Abstract
We report a case of a 30-year-old woman who experienced recurrent infections of the abdominal wall after travelling to Turkey from Germany to undergo abdominoplasty for aesthetic reasons. The patient's Mycobacterium fortuitum infection was successfully treated by surgery and antibiotic therapy. Surgical tourism—in this case, lipotourism—is resulting in an increasing number of patients in Europe who may present uncommon disease patterns.
Keywords: Abdominoplasty, atypical mycobacteria, lipotourist, Mycobacterium fortuitum, treatment
Introduction
In our daily clinical work we have noted an increasing number of cosmetic surgeries performed abroad in Eastern and Southern Europe for financial reasons, similar to American lipotourism, or liposurgery performed abroad, mostly in Latin America and the Caribbean. In our opinion this cosmetic-surgery tourism will lead to an increasing number of cases of rare infections in Europe, as has been observed in the United States [1–3].
We report a case of a 30-year-old woman, a German of Turkish origin, who was admitted to our hospital in February 2014 for recurrent infections of the abdominal wall. She reported that she had undergone abdominoplasty for aesthetic reasons in October 2012. The surgery had been performed in Turkey for financial reasons—the procedure was supposedly less expensive there. Three months after surgery, in January 2013, she first noticed skin lesions near the incision lines. In the meantime, before she was admitted to our hospital, she had consulted several physicians regarding various abscesses of the left abdominal wall, but the abscesses always recurred after a short period of time.
Case report
The patient was a nonsmoking woman with a normal body mass index of 21 kg/m2 and without a history of any serious disease or immunosuppression. Abdominal examination revealed extensive phlegmon and several locular abscesses in the abdominal wall (Fig. 1A). The laboratory values revealed mild anemia and no signs of general infection. We decided to relieve the largest abscess under local anesthesia, took a swab sample from an inner wound for microbiologic analysis, initiated antibiotic therapy with clarithromycin 500 mg twice a day and scheduled an extensive surgical debridement of the wounds with the patient under general anesthesia. Before surgery we performed magnetic resonance tomography (MRT) to exclude any deeper infections of the abdominal wall and to estimate the extent of the infection. The MRT revealed no infiltrations of the abdominal muscles; the two largest abscesses measured 9 × 1 cm and 5 × 2 cm.
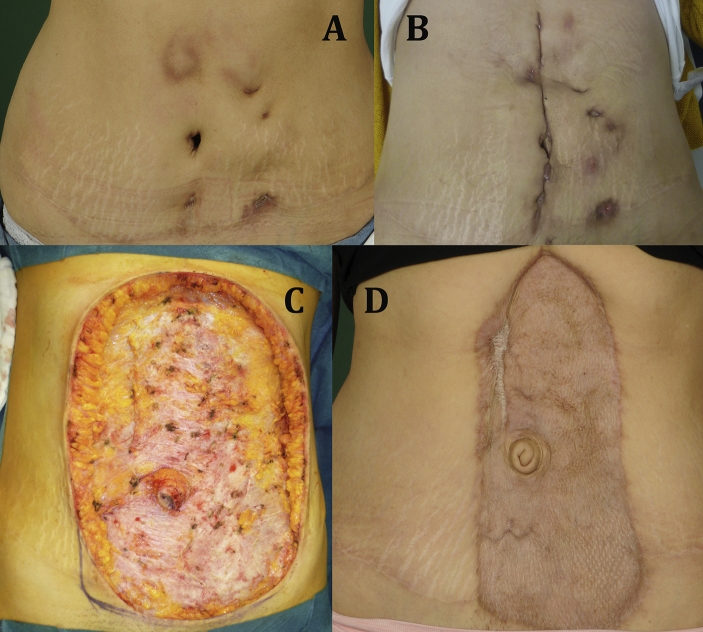
Fig. 1.
(A) Abdominal wall infection at first outpatient admission. (B) Abdominal wall reinfection 3 weeks after surgical debridement. (C) Abdominal wall after radical debridement. (D) Abdominal wall 5 months after skin mesh graft transplantation.
