice, and stored electronically on a central server. In total, data were available for 15 580 workers (15 021 men, 559 women) at baseline (where ‘baseline’ is defined as the first assessed health check-up, conducted in 2008) and 6496 workers (6320 men, 176 women) through 3-year, 4-year and 5-year follow-up surveillance points.
The COPPER Study is set in the Mimika District, located in the southern part of Papua Province (formerly Irian Jaya), Indonesia. The mining company operates one of the largest gold and copper mine production sites in the world and is the primary employer in the district. It directly or indirectly supports a primary and secondary hospital within the contract of work area, together with five community healthcare clinics and seven mine-site health posts. The mine itself is located at altitudes of up to 4200 m above sea level (asl) in one of the most remote locations and working environments on the planet—the mountainous western-central highlands of Papua Province. At the time of this study, the mining company employed approximately 22 558 people, of whom 71% worked at the highlands sites and 29% in the lowlands locations; males made up 94% of the workforce, 70% were below the age of 45 years, and approximately 98% were Indonesian nationals. Field workers, who constitute 63% of the total workforce, work in timed shifts (of either 8 h or 12 h intervals), and must engage in a significant amount of strenuous physical activity. Office workers comprise 37% of the total remaining workforce and spend most of their working time sitting while doing desk-bound activities, with minimal physical activity and no shift work duties. All workers live on site, where food is provided by the employer three times daily, with limited availability of external sources of intake.
To conduct the analyses, we secondarily used data that had already been obtained through the routine annual health examination process conducted through the health facilities. Thus, it was difficult to obtain informed consent retrospectively from the employees who had participated. As only anonymous data are used here, it was judged unnecessary to collect such consent for the purposes of this study. Study protocols were reviewed and approved by the Science Review Committee of International SOS.
Data collection and definition of NCD risk indicators
As part of annual medical check-ups, all participants underwent a physical examination including measurements of height (in bare feet without headwear, using a measuring tape against a wall), weight (in bare feet without heavy clothing, with standard weighing scale) and blood pressure (at the midpoint of the arm, with standard sphygmomanometer), as well as providing overnight (12 h) fasting blood samples. The associated blood tests included serum total cholesterol and fasting plasma glucose. On the basis of such measurements, metabolic risk factors for NCDs were assessed and defined as follows based on WHO criteria:24
Raised cholesterol: those aged 25 years or older having a total cholesterol value ≥190 mg/dL.
Raised blood glucose: those aged 25 years or older having a fasting plasma glucose value ≥126 mg/dL.
Raised blood pressure: those aged 25 years or older having systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg.
Overweight/obesity: those aged 20 years or older having a body mass index (BMI) ≥25 kg/m2 (with obesity defined as BMI ≥30 kg/m2), where BMI is calculated as weight in kilograms divided by height in metres squared.
Data were also collected on each worker's sex, birth date and starting date of employment with the company.
Statistical analyses
Student t tests and χ² analyses were used to compare baseline characteristics between workers included in the longitudinal analyses and those excluded from such analyses due to incomplete or missing follow-up data. Categorical data are presented as proportions, while numerical data are expressed as means±SD.
Separate one-way repeated measures analysis of variances (ANOVAs) with Bonferroni-corrected post-hoc tests were conducted for each NCD risk indicator to examine the main effect of time, comparing mean levels at baseline (T0), 3-year follow-up (T3), 4-year follow-up (T4) and 5-year follow-up (T5). Changes in prevalence of WHO-defined NCD risk indicators over time were further assessed using McNemar's χ² test (paired χ² test using Yates’ correction) for each time increment from baseline (ie, from T0 to T3, T4 and T5, respectively), with changes also depicted graphically by age bracket.
For investigating more directly the role of participants’ employment as miners in the observed progression of NCD risk over time, direct logistic regressions were performed to determine the impact of duration of employment (ie, time on site) on the likelihood that respondents would exhibit each of the measured metabolic risk indicators (ie, raised cholesterol, raised blood glucose, raised blood pressure, overweight/obesity) cross-sectionally at baseline and then, to account for possible reverse causality, prospectively at 3-year follow-up. For the prospective analyses, participants were analysed separately and outcomes presented in two groups: (1) as risk incidence (ie, new cases of elevated metabolic risk indicator levels) at 3-year follow-up among those with indicator levels falling below the designated risk threshold at baseline, and (2) as persistent risk (ie, continuing cases of elevated metabolic risk indicator levels) at 3-year follow-up among those with indicator levels meeting or exceeding the designated risk threshold at baseline. Separate regressions were modelled for each risk indicator, with categorical presence of the indicator (ie, raised vs normal) as the dependent variable and duration of employment at baseline as the primary independent variable of interest, tested in turn as both a categorical variable (≥10 vs<10 years) and as a continuous variable. Each such analysis was adjusted for gender and age bracket (25–29, 30–34, 35–39, 40–44, 45+ years) by inclusion of these variables as additional independent variables in the regression models.
