Abstract
There are very few international examples of the successful eradication of bovine tuberculosis (TB, caused by infection with Mycobacterium bovis) from a national cattle population. This paper presents a brief overview of the successful TB eradication programme in Australia from 1970, with primary emphasis on lessons of international relevance that were learned from the Australian experience. The national brucellosis and tuberculosis eradication campaign ran for 27 years from 1970 to 1997 and has been followed by ongoing abattoir surveillance. Rapid progress towards eradication was made in southern Australia, but proved much more challenging in extensive pastoral areas of northern Australia. Declaration of TB freedom was made on December 31, 1997. A range of factors were critical to this success, including a compelling rationale for eradication, an agreed final outcome, industry commitment and financial support, a business model for programme planning, implementation and review, consistent and transparent technical standards underpinned by a strict regulatory regime and applied research, the critical role of abattoir surveillance, effective elimination of residual infection and objective measures of programme progress. Although direct translation of some of these experiences may not be possible, many of the lessons learned from the Australian experience may be relevant to other countries.
EFFORTS to control bovine tuberculosis (TB, caused by infection with Mycobacterium bovis) began in Australia more than 100 years ago. At that time, controls were mainly motivated by public health concerns, and progress was made, albeit slowly, during the early part of the 20th century. After the Second World War, greater progress was made following the introduction of compensation for tuberculin reactor dairy cattle, and infection had become uncommon in Australian dairy herds by the 1960s (Mylrea 1990, Lehane 1996). However, these control efforts were not nationally coordinated, but rather state-based.
In the 1960s, there were increasing trade concerns for the beef and dairy industries, initially from the USA, because of the continuing presence of bovine brucellosis and TB. The US market was particularly important for Australian producers and it was in direct response to these concerns that the nationally coordinated brucellosis and tuberculosis eradication campaign (BTEC) commenced on January 1, 1970 (Lehane 1996). The campaign was launched with considerable optimism, in part because it followed shortly after a highly successful national programme to eradicate contagious bovine pleuropneumonia, which had commenced in 1961, with the last viable lesions being found in 1967 (Newton 1992, Turner 2011).
Aspects of BTEC have been reviewed on several occasions (Tweddle and Livingstone 1994, Lehane 1996, Neumann 1999, Cousins and Roberts 2001, Radunz 2006, Animal Health Australia 2009). Detailed information about BTEC and related programmes is also available (BTEC progress reports from 1976 to 1997; Box 1) (Anon 1989, Animal Health Australia 2000, 2005, 2007a).
Box 1: Resources developed in Australia during the national Brucellosis and Tuberculosis Eradication Campaign (BTEC) and subsequent programmes.
Standard definitions and rules
Brucellosis and tuberculosis eradication campaign
▪ 1975, Australian Bureau of Animal Health, Canberra
▪ 1979, Volume one. Australian Bureau of Animal Health, Canberra
▪ 1982, Volume one. Australian Bureau of Animal Health, Canberra
▪ 1984, Volume one. Australian Bureau of Animal Health, Canberra
▪ 1986, Volume one. Department of Primary Industry, Bureau of Rural Science, Canberra
▪ 1987, Volume two. Department of Primary Industry, Bureau of Rural Science, Canberra
▪ 1995, Department of Primary Industries and Energy, Canberra
Tuberculosis Freedom Assurance Programme
▪ 1998, Schedule L. Australian Animal Health Council Ltd, Canberra
Brucellosis and Tuberculosis Eradication Campaign, progress reports
▪ Number 1, Australian Bureau of Animal Health, 1976
▪ Number 2, Australian Bureau of Animal Health, 1978
▪ Number 3, Australian Bureau of Animal Health, 1979
▪ Number 4, Australian Bureau of Animal Health, 1979
▪ Number 5, Australian Bureau of Animal Health, 1980
▪ Number 6, 1979-80, Australian Bureau of Animal Health, Canberra, 1982
▪ Number 7, 1980-81, Australian Bureau of Animal Health, 1983
▪ Number 8, 1981-82, Australian Bureau of Animal Health, 1984
▪ Number 9, 1982-83, Bureau of Rural Science, Department of Primary Industry
▪ Number 10, 1983-84, Bureau of Rural Science, Department of Primary Industry
▪ Number 11, 1984-85, Bureau of Rural Science, Department of Primary Industry
▪ Number 12, 1985-86, Bureau of Rural Science, Department of Primary Industry
▪ Number 13, for the period 1986/87-1993/94, Department of Primary Industries and Energy, 1995
▪ Number 14, for the period 1994/95, Department of Primary Industries and Energy, 1996
▪ Number 15, for the period 1995/96, Department of Primary Industries and Energy, 1997
▪ Number 16, for the period 1996/97, Department of Primary Industries and Energy, 1998
▪ Summary report 1993-97, Department of Primary Industries and Energy, 1998
This paper presents a brief overview of the successful TB eradication programme in Australia from 1970, with primary emphasis on lessons of international relevance that were learned from the Australian experience.
Eradication of bovine tuberculosis
Brucellosis and tuberculosis eradication campaign (BTEC)
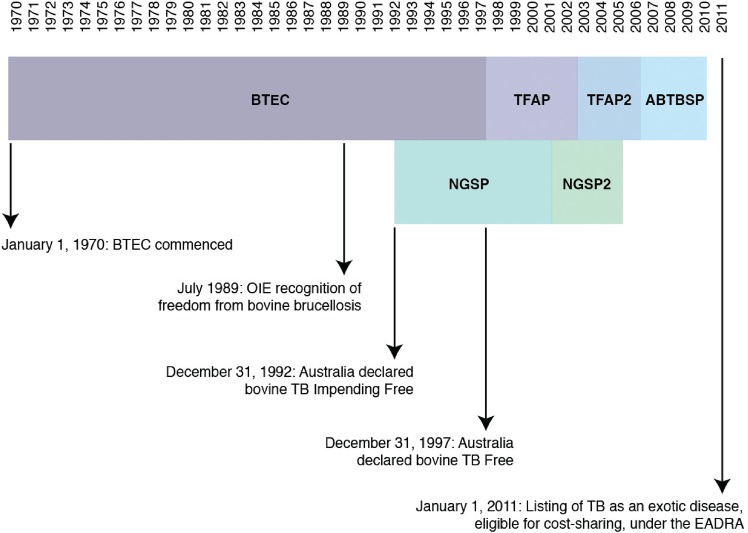
BTEC ran for 27 years from 1970 to 1997, and cost approximately AU$840 million (Neumann 1999) in operational expenditure. BTEC was nationally coordinated and worked under nationally agreed guidelines, with the national goal being the eradication of Mycobacterium bovis from Australia's cattle and water buffalo populations. Brucellosis (due to infection with Brucella abortus) and TB were tackled concurrently, with Australia exceeding equivalence to World Organisation for Animal Health (OIE) recognition of freedom from disease in 1989 and 1997, respectively (Fig 1).
