Abstract
Magnetic recording from five normal human adults demonstrates large 40-Hz coherent magnetic activity in the awake and in rapid-eye-movement (REM) sleep states that is very reduced during delta sleep (deep sleep characterized by delta waves in the electroencephalogram). This 40-Hz magnetic oscillation has been shown to be reset by sensory stimuli in the awake state. Such resetting is not observed during REM or delta sleep. The 40 Hz in REM sleep is characterized, as is that in the awake state, by a fronto-occipital phase shift over the head. This phase shift has a maximum duration of approximately 12-13 msec. Because 40-Hz oscillation is seen in wakefulness and in dreaming, we propose it to be a correlate of cognition, probably resultant from coherent 40-Hz resonance between thalamocortical-specific and nonspecific loops. Moreover, we proposed that the specific loops give the content of cognition, and a nonspecific loop gives the temporal binding required for the unity of cognitive experience.
Full text
PDF
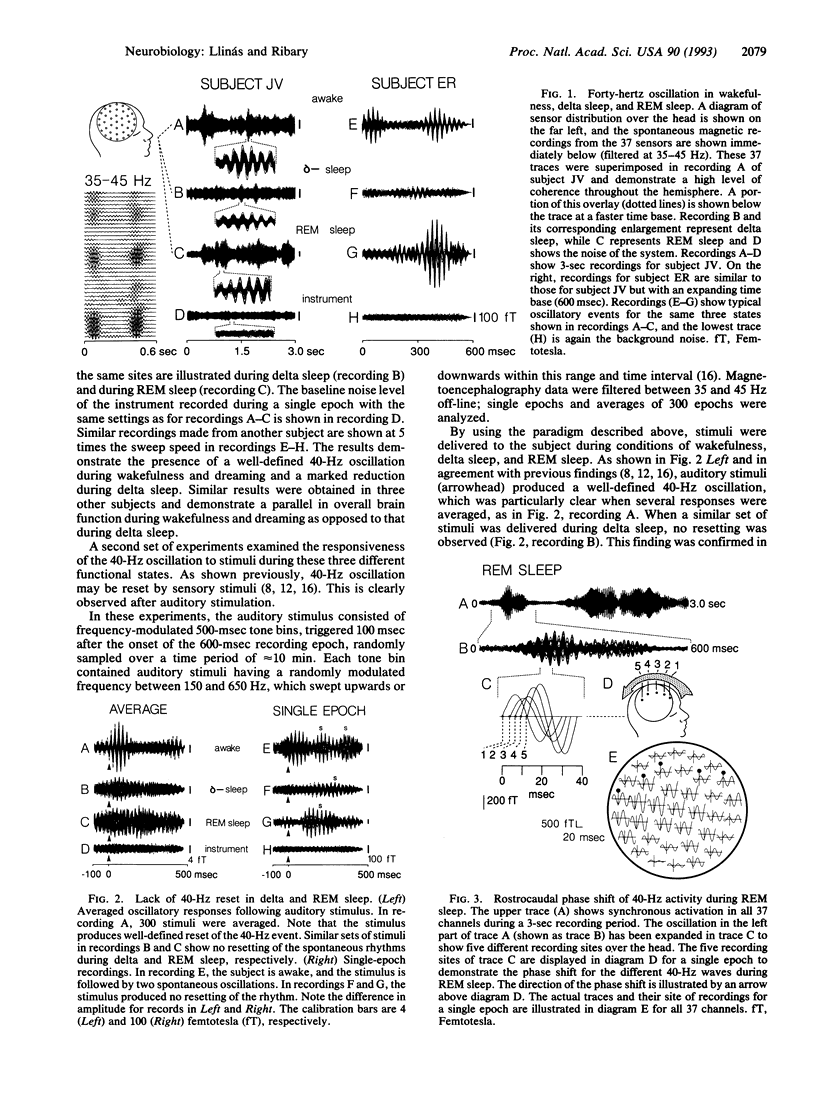

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouyer J. J., Montaron M. F., Vahnée J. M., Albert M. P., Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987 Sep;22(3):863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Bressler S. L., Freeman W. J. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980 Oct;50(1-2):19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Crick F., Koch C. Some reflections on visual awareness. Cold Spring Harb Symp Quant Biol. 1990;55:953–962. doi: 10.1101/sqb.1990.055.01.089. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Levay S. Laminar and synaptic organization of the projection from the thalamic nucleus centralis to primary visual cortex in the cat. J Comp Neurol. 1986 Dec 1;254(1):66–77. doi: 10.1002/cne.902540106. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- FACON E., STERIADE M., WERTHEIM N. Hypersomnie prolongée engendrée par des lésions bilatérales du système activateur médial; le syndrome thrombotique de la bifurcation du tronc basilaire. Rev Neurol (Paris) 1958 Feb;98(2):117–133. [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P. J. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose G. M., Freeman R. D. Oscillatory discharge in the visual system: does it have a functional role? J Neurophysiol. 1992 Nov;68(5):1558–1574. doi: 10.1152/jn.1992.68.5.1558. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R., Ilmoniemi R. J. Cerebral magnetic fields. Crit Rev Biomed Eng. 1986;14(2):93–126. [PubMed] [Google Scholar]
- Kristofferson A. B. Quantal and deterministic timing in human duration discrimination. Ann N Y Acad Sci. 1984;423:3–15. doi: 10.1111/j.1749-6632.1984.tb23413.x. [DOI] [PubMed] [Google Scholar]
- Llinás R. R., Grace A. A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R., Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44(3):521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- Pinault D., Deschênes M. Voltage-dependent 40-Hz oscillations in rat reticular thalamic neurons in vivo. Neuroscience. 1992 Nov;51(2):245–258. doi: 10.1016/0306-4522(92)90312-p. [DOI] [PubMed] [Google Scholar]
- Ribary U., Ioannides A. A., Singh K. D., Hasson R., Bolton J. P., Lado F., Mogilner A., Llinás R. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Paré D., Oakson G. Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk J., Ribary U., Cappell J., Yamamoto T., Llinás R. Anatomical localization revealed by MEG recordings of the human somatosensory system. Electroencephalogr Clin Neurophysiol. 1991 Mar;78(3):185–196. doi: 10.1016/0013-4694(91)90032-y. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Williamson S. J., Kaufman L., Nicholson C., Llinás R. Magnetic localization of neuronal activity in the human brain. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8732–8736. doi: 10.1073/pnas.85.22.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]