Multicollinearity that might significantly affect regression results was ruled out by examining pairwise correlations, variation inflation factors (VIFs) and tolerance values for each independent variable. All predictor intercorrelations and VIFs were well below the conservative recommended cut-offs of 0.8025 and 2,26 respectively, and all tolerance values greater than 0.40,27 indicating no serious multicollinearity bias. In particular, though age and duration of employment were significantly correlated (p<0.01), the degree was low-to-moderate (r=0.478). Across all regression models, the highest VIF was 1.359 and the lowest tolerance value 0.736.
Analyses involving assessments of metabolic risk were generally restricted to participants aged 25 years and older, in line with WHO standard indicators. Independent variables were entered into each regression analysis using a direct (simultaneous) entry method. All statistical tests were two-sided and evaluated as significant at the p<0.05 level, using SPSS V.22.0 for Windows (IBM SPSS Inc, Chicago, Illinois, USA).
Results
Baseline characteristics of study participants
Characteristics of the 15 580 study participants at baseline, stratified by gender, are presented in table 1. Relative to their female counterparts, male employees skewed significantly older (p<0.001) and had been employed with the company for longer periods of time (p<0.001). Male employees were also significantly more likely to exhibit impaired (>100 mg/dL) fasting blood glucose ranges (p<0.001) and raised blood pressure, though less likely to be either underweight (BMI<18.5) or obese (BMI≥30).
Table 1.
Baseline demographic and metabolic risk characteristics of male and female mining company employees
| Characteristic | Total (N=15 580) | Males (n=15 021) | Females (n=559) |
|---|---|---|---|
| Age, years, %* | |||
| 18–29 | 22.3 | 21.8 | 33.9 |
| 30–34 | 21.9 | 21.6 | 30.2 |
| 35–39 | 24.5 | 24.7 | 20.5 |
| 40–44 | 18.3 | 18.6 | 9.9 |
| 45–68 | 13.1 | 13.3 | 5.6 |
| Mean (SD) | 36.3 (7.4) | 36.4 (7.4) | 33.4 (6.8) |
| Duration of employment, years, mean (SD) | 9.2 (4.0) | 9.3 (4.0) | 8.1 (3.8) |
| Serum total cholesterol range, mg/dL, %† | |||
| Normal (<190) | 58.5 | 58.5 | 56.4 |
| Raised (190–240) | 33.3 | 33.3 | 34.0 |
| High (240+) | 8.3 | 8.2 | 9.7 |
| Mean (SD) | 183.9 (40.4) | 183.8 (40.4) | 187.0 (40.9) |
| Fasting blood glucose range, mg/dL, %‡ | |||
| Normal (70.2–100) | 90.0 | 89.8 | 94.7 |
| Impaired (101–125) | 7.5 | 7.7 | 2.9 |
| Raised (>125) | 2.5 | 2.5 | 2.4 |
| Mean (SD) | 87.1 (20.8) | 87.3 (20.8) | 82.5 (18.0) |
| SBP range, mm Hg, %§ | |||
| Normal (<120) | 52.3 | 52.0 | 60.1 |
| Prehypertensive (120–139) | 42.3 | 42.5 | 37.6 |
| Stage 1 hypertensive (140–159) | 4.6 | 4.8 | 1.6 |
| Stage 2 hypertensive (160–179) | 0.5 | 0.5 | 0.7 |
| Hypertensive emergency (180+) | 0.2 | 0.2 | 0 |
| Mean (SD) | 113.9 (12.9) | 113.9 (13.0) | 111.9 (11.3) |
| DBP range, mm Hg, %§ | |||
| Normal (<80) | 55.5 | 54.7 | 75.5 |
| Prehypertensive (80–89) | 33.3 | 33.8 | 19.3 |
| Stage 1 hypertensive (90–99) | 8.8 | 8.9 | 4.1 |
| Stage 2 hypertensive (100–109) | 1.9 | 1.9 | 0.7 |
| Hypertensive emergency (110+) | 0.6 | 0.6 | 0.4 |
| Mean (SD) | 74.9 (9.4) | 75.0 (9.4) | 71.9 (8.6) |
| Raised blood pressure, % (SBP≥140 mm Hg and/or DBP≥90 mm Hg) | 11.9 | 12.1 | 5.5 |
| Body mass index range, kg/m2, %¶ | |||
| Underweight (<18.5) | 2.1 | 2.0 | 4.0 |
| Normal (18.5–24.9) | 57.0 | 57.0 | 55.4 |
| Overweight (25–29.9) | 34.1 | 34.3 | 29.5 |
| Moderately obese (30–34.9) | 6.1 | 6.0 | 8.1 |
| Severely obese (35–39.9) | 0.6 | 0.5 | 2.7 |
| Very severely obese (40+) | 0.1 | 0.1 | 0.4 |
| Mean (SD) | 24.5 (3.6) | 24.5 (3.6) | 24.6 (4.3) |
*Baseline data on age were missing for 55 participants.
