Abstract
Animal models of focal cerebral ischemia are well accepted for investigating the pathogenesis and potential treatment strategies for human stroke. Occlusion of the middle cerebral artery (MCA) with an endovascular filament is a widely used model to induce focal cerebral ischemia. However, this model is not amenable to thrombolytic therapies. As thrombolysis with recombinant tissue plasminogen activator (rtPA) is a standard of care within 4.5 hours of human stroke onset, suitable animal models that mimic cellular and molecular mechanisms of thrombosis and thrombolysis of stroke are required. By occluding the MCA with a fibrin-rich allogeneic clot, we have developed an embolic model of MCA occlusion in the rat, which recapitulates the key components of thrombotic development and of thrombolytic therapy of rtPA observed from human ischemic stroke. The surgical procedures of our model can be typically completed within approximately 30 min and are highly adaptable to other strains of rats as well as mice for both genders. Thus, this model provides a powerful tool for translational stroke research.
Introduction
Stroke is a leading cause of death and disability, affecting 15 million people globally each year. Over 80% of stroke is caused by the obstruction of a cerebral artery by an endovascular embolus or thrombus formation. Unfortunately, treatment options for stroke are limited. Since approval by the FDA in 1996, intravenous administration of recombinant tissue plasminogen activator (rtPA) which dissolves the obstructive clot to restore blood flow1 remains the only treatment for patients within 4.5 hours of stroke onset. However, only a small percentage of patients with ischemic stroke are eligible for rtPA treatment, due to its narrow therapeutic window and the risk of brain hemorrhage2–4. In order to improve our understanding of the pathogenesis of stroke and to bring thrombolytic therapy and other potential therapeutic approaches to more stroke patients, suitable animal models that mimic the pathophysiology of ischemic stroke are required. Human stroke most frequently affects the territory of the middle cerebral artery (MCA)5. Spontaneous thrombolytic lysis occurs in human stroke6. Occlusion of the MCA by various methods in rodents generates reproducible ischemic brain damage and has been widely used to study the underlying molecular mechanisms and potential treatment strategies for stroke. Occlusion of the MCA with an intraluminal filament is the most frequently used minimally-invasive stroke model in rodents, which allows retraction of the filament from the MCA at variable durations to produce permanent or transient focal ischemia7. However, reperfusion achieved by retraction of the filament cannot model the biological events of thrombolysis8. There are additional models to induce stroke with its merits and limitations (Table 1). Importantly, large failures of neuroprotective clinical trials for acute stroke treatment highlight that neuroprotection alone without restoration of tissue perfusion and vascular integrity may not be adequate for treatment of acute stroke9–12. Treatment of acute stroke needs to restore the normal function of the neurovascular unit by rapidly reestablishing cerebral blood flow (CBF) in the ischemic cerebral microvascular bed, preserving vascular integrity, and minimizing cell death13. We developed a clinically relevant model of embolic ischemia in the rat that closely mimics the pathological features of thromboembolic stroke14–16. By precise delivery of a naturally formed allogeneic embolus to the origin of the MCA, we observe the dynamic development of thrombotic formation at the embolic occluded MCA site and the secondary thrombosis in the downstream cerebral microvessels of the MCA14–16. The ischemic cell damage is predictable and reproducible, which is well confined within the territory supplied by the MCA14–16. Longitudinal MRI analysis of this model reveals spontaneous recanalization of the occluded MCA approximately 24h or later after stroke17. Moreover, intravenous administration of rtPA within 2h of MCA occlusion (MCAO) promotes recanalization, substantially improves downstream microvascular perfusion and integrity, and considerably reduces infarct size, consequently leading to improvement of neurological outcomes18–20. Thus, this model provides the key components of thromboembolic development and of thrombolytic therapy of rtPA observed in human ischemic stroke21,22. This embolic model of focal cerebral ischemia has been widely used for evaluation of thrombolytic therapies and potential adjuvant agents to thrombolysis23–30. Here, we provide a detailed protocol on how to generate this model starting from the preparation of an embolus. The surgical procedures to occlude the MCA are minimally invasive and can be typically completed within 30 min.
Table 1.