FIG 1:
Chronology of key events in the brucellosis and tuberculosis eradication campaign (BTEC) and related programmes. ABTBSP Australian Bovine Tuberculosis Surveillance Project, EADRA Emergency Animal Disease Response Agreement, NGSP National Granuloma Submission Program, OIE World Organisation for Animal Health, TB Tuberculosis, TFAP Tuberculosis Freedom Assurance Programme
TB eradication during BTEC was conducted as a whole-herd test and slaughter programme, using the single intradermal (caudal fold) test (Fig 2), underpinned by a cattle tracing system that had been introduced during the early 1970s. This system was based on property identification, allocation of a unique property identification code (PIC), and the mandatory application of tail or ear tags bearing the PIC for defined cattle movements, particularly to sale yards and abattoirs (Animal Health Australia 2013). In northern extensive pastoral areas, firebranding of cattle on their property of birth was also a useful ancillary tracing tool. In southern (intensive farming) areas, whole herd testing was used as the primary method of TB detection until 1975. After this, it was discontinued in herds with no evidence of TB, except those without adequate abattoir surveillance. Testing continued in infected herds, and during trace-forward and trace-back activities associated with the detection of a TB case (Radunz 2006).
FIG 2:

Single intradermal (caudal fold) test, which formed the basis throughout BTEC for the whole-herd test and slaughter programme. Photo: Kevin de Witte
Abattoir surveillance played a central role throughout BTEC, and subsequently, in 1992, its role was further enhanced with the introduction of the National Granuloma Submission Program (NGSP) (Fig 1). This was an abattoir-based TB surveillance programme to maximise the number of granulomas submitted from cattle, buffalo, camels and deer detected during postmortem inspection, for laboratory analysis to preclude the possibility of TB. Throughout this programme, submissions were actively encouraged, as was feedback to inspectors and producers. From 2001, the programme shifted from bulk submission of granulomas (through the NGSP, which ran during 1992 to 2000) to targeting of higher-risk (older) animals and abattoir submission targets (one granuloma for every 1000 cattle slaughtered with two or more permanent teeth) (National Granuloma Submission Program 2 [NGSP2], which ran during 2001 to 2004) (Fig 1). During NGSP, 27,998 granulomas were examined from 82.4 million cattle (including calves) slaughtered (submission rate of one granuloma per 2942 cattle slaughtered). In the Northern Territory, Queensland, South Australia and Western Australia during NGSP2, 16,132 granulomas were examined from 13.9 million eligible cattle slaughtered (those with two or more permanent teeth) (submission rate of one granuloma per 864 eligible cattle slaughtered) (Animal Health Australia 2005).
Rapid progress towards TB eradication was made in farming areas in southern parts of Australia, in large part because of progress already made in the dairy industry before the start of BTEC (Smith 1959). The last confirmed primary cases of TB in bovids were detected in Tasmania in 1975, in Victoria in 1991, in New South Wales in 1995, and in South Australia in 1996. Secondary cases, following movement of animals from states or territories where infection had not yet been cleared, were subsequently detected in Victoria in 1996 (in imported buffalo from the Northern Territory) and in New South Wales in 2001 (on properties where cattle had moved following complete dispersal of a Queensland property, see Appendix C (adapted from More and Roe 2002) (available online as Supplementary Data).
Before BTEC, cattle control systems varied greatly across the extensive pastoral areas of northern Australia. On some stations, the management of cattle was limited to irregular harvesting from what were essentially feral cattle populations (Lehane 1996). Within-herd TB prevalence in the pastoral areas was often very low (less than 0.1 per cent). On some stations, however, within-herd prevalence was considerably higher, as a result of epidemiological circumstances that facilitated within-herd transmission, such as flooding or congregation at artificial watering points during the dry season.
In the northern pastoral areas, substantial modifications were needed to classical approaches to TB eradication. Improved property infrastructure, including internal fences and yards, was essential. Further, improved herd management was critical to achieve TB eradication. On many infected pastoral properties, a two-herd system was introduced, based on weaner and age segregation, in association with partial destocking or accelerated turnoff of high-risk cattle, generally the older breeder herd. To illustrate, in totally feral herds, initial efforts focused on the construction of some paddocks, followed by the mustering of stock from unfenced areas, the removal (to slaughter) of older cattle, the test and retention of younger, test-negative animals, the progressive establishment of a tested herd contained behind fences, and the depopulation of stock from unfenced areas.
Destocking (partial depopulation) of high-risk (older age) groups from paddocked areas and the removal of all cattle and buffalo from areas where a clean muster was not possible (ie, bush areas) was used extensively in northern Australia. This was initially undertaken using commercial mustering, by helicopter and on horseback (Fig 3), generally over a few years to optimise commercial use of the animals and provided that there was no detriment to an Approved Property Programme (APP), see below, on the property or to neighbouring properties. Then, helicopter shooting was conducted over an extended period, until it was no longer cost-beneficial to continue aerial operations. However, it became clear that in many areas a different strategy was needed, as many of the residual animals adopted a nocturnal grazing habit, staying under the cover of vegetation during daylight hours to evade the helicopters. From 1989, and depending on animal density and the nature of terrain and vegetation, radio-tracking was used to locate residual animals. The so-called ‘Judas’ cow technique (using a cow to lead others to a specific destination) was conducted over a number of years to complete bush destocking (Fig 4). In extensive pastoral areas, reactor animals were generally killed and a postmortem examination was carried out in the field (Fig 5).
FIG 3:

Helicopter mustering. Photo: Kevin de Witte
FIG 4:

The ‘Judas’ cow technique, which was used to locate residual animals. Photos: Kevin de Witte (a), Ron Glanville (b)
FIG 5:

In extensive pastoral areas, reactor postmortem examinations were conducted in the field. Photo: Kevin de Witte
Introduced in 1984, APPs became an essential component of eradication strategies throughout central and northern Australia (Lehane 1996). These programmes were developed in partnership with the owner, providing an agreed ‘road map’ for action towards TB freedom. The programmes specified long-term and interim milestones, agreed actions and annual written reviews. Eligibility to BTEC financial support was contingent on agreement to, and compliance with, the APP. Industry representatives played a key role in reviewing the proposed initial APPs with continuing review of progress, and also drove the development of peer support networks.
Depopulation of newly identified TB breakdown herds was adopted in all areas from 1990, a strategy that had been used in the southern farming areas since 1986. During the latter stages of the programme, measures were introduced to encourage the removal of all cattle previously exposed to TB-infected animals. This occurred despite the completion of the required eradication and confirmatory TB testing, and was an important strategy to minimise the risk of TB recrudescence and shorten the eradication programme (Radunz 2006).