†Baseline data on serum total cholesterol were missing for 449 participants.
‡Baseline data on fasting blood glucose were missing for 604 participants.
§Baseline data on blood pressure were missing for 36 participants.
¶Baseline data on body mass index were missing for 34 participants.
DBP, diastolic blood pressure; SBP, systolic blood pressure.
The total number of participants retained through all four waves of data collection and thus included in the longitudinal analyses was 6496, with a slightly higher retention rate in men than in women (p<0.001). Those excluded skewed younger (p<0.001) and had slightly lower mean values for cholesterol (p<0.001) and BMI (p<0.001).
Longitudinal trends in metabolic risk factors
Table 2 shows the trajectories from baseline to 3-year, 4-year and 5-year follow-up in mean levels of the assessed metabolic NCD risk factors, including Δ changes from baseline. One-way repeated measures ANOVA analyses revealed large, significant effects for time since baseline assessment for changes observed in serum total cholesterol (F[3,6086]=803.83, p<0.001, multivariate partial η2=0.28), systolic blood pressure (F[3,6381]=770.25, p<0.001, multivariate partial η2=0.27), diastolic blood pressure (F[3,6377]=603.65, p<0.001, multivariate partial η2=0.22) and BMI (F[3,6381]=544.53, p<0.001, multivariate partial η2=0.20); and a moderate, but significant effect for time since baseline assessment for fasting plasma glucose (F[3,6237]=186.15, p<0.001, multivariate partial η2=0.08).
Table 2.
Descriptive statistics for changes in metabolic risk factors across baseline and 3-year, 4-year and 5-year follow-ups
| Risk factor | Baseline (T0) | 3-Year follow-up (T3) | 4-Year follow-up (T4) | 5-Year follow-up (T5) |
|---|---|---|---|---|
| Serum total cholesterol (mg/dL) (N=6089) | ||||
| Mean (SD) | 187.6 (37.9) | 197.6 (37.7) | 196.2 (38.2) | 207.3 (40.2) |
| Mean (SE) Δ change from T0 | +10.0 (0.4) | +8.5 (0.4) | +19.7 (0.4) | |
| 95% CI for Δ change | (9.0 to 11.0)*** | (7.5 to 9.6)*** | (18.6 to 20.8)*** | |
| Fasting plasma glucose (mg/dL) (N=6240) | ||||
| Mean (SD) | 87.4 (20.3) | 90.7 (23.7) | 91.7 (24.4) | 94.4 (27.7) |
| Mean (SE) Δ change from T0 | +3.4 (0.3) | +4.4 (0.3) | +7.1 (0.3) | |
| 95% CI for Δ change | (2.7 to 4.1)*** | (3.6 to 5.1)*** | (6.3 to 7.9)*** | |
| Systolic blood pressure (mm Hg) (N=6684) | ||||
| Mean (SD) | 114.1 (13.3) | 118.0 (14.6) | 121.1 (15.6) | 123.9 (16.3) |
| Mean (SE) Δ change from T0 | +3.9 (0.2) | +7.0 (0.2) | +9.8 (0.2) | |
| 95% CI for Δ change | (3.3 to 4.4)*** | (6.4 to 7.5)*** | (9.2 to 10.3)*** | |
| Diastolic blood pressure (mm Hg) (N=6380) | ||||
| Mean (SD) | 75.0 (9.5) | 76.4 (10.6) | 79.3 (10.9) | 81.0 (11.2) |
| Mean (SE) Δ change from T0 | +1.4 (0.1) | +4.3 (0.2) | +6.0 (0.2) | |
| 95% CI for Δ change | (1.0 to 1.8)*** | (3.9 to 4.7)*** | (5.6 to 6.4)*** | |
| Body mass index (kg/m2) (N=6384) | ||||
| Mean (SD) | 24.7 (3.7) | 25.1 (3.6) | 25.4 (3.7) | 25.8 (3.7) |
| Mean (SE) Δ change from T0 | +0.4 (0.03) | +0.7 (0.03) | +1.1 (0.03) | |
| 95% CI for Δ change | (0.3 to 0.4)*** | (0.6 to 0.8)*** | (1.0 to 1.2)*** | |
p Values are based on one-way repeated measures analysis of variance analyses.
*p<0.05, **p<0.01, ***p<0.001.