Animal models of thromboembolic stroke
| Models | Advantages | Limitations |
|---|---|---|
| Photothrombosis59,60 | Reproducible ischemic brain damage | Often require craniotomy and ischemic lesion mainly localize to cortex |
| Artificial emboli61,62 | Minimally invasive and mimic the multifocal and heterogonous nature of human stroke | Un-controllable lodgment of emboli, high variation in infarct size, and not suitable for thrombolysis |
| Blood emboli63–65 | Susceptible to thrombolytic therapies | Un-controllable embolization site and occlusion time, and high variations in infarct size and location |
Advantages and limitations
Key advantages
A single embolus blocks the origin of the MCA. Thus, the ischemic cell damage is well confined within the territory supplied by the MCA, leading to predicable and reproducible focal cerebral ischemia.
Secondary thrombosis is formed at the site where the embolus resided and in downstream cerebral microvessels. Spontaneous recanalization of the occluded MCA occurs at 24h or later.
Impairment of sensorimotor function can be assayed;
Intravenous administration of rtPA within 2h onset of MCAO induces recanalization, increases downstream microvascular perfusion, and reduces ischemic cell damage.
Adjunct use of rtPA with neuroprotective agents extends therapeutic window of thrombolysis and reduces hemorrhagic transformation.
Limitations
Our model specifically blocks the origin of the MCA, which produces large ischemic lesion encompassing the entire MCA territory. However, with modification of the embolus length, a small cortical ischemic lesion in downstream MCA territory can be produced31.
Experimental design
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Hospital.
Preparation of embolus
The formation of a single fibrin-rich allogeneic clot is an essential step for generating reproducible ischemic brain damage. Young healthy rats are used as donors for arterial blood collection. An ex-vivo formed whole blood clot contains all blood elements, such as red blood cells (RBCs) and platelets. A red clot that contains many RBCs is relatively fragile. Therefore, to attain a single fibrin-rich white clot, a gentle wash with saline is needed to remove entrapped RBCs. In addition, it is necessary to maintain the flexibility of the clot, since platelet and RBCs modulate the structure and hardness of the clot and are resistant to thrombolysis32,33. All emboli should be used within 24 to 30 hours after blood collection.
Preparation of endovascular cathethers
To ensure successful delivery of a single clot to the orgin of the MCA, polyethylene catheters (PE-50, ID 0.58mm, OD 0.965mm) are modified by reducing the OD to 0.3mm at one end, which permits easy access to the intracranial portion of the internal carotid artery (ICA).
MCAO
To induce MCAO, it is critical to place a single embolus at the origin of the MCA, which reduces mortality by eliminating obstruction of the posterior communicating arteries (PCA) that are unavoidably blocked in the endovascular filament model. The procedures that we described herein are based on our extensive experience using male adult Wistar rats, in which the distance between the origin of the MCA and the site of arteriotomy on the external carotid artery (ECA, the distal entry site of catheter) are approximately 19–22mm. Thus, lodgment of the embolus at the origin of the MCA can be attained by advancing the catheter from the ECA arteriotomy site into the ICA until its tip is situated1–2mm proximal to the origin of the MCA. Due to the anatomic and surgical variations, the distance between the origin of the MCA and the arteriotomy may vary. Thus, the catheter is initially advanced 18mm, then further advanced gently for 1–3 mm until resistance is felt, which indicates the catheter has reached to the origin of the MCA due to the anatomic narrowing and tortuousity of artery anterior to the origin of the MCA. The catheter is then retracted 1–2mm to ensure the correct placement at1–2mm proximal to the origin of the MCA. Sham-operated rats are used as control in which rats are subjected to the identical surgical procedure but omit injection of the embolus. Although the procedures are primarily for young adult Wistar rats, applications of these procedures are highly adaptable to other strains of the rat and the mouse as well as to the aged rodent by adjusting the size of embolus and the length of the catheter30,31,34.
Materials
Animals
Male Wistar rats, 10–12weeks old and weighing 325–400g (Charles River Laboratory, strain code 003). The animal care facility at our institution is AALAC approved. Rats are housed separately in a temperature- and humidity-controlled room under a 12 h light-dark cycle with free access to food and water. CRITICAL Although the procedure was developed for young adult male Wistar rats, applications of these procedures are highly adaptable to other strains of the rat and the mouse as well as to female and aged rodent by adjusting the size of embolus and the length of the catheter30,31,34.