Throughout the Australian programme, M bovis infection was mainly limited to three animal species: cattle, water buffalo (Bubalis bubalis) and feral pigs (Sus scrofa) (Corner 2006). However, there were single reports of infection in goats co-grazing with infected cattle (Cousins and others 1993) and in fallow deer (Dama dama) where the source of infection could not be determined (Robinson and others 1989). Although infection was endemic in water buffalo across much of its range (McCool and Newton-Tabrett 1979, Hein and Tomasovic 1981), there was little interaction between cattle and water buffalo (Corner 2006). In contrast, feral pigs were found to be a spillover (or dead-end) host, with pigs becoming infected following the ingestion of infected tissues scavenged from the carcases of cattle and water buffalo (Corner 2006). TB was not detected in feral pigs following the removal of local TB-infected cattle and water buffalo populations (McInerney and others 1995). In contrast to the situation in New Zealand, infection with M bovis has never been reported in the common brushtail possum (Trichosurus vulpecula) in Australia. Given this background, throughout the eradication programme, emphasis was placed on the removal of all known infected cattle and water buffalo. Note that in Australia, water buffalo and feral pigs are classified as invasive animal species, with a major negative impact on Australia's environment (Department of the Environment 2015). Therefore, although Australia did not have any infected native wildlife reservoirs, equivalent challenges were faced in extensive pastoral areas with feral cattle and buffalo. Following the end of BTEC, all remaining buffalo were derived from buffalo populations where infection had never been present.
A number of risk-based strategies were used through BTEC and associated programmes, exceeding those required under the OIE Animal Health Code:
▪ First, risk was assessed at the level of the group (or herd or area) rather than at the level of the individual. This approach was taken cognisant of the epidemiology of infection (including the potential for latent or residual infection) and the imperfect sensitivity and specificity of available diagnostic tests.
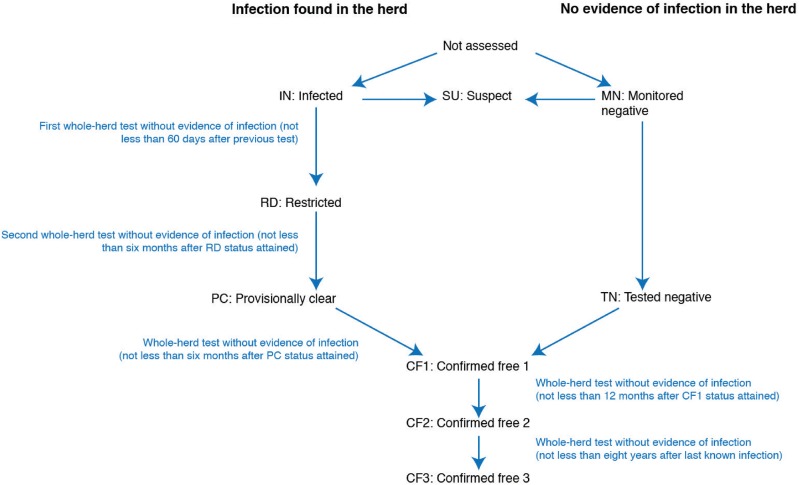
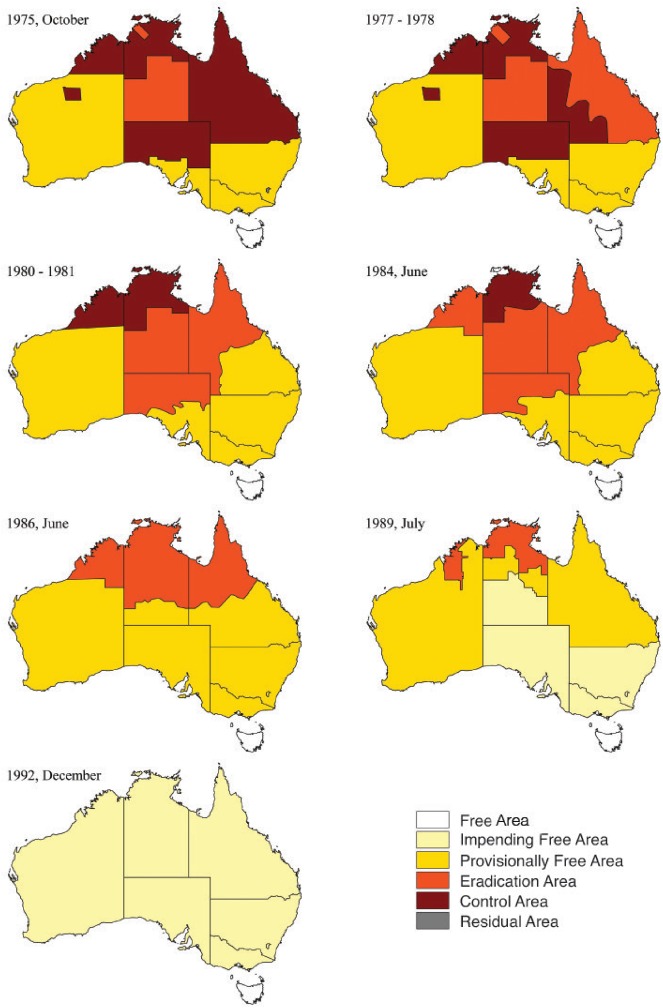
▪ Second, a dynamic system of risk-based herd and area classification was used throughout the programme (Table 1). The pathway for herd classification is presented in Fig 6. Infected (IN) herds took a minimum of 14 months and eight years to attain confirmed free (CF) 1 and CF3 status, respectively. Extended testing intervals were used throughout the pathway (a minimum of six months each from restricted [RD] to provisionally clear [PC] and from PC to CF1, 12 months from CF1 to CF2 [Fig 6]), to maximise test sensitivity by avoiding periods of desensitisation and allowing disease progression, if present. Area classification was based on both the apparent disease prevalence across the whole cattle population, and the proportion of herds in particular status classifications (Cousins and others 1998) (Table 1). The criteria of less than 5 per cent IN herds was used as part of the Provisionally Free area classification to account for the small number of large herds in pastoral areas. In the Northern Territory, for example, up to 50,000 cattle were managed on each of 350 properties over 1.2 million km2. Herd and area classification contributed substantially to both national and local TB management. These terms also became meaningful both for producers and programme managers, and were a powerful method to transparently measure progress (Cousins and others 1998). Fig 7 illustrates the change in TB area classification over time during BTEC.