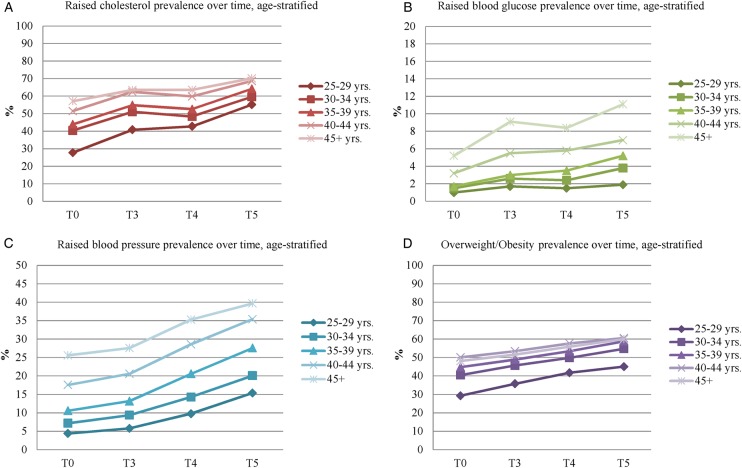
Assessed in terms of their clinically relevant, WHO-defined indicators, significant increases in prevalence rates from baseline were observed at each successive follow-up of all metabolic risk factors (ie, raised cholesterol, raised blood glucose, raised blood pressure and overweight/obesity), based on the McNemar's χ² test analyses (p<0.001). Similar increasing trends were observed in the age-stratified prevalence rates, as depicted in figure 1.
Figure 1.
Age-stratified trends in metabolic risk factors from baseline to 3-year, 4-year and 5-year follow-up. T0, baseline; T3, 3-year follow-up; T4, 4-year follow-up; T5, 5-year follow-up.
Analyses of association between duration of employment and NCD risk indicators
Associations between duration of employment and metabolic indicators of NCD risk were modelled both cross-sectionally and longitudinally among participants aged 25 years and older. The results of these adjusted analyses are shown in table 3. In summary, workers employed for 10 years or longer at baseline were 13% more likely at baseline and, among those with raised cholesterol at baseline, 26% more likely at 3-year follow-up, to exhibit persistent raised cholesterol; 33% more likely at 3-year follow-up to exhibit newly raised blood glucose and, among those with raised blood glucose at baseline, 62% more likely to exhibit persistent raised blood glucose; 16% more likely at baseline to exhibit raised blood pressure; and 14% more likely at baseline to be overweight or obese. Similar significant associations were found when duration of employment was assessed as a continuous variable, with significant incremental increases in NCD risk observed with each successive year of company employment. Across all regression analyses, the full model containing all predictors (employment duration, gender and age) was statistically significant based on both the Omnibus Tests of Model Coefficients (p≤0.001) and the Hosmer-Lemeshow Goodness of Fit Test (p>0.35). The models as a whole explained between 0.4–8.3% (Cox and Snell R2) and 0.4–11.2% (Nagelkerke R2) of the variance in NCD risk indicator status, and correctly classified 58.5–97.4% of cases (except in the case of the two prospective models of overweight/obesity status, in which none of the included predictors were significant and model fit was thus poor).
Table 3.
Logistic regression results for the association of baseline employment duration (≥10 years) with metabolic risk indicators in cross-sectional and prospective 3-year follow-up among participants aged 25 years and older, adjusted for gender and age
| Risk indicator | At baseline | Incident at 3-year follow-up† | Persistent at 3-year follow-up‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | AOR | (95% CI) | N | AOR | (95% CI) | N | AOR | (95% CI) | |
| Raised cholesterol (≥190 mg/dL) | 14 208 | 1.13 | (1.04 to 1.22)** | 7867 | 1.08 | (0.97 to 1.21) | 6244 | 1.26 | (1.08 to 1.47)** |
| Raised blood glucose (≥126 mg/dL) | 14 063 | 1.10 | (0.88 to 1.39) | 13 603 | 1.33 | (1.06 to 1.68)* | 368 | 1.62 | (1.01 to 2.58)* |
| Raised blood pressure (SBP≥140 mm Hg and/or DBP≥90 mm Hg) | 14 569 | 1.16 | (1.04 to 1.30)** | 12 751 | 0.91 | (0.81 to 1.02) | 1808 | 0.96 | (0.78 to 1.18) |
| Overweight or Obesity (≥25 kg/m2) | 14 569 | 1.14 | (1.05 to 1.23)** | 8352 | 1.03 | (0.90 to 1.17) | 6147 | 1.01 | (0.83 to 1.22) |
Logistic regression models all adjusted for gender and age bracket.
*p<0.05, **p<0.01, ***p<0.001.
†Among those with indicator levels below the designated metabolic NCD risk threshold at baseline.
‡Among those with indicator levels meeting or exceeding the designated metabolic NCD risk threshold at baseline.
AOR, adjusted OR; DBP, diastolic blood pressure; NCD, non-communicable disease; SBP, systolic blood pressure.