CAUTION All experimental procedures need to be approved by the Institutional Animal Care and Use Committee.
Reagents
Sodium chloride injectable 0.9% (Saline, wt/vol; Baxter, cat. no. 2B1302)
Cidex OPA, (Johnson & Johnson, cat. no. 20390) CAUTION Avoid direct contact with Cidex-OPA that may cause skin and eye irritation.
10% Povidone-Iodine Swabsticks (PDI, cat. no.M318)
Alcohol Prep Pads 70% (vol/vol; Kendall, cat. no. 6818)
Isoflurane (Baxter, cat. No. 1001936060) CAUTION Prolonged exposure to isoflurane potentially causes central nervous system depression. An exhaust system to eliminate excessive isoflurane needs to be operational during the surgery.
Equipment
Vannasmicrodissecting spring scissors (Roboz, cat. no.RS-5608)
Iris scissors (World Precision Instrument, cat. no. 500217)
Micro forceps (Codman,cat. no. 80–1807)
Mini aneurysm clip (Codman,cat. no. 20–1824)
Aneurysm clip applier (Codman,cat. no. 20–1855)
Micro-dissecting forceps (Stoelting Co, cat. no.52102–35)
Hartman mosquito forceps (Roboz, cat. no.RS-7101)
Mayo-Hegar needle holder (Codman, cat. No. 9505N)
Hamilton syringe 100 μl (Hamilton, cat. no. 710)
Tuberculin syringe with 27G needle (BD, cat. no. 23309623)
PE-10 tubing (Intramedic, cat. no. 14–170–12p)
PE-50 tubing (Intramedic, cat. no. 14–170–12B)
Sterile PE-50 tubing for blood collection (BD, cat. no. 427517)
Suture silk 4–0 for wound closure (ETHICON, cat. no. 683G)
Suture silk 4–0 for ligation (ETHICON,cat. no. L53G)
Petri dish (Corning, 100×20mm, cat. no.CLS430589)
Bunsen burner (Fisher, cat. no. 1201–21)
Nitrous oxide cylinder (Praxair, cas. no. 10024–97–2)
Oxygen cylinder (Praxair, cas. no. 7782–44–7)
Anesthesia gas blender with dual flow meter tubes (Surgivet, cat. no. 32375B4)
Isoflurane vaporizer (DatexOhmeda, cat. no. ISOTEC 5)
Table top surgical microscope (Ziess, cat. no. OPM1)
Procedures
Blood collection from the femoral artery
-
1
Anesthetize a donor rat with 3% isoflurane in 70% N2O and 30% O2 within an inducing chamber, and then transfer the rat to the surgical table with anesthesia maintained with 1.5% isoflurane throughout the surgical procedure.
Critical step: Avoid rats with fresh cuts, bruises, bite marks, and with signs of stress, because these events may compromise the clotting process.
-
2
Place the rat in supine position and retract both hind limbs posteriorly and laterally to expose the femoral triangle. Hold the limbs in position with surgical tapes and shave the inguinal region over the right femoral triangle with a clipper.
-
3
Apply 10% Povidone-Iodine and 70% Alcohol to disinfect the incision site.
-
4
Make an incision (approximate 10mm)along the femur above the femoral triangle.
-
5
Blunt dissection of connective tissues with a hemostatic forceps, and expose the femoral artery, vein, and nerve bundle.
-
6
Expose5–8mm femoral artery from the femoral neurovascular bundle by careful blunt dissection of the femoral nerve and vein with a hemostatic forceps.
-
7
Ligate the femoral artery distally with a 4-0 silk suture, and transiently block blood flow with an aneurysm clip at the proximal end of the femoral artery.
-
8
Place a 4-0 silk suture loosely around the femoral artery between the ligation and the clamped site.