TABLE 1:
Area classification as used during the Australian brucellosis and tuberculosis eradication campaign. From Lehane (1996) and Cousins and others (1998)
| Area classification | How classified |
|---|---|
| Residual | An area not included in any of the classifications below (only applicable early in the campaign) |
| Control | Quarantine of IN herds An approved monitoring system in place |
| Eradication | As for Control, plus active disease control |
| Provisionally Free | As for Eradication, plus apparent disease prevalence less than 0.1 per cent Less than 5 per cent IN herds |
| Impending Free (from 1986) | Previously a Provisionally Free area No known IN or RD herds at the time of declaration Capacity to eradicate any breakdown within 24 months of detection |
| Free | Impending Free for at least five years Approved abattoir monitoring system and granuloma submission programme in place TB believed eradicated No herds classified as IN, RD or PC at the time of declaration Movement controls in place for cattle from herds that had TB and achieved CF1 status |
CF1 Confirmed free 1, IN Infected, PC Provisionally clear, RD Restricted
FIG 6:
The pathway for change in herd classification, based on McGuin (1986) and the final (1995) version of the standard definitions and rules (Box 1). This figure represents the status pathway used during BTEC and should not be strictly interpreted as a hierarchy of risk. For example, depending on herd history, MN status was generally (but not always) considered to represent a lower TB risk than CF
FIG 7:
Changes in TB area classification over time during the Australian brucellosis and TB eradication campaign. Modified from BTEC progress reports number 1 (October 1975), 4 (1977-78), 7 (1980-81), 10 (June 1984) and 13 (June 1986, July 1989, December 1992). Definitions for area classification are presented in Table 1
▪ Third, restrictions on the movement of cattle between herds and between areas was determined on the basis of herd and area risk, to limit the potential for spread of infection to lower-risk herds and areas (Table 2, 3). There were marketing benefits for an owner achieving eradication on their property. During the early stages of BTEC, there were also low risk options such as ‘all in, all out’ bullock depots and approved feedlots to enable a cooperating owner to minimise the negative cash flow impacts of an APP. Towards the latter stages of the eradication programme, there were severe restrictions on the movement of cattle from IN, RD and PC herds in Provisionally Free, Impending Free and Free Areas, and from CF1 herds in Provisionally Free Areas (Table 2, 3).
TABLE 2:
Restrictions imposed on the movement of cattle into Impending Free Areas, from Free or Impending Free Areas and from Provisionally Free Areas, by herd status of the exporting herd
| Movement into Impending Free Areas | ||
|---|---|---|
| Movement from: | Direct to abattoir for immediate slaughter | For fattening or breeding |
| Provisionally Free Areas | ||
| SU, IN, RD, PC | No movement allowed, except with CVO approval in special circumstances and under specified conditions | No movement allowed |
| CF1 | No movement allowed, except with CVO approval in special circumstances and under specified conditions | One negative test |
| CF2, CF3, TN, MN | No movement test | One negative test |
| Free or Impending Free areas | ||
| SU, IN, RD, PC | Prior CVO permission and notification of movement required between administrative areas, otherwise no restrictions. Suitable visible identification required | No movement permitted. Within these areas, movements only with CVO permission |
| CF1 | No restriction | Movement test and cattle retain CF1 status. The movement cattle must be held in isolation until completion of CF2 test at the approved interval. Prior CVO approval for movement to other administrative areas |
| CF2 | No restriction | Conditions may be applied |
| MN, TN, CF3 | No restriction | No restriction |
These are as outlined in the final (1995) version of the standard definitions and rules. Definitions for area classification and herd status are presented in Table 1 and Fig 6, respectively.
CF1 Confirmed free 1, CF2 Confirmed free 2, CF3 Confirmed free 3, CVO Chief Veterinary Officer, IN Infected, MN Monitored negative, PC Provisionally clear, RD Restricted, SU Suspect, TN Tested negative
TABLE 3:
Restrictions imposed on the movement of cattle within and into a Provisionally Free Area, by herd status of the exporting herd
| Movement into Provisionally Free Areas | ||
|---|---|---|
| Movement from: | Direct to abattoir for immediate slaughter | For fattening or breeding |
| SU, IN, RD, PC, CF1 | No movement test required | No movement allowed |
| CF2, CF3, TN, MN | No movement test required | No movement test required |
These are as outlined in the final (1995) version of the standard definitions and rules. Definitions for area classification and herd status are presented in Table 1 and Fig 6, respectively.
CF1 Confirmed free 1, CF2 Confirmed free 2, CF3 Confirmed free 3, IN Infected, MN Monitored negative, PC Provisionally clear, RD Restricted, SU Suspect, TN Tested negative
▪ Fourth, a broad range of strategies were used to effectively manage residual TB risk, including:
a focus on infection risk in the group (or herd or area) rather than the individual;
a risk-based approach to herd and area classification (Table 1, Fig 6) and to movement restrictions (Table 2, 3), management of infected herds to minimise the population of cattle with TB exposure; and
a progressive tightening of controls as the programme progressed.
Nonetheless, an ongoing risk of residual infection was assumed until all cattle exposed to an infected animal had been slaughtered. Consequently during the first Tuberculosis Freedom Assurance Programme (TFAP, see below) (Box 1), the risk was managed by early turn off to slaughter to minimise the risk of recrudescence.
Financial support measures within BTEC evolved over time. Reactor compensation was available from the start of the campaign. Compensation for destocking (paddock checks, age destocking, bush destocking) became available from the early 1980s with additional assistance from 1984. The latter included a subsidy to hold cattle for the TB test (in yards/holding paddocks), a restocking freight rebate, low interest loans for infrastructure necessary for eradication, and an interest subsidy. From 2000, a freight subsidy was provided for three years to assist in the slaughter of cattle exposed to TB-infected animals younger than the normal slaughter age despite the completion of the required eradication testing (Radunz 2006). Producers also had access to professional support for farm-based economic decision-making.
In northern Australia, the last confirmed primary cases of TB in cattle were detected in Western Australia in 1998, in the Northern Territory in 1999, and in Queensland in 2000 (Fig 8) (Radunz 2006). The last known cases of TB in Australia were detected in 2002: two primary cases in adjacent buffalo herds in the Northern Territory (Radunz 2006, Table 5) and a secondary case in a cattle herd in Queensland (More and Roe 2002, Animal Health Australia 2006). Further details about the latter case is presented in Appendix D (adapted from More and Roe 2002) (available online as Supplementary Data). The whole of Australia was declared Impending Free in 1992 and Free on December 31, 1997. From 2011, infection with M bovis was classified as an exotic disease of cattle.