Discussion
Findings from the COPPER Study point to an unmistakeable heavy and mounting burden of NCD risk among those employed in mining operations in Papua, Indonesia. Recorded rates and upward-rising trends of metabolic risk factors reflect the wider context of changing disease epidemiology in Indonesia, the SEA region and, more broadly, LMICs worldwide.1 3 Moreover, results suggest a markedly elevated level of NCD risk specific to working and living in this isolated mining community.
From baseline through 5-year follow-up, increasing trends in NCD risk factors were observed in terms of both mean levels and indicator prevalence rates. Changes were statistically as well as clinically significant. Even with regard to raw mean values for the study sample, two of the risk factors—total plasma cholesterol and BMI—progressed from being just below the WHO-defined cut-off point at baseline to falling within the designated high-risk range (progressing from 187.6 to 207.3 mg/dL for cholesterol and from 24.7 to 25.8 kg/m2 for BMI) after 5 years. Moreover, based on Ketola et al's28 classification using 19.33 mg/dL as the cut-off point for clinical relevance in total cholesterol changes, the 19.72 mg/dL individual-level change in total cholesterol observed from baseline to 5-year follow-up in the present study may be regarded as both statistically and clinically meaningful.
Over the past few decades, traditional societies in many developing countries have experienced rapid and poorly planned urbanisation, leading to lifestyles characterised by unhealthy nutrition, reduced physical activity and increased tobacco consumption—among the primary behavioural risk factors contributing to NCDs.13 14 These risk factors are especially prevalent in the SEA region, home to nearly 250 million smokers and an equal number of smokeless tobacco users.7 29 The consequences have been manifested clearly in NCD risk factor trajectories. For example, a study using multicountry data between 1980 and 2008 has shown a decreasing trend of mean blood pressure in western countries, but an increasing trend in SEA and Oceania.30 Furthermore, obesity is rapidly increasing across both developed and developing countries, particularly in urban settings.31 In this sense, the observed prevalence rates and longitudinal trends seen in our study population are in line with broader population-level transitions.
Yet the COPPER Study findings further suggest that the high and rising levels of NCD risk factors observed among the mine employees extend beyond general population-level and regional trends, indicating possible exposures specific to the environment experienced by mining employees in Papua. Compared with 2008 Indonesian national population prevalence estimates,14 baseline rates in the observed mine workers were higher for raised cholesterol (41.6% vs 35.1%) and nearly doubled for overweight/obesity indices (40.9% vs 21.0%). Moreover, our cross-sectional and longitudinal analyses found that the observed population's risk for raised cholesterol, raised blood glucose, raised blood pressure and overweight/obesity increased with every year spent on site, both cross-sectionally at baseline and prospectively at 3-year follow-up. This result implies that participants employed for longer durations experienced a longer duration of exposure to conditions contributing to NCD risk. Although our data do not address specific causes, several possible dynamics might be proposed based on previous studies and knowledge on common behavioural risk factors for NCDs.
Compared with most other industries, the mining workforce has been identified as having a high (though unqualified) proportion of chronic health problems.32 Chronic illnesses may be caused through exposure to a range of physical, chemical, biological, ergonomic and psychosocial hazards common to mining activities including shift work and nocturnal shifts, air pollution, silica dust, high altitudes, and elevated trauma and stress levels—all of which may interact in complex ways leading to elevated cholesterol, blood glucose, blood pressure and BMI through diverse pathophysiological mechanisms including direct physiological effects, dysregulation of neuroendocrine pathways and behavioural modification towards unhealthy lifestyle habits.16–18 32 These occupational hazards form part of a complex matrix of risk factors that are a function of technological development as well as social, economic and demographic factors, exacerbated by the regional and remote location of sites and various organisational issues influencing work demands.
The mining site in the present study provides a semicontrolled environment as far as lifestyle factors are concerned. Importantly, studies under similar worksite conditions have observed that obesity and unbalanced eating patterns increase with eating in staff canteens, even after adjusting for demographic and socioeconomic variables,33 yet some studies have also demonstrated the capacity for worksite canteens to increase workers’ intake of fruits and vegetables,34 pointing to the potential for positive impact through workplace interventions.35
The continued role for the private sector in promoting workplace wellness was explicitly called for in the United Nations Declaration on NCDs,36 providing opportunities such as peer networks and employer incentives.37 At the same time, potential negative aspects also need to be carefully considered including the potential for faulting the workers, coercion and conflicts of interest.37
Particularly in the Indonesian/SEA region context, the private sector may also play a critical role in addressing important gaps in local knowledge and response systems.38 Reflecting a growing recognition of such widely untapped potential, the World Health Assembly, in 2010, passed a resolution calling on countries to “constructively engage the private sector in providing essential health-care services”.39 In remote and low-resource settings where health information systems and monitoring and evaluation (M&E) mechanisms may be sparse or absent, companies with the resources and infrastructure to do so have the opportunity to contribute quality up-to-date data on NCD risk trends and pilot more innovative or resource-intensive initiatives contributing to multiple objectives under the new WHO Global NCDs Action Plan.40
There is now compelling evidence that workplace-based programmes incorporating health risk assessments used in combination with other interventions (eg, targeting dietary intake and increased physical activity) are effective in relation to lowering tobacco use, alcohol use, dietary fat intake, blood pressure, cholesterol, and control of overweight and obesity, with concomitant improvements in medical parameters as well as in psychological and physical well-being.35 41–44 For the employer, improved health outcomes may also lead to reduced absenteeism and sick leave, increased productivity and reduced healthcare expenditures.42
In view of the escalating burden of NCD risk factors observed among mine workers residing in Papua, implementation of an effective intervention programme is critical and timely. Building on the COPPER Study findings, further research will be needed to more thoroughly explicate the vulnerabilities and needs of the mining company employees, recognising the multifactorial nature of NCD risk.