During the inpatient treatment we performed a surgical debridement of the wound and initiated a vacuum-assisted closure (VAC) therapy. In the following 2 weeks we changed the VAC system twice and achieved secondary wound closure. The patient was discharged with inconspicuous wounds and in overall good health.
In the meantime the microbiologic analysis identified Mycobacterium fortuitum. Antimicrobial susceptibility testing by the gradient diffusion method found the following minimum inhibitory concentrations: amikacin 1.0 μg/mL, levofloxacin 0.125 μg/mL, moxifloxacin 0.047 μg/mL, clarithromycin 1.5 μg/mL, linezolid >256 μg/mL, rifampin >32 μg/mL, tetracycline 0.064 μg/mL, imipenem 4 μg/mL, meropenem 3 μg/mL, ampicillin >256 μg/mL and amoxicillin/clavulanic acid >256 μg/mL. Histology did not reveal any malignancy of the excised tissue.
Two weeks after discharge the patient again presented with abdominal wall pain and indurations of the skin (Fig. 1B). We reinitiated antibiotic therapy with clarithromycin 500 mg twice a day and reevaluated the surgical and medical treatment options in close cooperation with the Institute of Medical Microbiology of the University Hospital Münster. The swab sample taken from the wound at this time again grew M. fortuitum. Additionally, multiple flora were identified, including Prevotella bergensis, Actinomyces europaeus, Staphylococcus epidermidis, Micrococcus luteus, Lactobacillus sp. and Peptostreptococcus sp.
Because of the continuous progression of the disease over the last months and the proximity to the internal abdominal organs, interdisciplinary discussion led to the conclusion that the case was a potentially life-threatening illness. It was decided that inpatient treatment with radical debridement of the infected abdominal soft tissue, in combination with targeted antimicrobial therapy according to the results of susceptibility testing, was necessary.
For antimicrobial therapy we decided to treat the patient intravenously for 14 days with amikacin (15 mg/kg) and moxifloxacin 400 mg once per day, clarithromycin 500 mg twice a day and ampicillin/sulbactam 3 g every 8 hours.
After that she received oral antimicrobial therapy with moxifloxacin 400 mg once a day and clarithromycin 500 mg twice a day for 3 months.
We began the hospital treatment with this extended antibiotic therapy and radical debridement (Fig. 1C), initiating VAC therapy followed by several revisions with continued VAC therapy to prepare the wound for skin grafting. Three weeks later we closed the wound with a large skin mesh graft. The skin mesh graft adhered successfully (Fig. 1D).
The patient was discharged from hospital with prescribed oral antibiotics and was regularly followed up. At the 1-year follow-up there was no sign of reinfection, and no pathologic findings were found in the blood tests.
Discussion
M. fortuitum is a rapidly growing mycobacterium that can cause severe infections leading to unsuccessful wound healing, wound dehiscence and infection recurrence [4]. Suspicion arises with a lack of response to conventional antibiotic regimens and if standard bacterial cultures remain negative. M. fortuitum is found in lakes, rivers, tap water, wastewater, and dust and dirt, and it can infect a wound when exposed to contaminated tap water [5]. Hospital-acquired disease caused by poor hygiene of sanitary facilities has previously been reported, but these infections usually present as disseminated skin lesions and soft tissue lesions, predominantly occurring in the setting of severe immunosuppression, especially in AIDS [6–8].
The involvement of M. fortuitum in surgical site infections is well documented. It can be the cause of multiple types of infections such as sternal wound infections after cardiothoracic surgery or after breast augmentation surgery, e.g. due to contamination of the wound with contaminated tap water [4,6,7,9]. Recent outbreaks have also been described in immunocompetent hosts after use of contaminated whirlpool foot baths in nail salons [10]. M. fortuitum infections after liposurgery have been reported in different continents [11] but to our knowledge not yet in Europe.