FIG 8:

The final round of testing on the last quarantined infected property in Queensland, Australia. For further information, see Appendix B (adapted from More and Roe 2002) (available online as Supplementary Data). Photo: Rod Robertson
TABLE 5:
Confirmed primary cases of bovine TB during the final stages of tuberculosis eradication in Australia (Radunz 2006)
| During the final years of BTEC (1993-1997) | During TFAP (1998-2002) | During TFAP2 (2003-2006) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 93 | 94 | 95 | 96 | 97 | 98 | 99 | 00 | 01 | 02 | 03 | 04 | 05 | 06 | |
| Southern states | ||||||||||||||
| Tasmania | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Victoria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New South Wales | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| South Australia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northern states | ||||||||||||||
| Western Australia | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Queensland | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northern Territory | 6 | 5 | 5 | 3 | 4 | 2 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Total | 8 | 7 | 8 | 6 | 7 | 4 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 |
BTEC Brucellosis and Tuberculosis Eradication Campaign, TFAP Tuberculosis Freedom Assurance Programme
Programmes subsequent to BTEC
Recognising that isolated cases of TB might still occur after freedom from disease was declared, the first TFAP commenced in 1998 and ran through until 2002. This was followed by TFAP2 (which ran from 2003 to 2006). These programmes provided a mechanism to effectively manage the few new cases that arose after 1997 (Anon 2001). The Australian Bovine Tuberculosis Surveillance Project (ABTBSP) was conducted during 2007 to 2010, once TFAP2 had concluded, providing a nationally integrated approach to surveillance for bovine TB (Anon 2014). During TFAP and TFAP2, particular emphasis was placed on the monitoring of all herds known to have been infected since 1988, to ensure that all cattle known to have been exposed to TB infection were sent to slaughter. This was based on the assumption that the maximum productive age of cows in northern areas was about 10 years. In these herds, annual reviews were also conducted and financial assistance was available.
TB as an exotic disease
From the start of 2011, bovine TB was included in Australia's Emergency Animal Disease Response Agreement (EADRA), providing the mechanism for government and industry cost-sharing in the event of discovery of what is now considered an exotic disease. It is currently classified as a Category 4 disease (private benefits considered greater than public benefits: 20 per cent government, 80 per cent industry funding in the event of an emergency response) (Table 4). A manual is now available describing the proposed Australian response to a TB incident (Animal Health Australia 2009).
TABLE 4:
Cost-sharing arrangements as part of Australia's Emergency Animal Disease Response Agreement (Animal Health Australia 2001)
| Source of funding (per cent) | ||
|---|---|---|
| Category of emergency animal disease | Government | Industry |
| Category 1: Public benefits only* | 100 | 0 |
| Category 2: Public benefits greater than private benefits† | 80 | 20 |
| Category 3: Proportion of public to private benefits is roughly equal‡ | 50 | 50 |
| Category 4: Private benefits are greater than public benefits$ | 20 | 80 |
There is no category where only private benefits exist. Cost-sharing applies in respect to salaries and wages, operating expenses, capital costs and compensation, but with some clarification. * Including Australian bat lyssavirus, rabies, † Including foot and mouth disease, sheep and goat pox, ‡ Including African swine fever, lumpy skin disease, $ Including Aujeszky's disease, equine influenza
Subsequent impact on animal health in Australia
Experiences from the TB eradication program have had a substantial and enduring impact on animal health and welfare in Australia. BTEC was a major driver of development in the northern cattle industry, as discussed later. Further, major developments in Australia's animal health systems were built on the approaches and partnerships that developed under BTEC. The model of the industry and government partnerships developed during BTEC fostered the establishment of Animal Health Australia (AHA), a not-for-profit company established in 1996 by the Commonwealth, state and territory governments and major national livestock industry organisations. Key outputs included the EADRA and the development and coordination of the delivery of national animal health programmes (Animal Health Australia 2015). The work of this company is guided by government animal health policy, developed in consultation with industry. Further, the Federal and State governments retain legislative authority for animal disease control (Black 2012). Key activities of AHA include the development of strategic and long-term plans for animal health services, and the management of national animal health programmes (Neumann 2002, Animal Health Australia 2014a). Service delivery under these programmes is generally performed by other organisations, particularly federal and state governments.
Key lessons learned
The following provides an outline of some of the key lessons learned during BTEC and accompanying programmes. Some have been raised previously (Lehane 1996, Cousins and others 1998, Radunz 2006).
A compelling rationale, both nationally and for individual farmers
In Australia, the cattle industry is highly export-oriented. Throughout the 1960s and beyond, there were very real concerns that international trade, initially to the USA, would be threatened as a consequence both of TB infection in the national herd and progress being made towards TB eradication in importing countries. This was a pressing issue, noting the critical role of the US export market for Australian cattle producers (47 per cent of total beef and veal exports in 1959 to 1960 were destined for the USA, increasing to 71.6 per cent in 1969 to 1970 [Australian Bureau of Statistics 2007]). Therefore, there was a compelling rationale for national TB eradication. Further, throughout the campaign, farm-based trading restrictions were closely linked with infection risk, providing substantial advantages to individual producers to progress towards freedom. It is unlikely that TB eradication would have been achieved if TB had not been recognised as an industry problem.
There was an additional, but unintended, benefit from BTEC, both to the industry as a whole, and to individual producers, as a result of substantial improvements to cattle productivity in northern Australia. These were achieved through improved husbandry (for example, two annual musters rather than one, controlled mating, removal of feral bulls, enforced weaning, mineral supplementation) and cattle controls through improved fencing. Reproductive rates improved and mortality decreased, allowing heifer selection and the slaughter of cull cows for beef. To illustrate, similar output from the Australian beef cattle population was achieved in the early 1990s with around 24 million cattle as in 1974 to 1975 with 32 million cattle (Lehane 1996). Other aspects of cattle production in northern Australia also fundamentally changed, primarily as a result of BTEC, including cattle movements and marketing patterns, and the genetics of animals in the northern cattle industry.
A clear, agreed outcome
Throughout BTEC, there was a clear, agreed final outcome, namely the eradication of M bovis from the Australian cattle and buffalo population. Among both government and industry, there was a shared purpose to a common goal. The criteria for area freedom as used by Australia, is outlined in Table 1. This outcome is in compliance with, but more stringent than, the International Animal Health Code of the OIE, which requires that 99.8 per cent of herds and 99.9 per cent of cattle are free of bovine TB. In comparison, under EU legislation officially TB free (OTF) status is achieved for a region when 99.9 per cent of herds are free of TB each year for six consecutive years (Anon 1964). None of these standards exclude the possibility that TB may still exist in the cattle population, hence the OIE requirement for continuing abattoir surveillance and effective trace back (Tolson and others 2001).
Industry commitment and support
Genuine industry commitment
Several authors have highlighted the critical role played by industry in BTEC's success. Indeed, Lehane (1996) says of the key industry organisations that ‘their initiative in gaining a strong voice for industry in the campaign's management, and their advocacy of various assistance measures for producers, helped ensure a successful outcome’. Further, McCormick (2001) suggested that ‘TFAP enjoy[ed] industry “ownership” and involvement at all levels of management.’ These statements reflect a strong and constructive relationship between government and industry, noting that TB eradication was largely administered by state governments under state legislation, but working under nationally agreed guidelines. However, this was not always the case, with industry playing a relatively minor role in decision-making during the initial stages of the programme. This changed fundamentally in 1984, coinciding with rising industry opposition to the use of mass destocking to tackle TB (and brucellosis) in difficult northern areas. Following federal intervention at that time, industry subsequently played a central role in BTEC decision-making, on the national BTEC committee, on state and regional advisory committees, and on teams tasked to review approved property programmes (Lehane 1996, Radunz 2006). In addition, industry champions, working closely – in a paid role – with both industry and government, were central to BTEC success (Lehane 1996).