This study, despite its advantages of a large sample size and historical prospective design, does present some methodological limitations that must be considered in interpreting the present findings. First, there is insufficient epidemiological evidence to draw meaningful conclusions about the existence of a causal association between specific occupational exposures and the development of NCD risk. Results do not, however, rule out important associations in this direction, and the contributory evidence provided by multivariable regression analyses and comparisons with national population prevalence points to mechanisms that may link on-site exposures to such risk. Yet the extent to which occupational exposures versus lifestyle or environmental factors underlie these differences is not clear, and the current study lacked data to directly explore or control for either.
In a related vein, information collected on potential confounding risk factors was limited. We did not take into account, for example, participants’ complete sociodemographic profiles, diversity across multiple work environments and job categories, or detailed work histories, which may have explained a proportion of the variability in exposure. Consequently, competing non-causal explanations are possible. For example, the effect of duration of employment may be confounded by physical activity requirements being greater in the workers with the least seniority, or by tendencies for sicker workers to remain with the company in the interests of retaining health insurance benefits. Further studies incorporating a broader consideration of potential confounders as well as relevant control groups will be necessary to clarify the important exposures at play along with their causal interactions.
As a further limitation, loss to follow-up may have introduced selection bias and limited somewhat the generalisability of our findings. Attrition analysis revealed that those participants who did not undergo follow-up measurements exhibited significant differences in gender, age, mean cholesterol and mean BMI, though not in other key variables. Finally, comparison of the morbidity experience of an occupational cohort to the general population may be biased and results of this study thus diluted by the “healthy worker effect”,45 with workers tending to exhibit lower rates of chronic disease than the population at large. However, the longitudinal nature of our analyses might be expected to counteract such biases, at least partially, and rates of raised cholesterol and overweight/obesity were still found to be higher than the general population.
Conclusions
Our findings, obtained from a large worksite cohort, highlight high and upward-trending rates of NCD risk, and provide evidence of a potential relationship between mining community residence and certain NCD risk factors in Papua, Indonesia. Unfortunately, national health promotion activities are currently weak, leaving room for much positive action towards improved and expanded prevention and control of NCDs within the private sector. Primary and secondary prevention as well as control strategies in the workplace are needed to change unhealthy lifestyle habits such as poor diet and lack of physical activity towards reducing heavy burdens of metabolic risk factors among mine employees, particularly high cholesterol and overweight/obesity. To this end, evidence-based interventions such as traffic light food labelling in company cafeterias to promote better dietary habits,46 a portable patient record for improved management of NCDs47 such as diabetes, mHealth interventions for a range of NCDs prevention and self-management aspects, and smoking cessation counselling and therapy programmes, combined with regular M&E of relevant metabolic and behavioural risk indicators (eg, sodium intake), should be explored for systematic on-site implementation.
Footnotes
Twitter: Follow Francesca Viliani at @fravili
Contributors: All the authors contributed to the various stages of this study. RR-F conceptualised the topic, led the development of the manuscript and wrote the original draft. ER and HK participated in the interpretation of the study findings. FV and MJB reviewed and edited the manuscript. RMA researched the literature, performed all of the statistical analyses and revised the manuscript. All the authors read and commented on the drafts, and approved the final version of the manuscript for submission.
Funding: PT Freeport, Freeport McMoRan Copper & Gold Inc.
Competing interests: None declared.