There have been reported outbreaks of Mycobacterium abscessus wound infections in lipotourists from the United States who underwent abdominoplasty in the Dominican Republic [1]. Travelling to foreign countries, with their supposedly lower costs for cosmetic surgery, is relatively common in the United States; these cosmetic surgeries are performed mostly in Latin America and the Caribbean [1–3,11]. In recent years this kind of tourism seems to have recruited an increasing number of patients in Europe too, which may confront physicians with uncommon disease patterns, especially physicians in private practice and those practicing in general medicine. The European destinations for such surgical tourism are mostly in Eastern and Southern Europe. Therefore, it is important to execute a full history of the patient and to know the significant signs of this rare infection. In particular, a negative standard bacterial culture, a lack of response to conventional antibiotic regimens and infection recurrence, particularly months after surgery, should set physicians' alarm bells ringing.
We report a successful surgical and antibiotic treatment of a M. fortuitum infection after abdominoplasty. In our case neither surgical treatment nor antibiotic treatment alone was successful. We could only achieve cure by close interdisciplinary collaboration of both medical specialties. Awareness among physicians will, we hope, result in faster identification and treatment of these cases. It is important to make patients appreciate the higher standards of hygiene and the more skilled performance of these procedures even though they are associated with higher cost. Bargain hunters should be made aware that in the end the price of their abdominoplasty may be higher than expected.
Conflict of interest
None declared.
References
- 1.Furuya E.Y., Paez A., Srinivasan A., Cooksey R., Augenbraun M., Baron M. Outbreak of Mycobacterium abscessus wound infections among “lipotourists” from the United States who underwent abdominoplasty in the Dominican Republic. Clin Infect Dis. 2008;46:1181–1188. doi: 10.1086/529191. [DOI] [PubMed] [Google Scholar]
- 2.Bax H.I., van Ingen J., Dwarkasing R.S., Verbon A. [Lipotourism, not without risks: a complication of cosmetic surgery abroad] Ned Tijdschr Geneeskd. 2014;158:A7926. [PubMed] [Google Scholar]
- 3.Newman M.I., Camberos A.E., Ascherman J. Mycobacteria abscessus outbreak in US patients linked to offshore surgicenter. Ann Plast Surg. 2005;55:107–110. doi: 10.1097/01.sap.0000168030.87804.93. [DOI] [PubMed] [Google Scholar]
- 4.Olalla J., Pombo M., Aguado J.M., Rodríguez E., Palenque E., Costa J.R. Mycobacterium fortuitum complex endocarditis—case report and literature review. Clin Microbiol Infect. 2002;8:125–129. doi: 10.1046/j.1198-743x.2001.00397.x. [DOI] [PubMed] [Google Scholar]
- 5.Toro A., Adekambi T., Cheynet F., Fournier P.E., Drancourt M. Mycobacterium setense infection in humans. Emerg Infect Dis. 2008;14:1330–1332. doi: 10.3201/eid1408.080179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piersimoni C., Scarparo C. Extrapulmonary infections associated with nontuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351–1358. doi: 10.3201/eid1509.081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 8.Clegg H.W., Foster M.T., Sanders W.E., Jr., Baine W.B. Infection due to organisms of the Mycobacterium fortuitum complex after augmentation mammaplasty: clinical and epidemiologic features. J Infect Dis. 1983;147:427–433. doi: 10.1093/infdis/147.3.427. [DOI] [PubMed] [Google Scholar]
- 9.Thibeaut S., Levy P.Y., Pelletier M.L., Drancourt M. Mycobacterium conceptionense infection after breast implant surgery, France. Emerg Infect Dis. 2010;16:1180–1181. doi: 10.3201/eid1607.090771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vugia D.J., Jang Y., Zizek C., Ely J., Winthrop K.L., Desmond E. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616–618. doi: 10.3201/eid1104.040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Centers for Disease Control and Prevention Rapidly growing mycobacterial infection following liposuction and liposculpture—Caracas, Venezuela, 1996–1998. MMWR Morb Mortal Wkly Rep. 1998;47:1065–1067. [PubMed] [Google Scholar]