Industry continues to play a central role in decision-making in animal health in Australia. The trust built between government and industry during BTEC and associated programmes, and the lessons learned, has played a key role in the establishment and ongoing operation of animal health programmes in Australia, including AHA.
Cost-sharing by government and industry
From a very early stage, industry was a major financial contributor to BTEC (Radunz 2006). Industry funding commenced with the introduction of levies in 1973 on cattle slaughterings and, shortly after, on live exports (Cousins and others 1998). The levy varied over time, to a high of AU$4 in 1982. This was replaced by a transaction levy in 1991, covering live sales as well as sales for slaughter (Lehane 1996). Cost-sharing evolved during BTEC, and it was not until February 1988 that it was formally agreed that industry would cover 50 per cent of the programme costs, with the states paying 30 per cent and the Commonwealth 20 per cent. These costs covered operations, compensation and additional assistance measures. The five years between 1988 and 1992 (when national Impending Freedom was achieved) were perhaps the most critical during BTEC as there was considerable urgency from industry to successfully finalise the eradication effort (Lehane 1996).
Throughout BTEC, all producers paid the above-mentioned levies, in recognition of the industry-wide benefit that would accrue once TB eradication was achieved. Therefore, southern producers were key contributors to eradication efforts in northern Australia, where eradication proved more difficult and costly. To illustrate, an estimated 20 per cent of overall BTEC costs were incurred in the Northern Territory, where approximately 5 per cent of the national cattle population were found.
The programme was supported by legislation, including Stock Diseases Acts in the states and territories, and the National Cattle Disease Eradication Trust Account Act, thereby ensuring that all producers took part in the campaign and shared in the costs (Anon 1982). Under Commonwealth legislation, industry levy monies were held in this trust account for the specific purpose of meeting costs incurred by government ‘for the purpose of the eradication of any disease of cattle that is endemic in Australia’ (Anon 1991). All sectors of the Australian cattle industry, including all producers, had a substantial stake in programme success.
Building on the BTEC experience, cost-sharing is now the norm in national animal health programmes in Australia, with a beneficiary pays approach being used as the basis for cost-sharing between government and industry. To illustrate, a sliding scale is used to calculate cost-sharing as part of EADRA (Table 4) (Animal Health Australia 2001).
A business model for programme planning, implementation and review
Detailed forward planning became a key feature of BTEC and associated programmes, including the development of multi-annual strategic plans and annual operational plans underpinned by legal agreements between the Australian government, state or territory governments and relevant industry organisations. These plans included long-term goals, interim targets, likely activities and associated budgets. This process proved critical in engaging both government and industry, and allowing ongoing critical review of progress. Sequential strategic plans and budgets, with mid-term reviews, were agreed over multi-annual periods: during 1975 to 1984 with a stated outcome of national Provisional Freedom, 1984 to 1992 to national Impending Freedom, and 1992 to 1997 to national Free Area status, respectively (Lehane 1996).
These experiences have contributed substantially to current approaches in Australia, where national animal health programmes are now developed and managed using agreed business processes. For example, Australia's EADRA is a legally binding, contractual arrangement that brings together government (the Commonwealth, the states and territories) and livestock industry groups to collectively and significantly increase Australia's capacity to both prepare for and respond to emergency animal disease (EAD) incursions (Animal Health Australia 2014b). EADRA forms just one part of AHA's strategic plan for 2010 to 2015 (outlining the organisation's mission, values and strategic priorities) (Animal Health Australia 2010), with defined three-year rolling business plans (incorporating objectives and scope, management plan, activities, stakeholder communications, financial management, strategy, evaluation, business rules) (Animal Health Australia 2014c). A range of other resources has been developed relevant to EAD preparedness including management manuals, disease strategies and operational manuals (Animal Health Australia 2001, 2015). These are also underpinned by three-year rolling business plans.
Consistent and transparent technical standards, underpinned by a strict regulatory regime, as well as applied research
Throughout BTEC (1975, 1979, 1982, 1984, 1986, 1995) and TFAP (1998), standard definitions and rules (SDRs) were developed, providing the minimum national standards agreed by all states and territories for the conduct of the campaign (Lehane 1996) (Box 1). First published in 1975, the SDRs were subject to regular technical review. These documents focused on definitions (such as herd classification and pathways), rules (including movement between areas), animal identification, breakdowns, monitoring and imports. Standard laboratory procedures were also defined from 1975.
The national programme was underpinned by an active research programme, which was funded by both government (Commonwealth, states and territories) and the cattle industry. Two key research strands were pursued, including an improved understanding of TB epidemiology and diagnostics. The role of feral pigs in TB epidemiology was of particular concern (Corner and others 1981, McInerney and others 1995). Research into diagnostics focused on optimisation of the single caudal fold test (Lepper and others 1977), improved culture methods, new methods for agent identification, DNA typing of M bovis isolates and development of the interferon-gamma assay (Cousins and others 1998). DNA typing and the interferon-gamma assay did not become available until the latter stages of the programme, and had little impact on the final outcome.
Critical role of abattoir surveillance
Abattoir surveillance rapidly emerged as the primary surveillance method for the detection of infection in herds not previously known to be infected, with abattoir surveillance being the primary method for the detection of new TB case herds from 1975. A number of strategies were used to maximise the sensitivity of abattoir surveillance, which were later formalised within the NGSP. These included efforts to raise awareness, encouraging submissions from, and providing feedback to, meat inspectors, and the use of risk-based sampling (during NGSP2) and submission targets. Detailed information about these submissions is published annually (for example, Animal Health Australia 2014d). The cattle-tracing system was critical to the success of the overall eradication programme. Introduced during the early 1970s, it enabled tracing to the property of origin. This system was based on property identification, allocation of a unique PIC and the mandatory application of tail or ear tags bearing the PIC for most cattle movements, particularly to saleyards and abattoirs (Animal Health Australia 2013).
Effective elimination of residual infection
The number of confirmed primary cases of TB during the final stages of eradication in Australia is presented in Table 5. Case studies of primary and secondary cases from Queensland during 1998 to 2002 are presented in Appendices A to D (adapted from More and Roe 2002) (available online as Supplementary Data). Appendix B was typical of most TB cases detected during the latter parts of the programme throughout northern Australia, where infected animals were most commonly associated with previous infection in the same herd. In these herds, generally only a very small number of infected animals (usually older cows) were detected, with no evidence in approximately 80 per cent of cases of further within-herd transmission. Nonetheless, animals potentially exposed to these TB cases were identified for early removal to slaughter. Appendices A, C and D were not typical, highlighting spread to other farms. Collectively, these case studies illustrate some of the challenges faced during the management of infected (sub)groups, in particular the potential for residually infected cattle.