Ethics approval: Freeport Public Health and Malaria Control, International SOS, Science Review Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. . A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Global burden of disease. WHO, 2012. http://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html [Google Scholar]
- 4.Abegunde DO, Mathers CD, Adam T, et al. . The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007;370:1929–38. 10.1016/S0140-6736(07)61696-1 [DOI] [PubMed] [Google Scholar]
- 5.WHO. Preventing chronic diseases: a vital investment: WHO Global Report. Geneva: World Health Organization, 2005. http://www.who.int/chp/chronic_disease_report/en/ [Google Scholar]
- 6.Bloom DE, Cafiero ET, Jané-Llopis E, et al. . The global economic burden of non-communicable diseases. Geneva: World Economic Forum, 2011. [Google Scholar]
- 7.WHO Regional Office for South-East Asia Noncommunicable diseases in the South-East Asia region: 2011 situation and response. New Delhi: WHO, 2011. http://www.searo.who.int/entity/noncommunicable_diseases/documents/9789290224136/en/ [Google Scholar]
- 8.WHO Noncommunicable diseases country profiles 2014: Indonesia. Geneva: WHO, 2014. http://www.who.int/nmh/countries/idn_en.pdf [Google Scholar]
- 9.WHO Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: WHO, 2009. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf [Google Scholar]
- 10.Must A, Spadano J, Coakley EH, et al. . The disease burden associated with overweight and obesity. JAMA 1999;282:1523–9. 10.1001/jama.282.16.1523 [DOI] [PubMed] [Google Scholar]
- 11.McMillan DC, Sattar N, McArdle CS. ABC of obesity. Obesity and cancer. BMJ 2006;333:1109–11. 10.1136/bmj.39042.565035.BE1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawes CM, Vander Hoorn S, Rodgers A. International society of hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008;371:1513–18. 10.1016/S0140-6736(08)60655-8 [DOI] [PubMed] [Google Scholar]
- 13.Kontis V, Mathers CD, Rehm J, et al. . Contribution of six risk factors to achieving the 25×25 non-communicable disease mortality reduction target: a modelling study. Lancet 2014;384:427–37. 10.1016/S0140-6736(14)60616-4 [DOI] [PubMed] [Google Scholar]
- 14.WHO Global Status Report on Noncommunicable Diseases 2010. Geneva: WHO, 2011. http://www.who.int/nmh/publications/ncd_report2010/en/ [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, et al. . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 16.Davies HW, Teschke K, Kennedy SM, et al. . Occupational exposure to noise and mortality from acute myocardial infarction. Epidemiology 2005;16:25–32. 10.1097/01.ede.0000147121.13399.bf [DOI] [PubMed] [Google Scholar]
- 17.Björ B, Burström L, Eriksson K, et al. . Mortality from myocardial infarction in relation to exposure to vibration and dust among a cohort of iron-ore miners in Sweden. Occup Environ Med 2010;67:154–8. 10.1136/oem.2009.046599 [DOI] [PubMed] [Google Scholar]
- 18.Dominici F, Peng RD, Bell ML, et al. . Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–34. 10.1001/jama.295.10.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enterline PE. A review of mortality data for American coal miners. Ann NY Acad Sci 1972;200:260–72. 10.1111/j.1749-6632.1972.tb40192.x [DOI] [PubMed] [Google Scholar]
- 20.Stadler K. Dietary intake, physical activity and risk for chronic diseases of lifestyle among employees at a South African open-cast diamond mine [Master's thesis]. Department of Human Nutrition: Stellenbosch University, 2006. [Google Scholar]
- 21.Stephens C, Ahern M. Worker and community health impacts related to mining operations internationally: a rapid review of the literature. London: London School of Hygiene & Tropical Medicine, 2001. [Google Scholar]
- 22.Jenkins WD, Christian WJ, Mueller G, et al. . Population cancer risks associated with coal mining: a systematic review. PLoS ONE 2013;8:e71312 10.1371/journal.pone.0071312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mincheva L, Khadzhiolova I. An occupational physiology study at the Asarel Mining and Milling Works—screening for risk factors of the cardiovascular system in workers in an open-pit mine. Probl Khig 1995;20:47–59. [PubMed] [Google Scholar]
- 24.WHO Noncommunicable disease country profiles 2011. Geneva: WHO, 2011. http://www.who.int/nmh/publications/ncd_profiles2011/en/ [Google Scholar]
- 25.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariate methods. 2nd edn Boston, MA: PWS-Kent, 1988. [Google Scholar]
- 26.Fox J. Regression diagnostics. Beverly Hills, CA: Sage, 1991. [Google Scholar]
- 27.Allison P. Logistic regression using the SAS System: theory and application. Cary, NC: SAS Institute, Inc, 1999. [Google Scholar]