With industry support, programme managers imposed relatively draconian measures, when needed, to minimise infection risk from known infected herds. A large number of methodological risk-based approaches were used, as outlined previously, underpinned by innovative thinking, meticulous (and at times, ruthless) application and ongoing critical review. Throughout southern Australia, robust movement controls, animal traceability and abattoir surveillance were in place from the early years of BTEC. Further, there was industry support, from the early years of the programme, for whole-herd slaughter in herds where TB clearance proved particularly difficult. In northern Australia, additional methods were introduced for extensive pastoral areas, including the use of helicopter mustering, paddock checks (also using helicopters), completing of bush destocking using destruction from helicopters (and at times, ‘Judas’ cows), herd segregation and accelerated culling of high-risk groups (Lehane 1996).
Objective and readily understood measures of programme progress
Measures of programme progress were both objective and readily understood. Many of these measures were based on herd and area classification, providing clear evidence of progress towards eradication, both nationally (Fig 7) and locally. Defined areas were progressively cleared of TB, to focus available resources and provide confidence among other producers about the progress that could be achieved. Milestones were used extensively, such as declaration of national Impending Freedom (on December 31, 1992). Changes in herd and area classification also directly impacted on individual producers.
The Australian National Disease Information System (ANADIS) computer program was installed in 1976 to 1977 primarily in support of brucellosis eradication (Lehane 1976). However, it also played a valuable role in the tracking of herds and animals for TB eradication. It was later replaced by systems developed within individual states. From the start of 1993, immediately after Australia had declared Impending Freedom, a TB case herd register was developed, with information on all incidents, including confirmed primary cases, suspect primary cases (not confirmed by laboratory testing) and secondary cases (further infection arising from animal movement from known infected herds) (Animal Health Australia 2007).
Conclusions
There are few international examples of the successful eradication of TB from a national cattle population. Australia achieved freedom following a 27-year campaign, followed by ongoing abattoir surveillance. Unique challenges were faced in Australia, noting that about half of the national cattle herd is husbanded in an extensive pastoral management system. Direct translation of some of these experiences to other countries may not be possible. Nonetheless, there are many lessons to be learned from the Australian experience that may be relevant to other countries. It is important that these lessons are not forgotten.
Supplementary Material
Acknowledgments
A large number of people have assisted during the preparation of this paper, providing their perspectives of aspects of TB eradication in Australia. They include Bob Biddle, Andrew Cupit, Kevin de Witte, Iain East, Graeme Garner, Louise Kench, Robyn Martin, Bill Matthews, Andrew Moss, John Roberts, Richard Rubira, Bill Scanlan, Mark Schipp and John Stewart. Their input was hugely appreciated. Thanks too to Graeme Garner, Penny Phillips and Rachel Wicks for assistance during the first author's visit to Canberra, and to Daniel Collins for expert assistance with mapping.
References
- 1.ANON (1982) Industries Assistance Commission Report. Bovine brucellosis and tuberculosis eradication campaign. Australian Government Publishing Service [Google Scholar]
- 2.ANON (1989) Operational management review. Report on the bovine brucellosis and tuberculosis eradication campaign. BTEC Committee [Google Scholar]
- 3.ANON (1991) National Cattle Disease Eradication Trust Account Act 1991. ComLaw, Australian Government. www.comlaw.gov.au/Details/C2004C05447. Accessed August 13, 2015 [Google Scholar]
- 4.ANON (2001) Tuberculosis Freedom Assurance Program. Mid term review. Parts 1 & 2. Animal Health Australia. ISBN 1 87671407 7 [Google Scholar]
- 5.ANON (2014) Australian Bovine Tuberculosis Surveillance Project. Animal Health Australia. www.animalhealthaustralia.com.au/programs/disease-surveillance/australian-bovine-tuberculosis-surveillance-project/ Accessed August 13, 2015 [Google Scholar]
- 6.ANIMAL HEALTH AUSTRALIA (2000) Tuberculosis Freedom Assurance Program. Mid term review. Parts 1 & 2. Animal Health Australia [Google Scholar]
- 7.ANIMAL HEALTH AUSTRALIA (2001) Government and livestock industry cost sharing deed in respect of emergency animal disease responses. www.animalhealthaustralia.com.au/wp-content/uploads/2011/04/EADRA-Version-11-01-%E2%80%93-28-06-2011.pdf Accessed August 13, 2015 [Google Scholar]
- 8.ANIMAL HEALTH AUSTRALIA (2005) National Granuloma Submission Program. Final report March 1992 to December 2004. Animal Health Australia [Google Scholar]
- 9.ANIMAL HEALTH AUSTRALIA (2006) Animal Health in Australia 2006. Animal Health Australia. www.animalhealthaustralia.com.au/wp-content/uploads/2011/05/AHIA-2006.pdf. Accessed August 18, 2015 [Google Scholar]
- 10.ANIMAL HEALTH AUSTRALIA (2007) Tuberculosis Freedom Assurance Program 2. Final report. Eradicating bovine tuberculosis from Australian livestock. Animal Health Australia [Google Scholar]
- 11.ANIMAL HEALTH AUSTRALIA (2009) Bovine tuberculosis case response manual. Managing an incident of bovine tuberculosis. Edition 2, 2009. Primary Industries Ministerial Council. www.animalhealthaustralia.com.au/wp-content/uploads/2011/03/TB-Case-Response-Manual-Ed2.pdf Accessed August 18, 2015 [Google Scholar]
- 12.ANIMAL HEALTH AUSTRALIA (2010) Strategic Plan 2010-15. Working together for animal health. www.animalhealthaustralia.com.au/wp-content/uploads/2011/05/AHA-Strategic-Plan-2010-2015.pdf Accessed August 13, 2015 [Google Scholar]
- 13.ANIMAL HEALTH AUSTRALIA (2013) History of livestock identification and traceability. www.animalhealthaustralia.com.au/programs/biosecurity/national-livestock-identification-system/history-of-livestock-identification-and-traceability/ Accessed August 13, 2015 [Google Scholar]
- 14.ANIMAL HEALTH AUSTRALIA (2014a) Annual Report 2013-14. www.animalhealthaustralia.com.au/wp-content/uploads/2014/11/AHA-AR-2013-2014-AccessibleWeb.pdf Accessed August 13, 2015 [Google Scholar]
- 15.ANIMAL HEALTH AUSTRALIA (2014b) EAD Response Agreement. www.animalhealthaustralia.com.au/programs/emergency-animal-disease-preparedness/ead-response-agreement/. Accessed August 13, 2015 [Google Scholar]
- 16.ANIMAL HEALTH AUSTRALIA (2014c) EADRA Business Plan (2014/15-2016/17). Version No: 4.0. www.animalhealthaustralia.com.au/wp-content/uploads/2011/04/EADRA-business-plan-14-15-v4_0_110614_FINAL.pdf Accessed August 13, 2015 [Google Scholar]
- 17.ANIMAL HEALTH AUSTRALIA (2014d) Animal health in Australia 2013, Animal Health Australia [Google Scholar]
- 18.ANIMAL HEALTH AUSTRALIA (2015) AUSVETPLAN. www.animalhealthaustralia.com.au/programs/emergency-animal-disease-preparedness/ausvetplan/. Accessed August 13, 2015 [Google Scholar]
- 19.ANON (1964) Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra-Community trade in bovine animals and swine. Official Journal of the European Communities L121, 1977-2012 (including subsequent corrections and amendments) [Google Scholar]
- 20.AUSTRALIAN BUREAU OF STATISTICS (2007) Australia's beef cattle industry. www.abs.gov.au/ausstats/abs@.nsf/Previousproducts/1301.0Feature%20Article232005?opendocument Accessed August 18, 2015 [Google Scholar]
- 21.BLACK P. F. (2012) Good governance of animal health systems and public-private partnerships: an Australian case study. Revue Scientifique et Technique/Office International des Épizooties 31, 699–708 [DOI] [PubMed] [Google Scholar]
- 22.CORNER L. A. (2006) The role of wild animal populations in the epidemiology of tuberculosis in domestic animals: How to assess the risk. Veterinary Microbiology 112, 303–312 [DOI] [PubMed] [Google Scholar]
- 23.CORNER L. A., BARRETT R. H., LEPPER A. W., LEWIS V., PEARSON C. W. (1981) A survey of mycobacteriosis of feral pigs in the Northern Territory. Australian Veterinary Journal 57, 537–542 [DOI] [PubMed] [Google Scholar]
- 24.COUSINS D. V., CORNER L. A., TOLSON J. W., JONES S. L., WOOD P. R. (1998) Eradication of Bovine Tuberculosis from Australia. Key Management and Technical Aspects. CSL Ltd [Google Scholar]
- 25.COUSINS D. V., FRANCIS B. R., CASEY R., MAYBERRY C. (1993) Mycobacterium bovis infection in a goat. Australian Veterinary Journal 70, 262–263 [DOI] [PubMed] [Google Scholar]
- 26.COUSINS D. V., ROBERTS J. L. (2001) Australia's campaign to eradicate bovine tuberculosis: the battle for freedom and beyond. Tuberculosis 81, 5–15 [DOI] [PubMed] [Google Scholar]
- 27.DEPARTMENT OF THE ENVIRONMENT (2015) Invasive species. www.environment.gov.au/biodiversity/invasive-species. Accessed August 14, 2015 [Google Scholar]
- 28.HEIN W. R., TOMASOVIC A. A. (1981) An abattoir survey of tuberculosis in feral buffaloes. Australian Veterinary Journal 57, 543–547 [DOI] [PubMed] [Google Scholar]
- 29.LEHANE R. (1996) Beating the Odds in a Big Country. The Eradication of Bovine Brucellosis and Tuberculosis in Australia. CSIRO Publishing [Google Scholar]
- 30.LEPPER A. W., NEWTON-TABRETT D. A., CORNER L. A., CARPENTER M. T., SCANLAN W. A., WILLIAMS O. J., HELWIG D. M. (1977) The use of bovine PPD tuberculin in the single caudal fold test to detect tuberculosis in beef cattle. Australian Veterinary Journal53, 208–213 [DOI] [PubMed] [Google Scholar]
- 31.MCCOOL C. J., NEWTON-TABRETT D. A. (1979) The route of infection in tuberculosis in feral buffalo. Australian Veterinary Journal 55, 401–402 [PubMed] [Google Scholar]
- 32.MCCORMICK B. (2001) Stockguard Cattle. Strategic assessment – bovine tuberculosis. Department of Agriculture, Western Australia [Google Scholar]
- 33.MCGUIN C. (1986) Taming the Outback. NT Brucellosis and Tuberculosis Eradication campaign. BTEC Unit, Department of Primary Production [Google Scholar]
- 34.MCINERNEY J., SMALL K. J., CALEY P. (1995) Prevalence of Mycobacterium bovis infection in feral pigs in the Northern Territory. Australian Veterinary Journal 72, 448–451 [DOI] [PubMed] [Google Scholar]
- 35.MORE S., ROE D. (2002) Review of tuberculosis surveillance for TFAP2. Animal Health Australia [Google Scholar]
- 36.MYLREA P. J. (1990) Eradication of bovine tuberculosis from New South Wales — A century of endeavour. Australian Veterinary Journal 67, 104–107 [DOI] [PubMed] [Google Scholar]
- 37.NEUMANN G. B. (1999) Bovine tuberculosis - an increasingly rare event. Australian Veterinary Journal 77, 445–446 [DOI] [PubMed] [Google Scholar]
- 38.NEUMANN G. B. (2002) Setting the standards for Australia's animal health system. Australian Veterinary Journal 80, 329 [Google Scholar]
- 39.NEWTON L .G. (1992) Contagious bovine pleuropneumonia in Australia: some historic highlights from entry to eradication. Australian Veterinary Journal 69, 306–317 [DOI] [PubMed] [Google Scholar]
- 40.RADUNZ B. (2006) Surveillance and risk management during the latter stages of eradication: experiences from Australia. Veterinary Microbiology 112, 283–290 [DOI] [PubMed] [Google Scholar]
- 41.ROBINSON R. C., PHILLIPS P. H., STEVENS G., STORM P. A. (1989) An outbreak of Mycobacterium bovis infection in fallow deer (Dama dama). Australian Veterinary Journal 66, 195–197. [DOI] [PubMed] [Google Scholar]
- 42.SMITH W. S. (1959) Tuberculosis in beef cattle in South Australia. Australian Veterinary Journal 35, 116–119 [Google Scholar]
- 43.TOLSON J., GARNER G., HAWKINS C., DEWITTE K. (2001) The monitoring requirements for bovine tuberculosis in Australia after 2002. Animal Health Australia [Google Scholar]
- 44.TURNER A. J. (2011) Endemic disease control and regulation in Australia 1901-2010. Australian Veterinary Journal 89, 413–421 [DOI] [PubMed] [Google Scholar]
- 45.TWEDDLE N. E., LIVINGSTONE P. (1994) Bovine tuberculosis control and eradication programs in Australia and New Zealand. Veterinary Microbiology 40, 23–39 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.