- 28.Ketola E, Sipilä R, Mäkelä M. Effectiveness of individual lifestyle interventions in reducing cardiovascular disease and risk factors. Ann Med 2000;32:239–51. 10.3109/07853890009011767 [DOI] [PubMed] [Google Scholar]
- 29.WHO Regional Office for South-East Asia Regional strategy for tobacco control. New Delhi: WHO, 2012. http://www.searo.who.int/entity/tobacco/documents/sea_tobacco_45/en/ [Google Scholar]
- 30.Danaei G, Finucane MM, Lin JK, et al. . National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 2011;377:568–77. 10.1016/S0140-6736(10)62036-3 [DOI] [PubMed] [Google Scholar]
- 31.Swinburn BA, Sacks G, Hall KD, et al. . The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804–14. 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- 32.Donoghue AM. Occupational health hazards in mining: an overview. Occup Med (Lond) 2004;54:283–9. 10.1093/occmed/kqh072 [DOI] [PubMed] [Google Scholar]
- 33.Kjøllesdala MR, Holmboe-Ottesena G, Wandel M. Frequent use of staff canteens is associated with unhealthy dietary habits and obesity in a Norwegian adult population. Health Nutr 2011;14:133–41. 10.1017/S1368980010001473 [DOI] [PubMed] [Google Scholar]
- 34.Lassena A, Thorsena AV, Ellen Trolle E. Successful strategies to increase the consumption of fruits and vegetables: results from the Danish ‘6 a day’ Work-site Canteen Model Study. Public Health Nutr 2004;7:263–70. [DOI] [PubMed] [Google Scholar]
- 35.Arena R, Guazzi M, Briggs PD, et al. . Promoting health and wellness in the workplace: a unique opportunity to establish primary and extended secondary cardiovascular risk reduction programs. Mayo Clin Proc 2013;88:605–17. 10.1016/j.mayocp.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UN General Assembly Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. A/66/L.1. September 16, 2011. http://www.un.org/ga/search/view_doc.asp?symbol=A/66/L.1.
- 37.Green KL. Issues of control and responsibility in workers’ health. Health Educ Q 1988;15:473–86. 10.1177/109019818801500407 [DOI] [PubMed] [Google Scholar]
- 38.Forsberg BC, Montagu D, Sundewall J. Moving towards in-depth knowledge on the private health sector in low- and middle-income countries. Health Policy Plan 2011;26(Suppl 1):i1–3. 10.1093/heapol/czr050 [DOI] [PubMed] [Google Scholar]
- 39.WHO Strengthening the Capacity of Governments to Constructively Engage the Private Sector in Providing Essential Health-care Services: Report by the Secretariat, 63rd World Health Assembly, Provisional Agenda Item 11.22, A63/25. March 25, 2010. http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_25-en.pdf
- 40.WHO Follow-up to the Political Declaration of the High-level Meeting of the General Assembly on the Prevention and Control of Non-communicable Diseases. 66th World Health Assembly Agenda item 13.1. May 27, 2013. http://apps.who.int/gb/ebwha/pdf_files/WHA66/A66_R10-en.pdf.
- 41.Kuoppala J, Lamminpää A, Husman P. Work health promotion, job well-being, and sickness absences—a systematic review and meta-analysis. J Occup Environ Med 2008;50:1216–27. 10.1097/JOM.0b013e31818dbf92 [DOI] [PubMed] [Google Scholar]
- 42.Sockoll I, Kramer I, Bödeker W. Effectiveness and economic benefits of workplace health promotion and prevention, Summary of the scientific evidence 2000 to 2006, IGA—Report 13e, BKK 2009.http://www.neaygeia.gr/UserFiles/File/iga_report_13e.pdf http://www.iga-info.de/fileadmin/Veroeffentlichungen/iga-Reporte_Projektberichte/iga-Report_13e_effectiveness_workplace_prevention.pdf.
- 43.Soler RE, Leeks KD, Razi S, et al. . A systematic review of selected interventions for worksite health promotion. The assessment of health risks with feedback. Am J Prev Med 2010;38:S237–62. 10.1016/j.amepre.2009.10.030 [DOI] [PubMed] [Google Scholar]
- 44.Pelletier KR. A review and analysis of the clinical and cost-effectiveness studies of comprehensive health promotion and disease management programs at the worksite: update VIII 2008 to 2010. J Occup Environ Med 2011;53:1310–31. 10.1097/JOM.0b013e3182337748 [DOI] [PubMed] [Google Scholar]
- 45.McMichael AJ. Standardized mortality ratios and the “healthy worker effect”: scratching beneath the surface. J Occup Med 1976;18:165–8. 10.1097/00043764-197603000-00009 [DOI] [PubMed] [Google Scholar]
- 46.Geaney F, Scotto DI Marrazzo FJ, Kelly C, et al. The food choice at work study: effectiveness of complex workplace dietary interventions on dietary behaviours and diet-related disease risk <n dash> study protocol for a clustered controlled trial. Trials 2013;14:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peiris D, Praveen D, Johnson C. Use of mHealth Systems and Tools for Non-Communicable Diseases in Low- and Middle-Income Countries: a Systematic Review. J Cardiovasc Transl Res 2014;7:677–91. [DOI] [PubMed] [Google Scholar]