-
9
Create a partial arteriotomy between the two sutures with a micro scissors, and then insert a sterilePE-50 tubing (400–500mm) along the artery towards the proximal direction until it reach the clamped site. Tighten the 4-0 silk suture between the ligation and clip to secure the tubing. Arterial blood collected in this length of the PE-50 tubing commonly generates ~10 individual clots suitable for MCAO. The length of the tubing can be varied depending on how many rats are scheduled for MCAO.
-
10
Release the clip slowly to allow the blood to spontaneously flow into the tubing, and re-apply the clip immediately after the tubing is filled with blood.
Critical step: In order to generate a uniform clot, it is essential that blood should flow smoothly and directly into the tubing. Any manipulations such as forcibly withdrawing the blood with a syringe during blood collection, will lead to formation of fragile segments of clots 24h after collection, which are not suitable for MCAO.
-
11
Remove the PE-50 tubing, and immediately tie off the suture.
-
12
Remove the clip from the artery and close the incision with 4-0 silk suture.
-
13
Place the rat back into its home cage and allow recovery from anesthesia.
-
14
Place blood containing PE-50 tubing in a Petri dish at 37°C for 2h, and subsequently retain it at 4°C for an additional 22h.
Critical step: Lay blood filled tubing flat and avoids unnecessary agitation during the 24h incubation period.
Preparation of endovascular cathethers
-
15
Turn on a gas burner, and adjust the fire to a low to medium flame.
-
16
Hold the a PE-50 tubing (150mm in length) by hand approximately 200 mm above the flame until the medial portion of the tubing becomes soft and flexible.
-
17
Move the tubing away from the fire and immediately stretch the tubing laterally until the medial portion of the tubing is elongated and its OD is reduced. Measure the medial portion of the modified PE-50 tubing with a dial caliber to the OD between 0.3 – 0.4 mm. Cut and discard the portion with an OD less than 0.3mm.
-
18
Sterilize the modified PE-50 tubing in Cidex OPA solution overnight, and rinse with sterile saline at least 3 times before use.
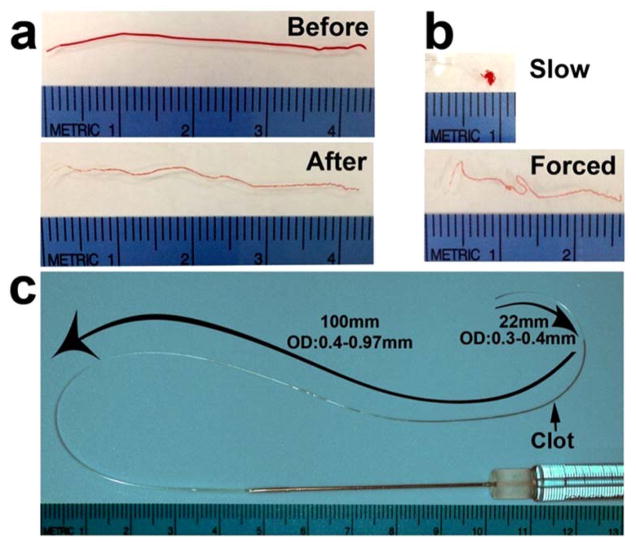
Critical step: For easy advancement the catheter to the origin of the MCA, the entire length of the modified tubing should be at least 120 mm including ~ 22mm long portion from the tip with an OD of 0.3–0.4mm (Fig. 1c).
Figure 1. Preparation of clot and measurement of catheter.
(a) Photos show a clot before and after wash; (b) A clot forms into a ball-like shape after it is slowly injected out of the modified PE-50 catheter, whereas the clot becomes a thread-like shape after forced injection; (c) A photo shows the length and OD of a modified PE-50 catheter containing a washed clot. The ruler on the individual images has a millimeter scale.
Preparation of embolus
-
19
Fill a Petri dish (100×20mm) with sterile saline, and prepare a ~500mm PE-10 tubing with one end connected with a 1cc saline filled syringe.
-
20
Cut the clot containing PE-50 tubing into ~50 mm segments in length, and connect the segment with the PE-10 tubing. Then push the syringe to flush the clot out of the PE-50 tubing into the saline filled Petri dish. If multiple MCAO procedures are planned in the same day, a longer segment of clot containing PE-50 tubing up to 300 mm could be used, which can generate ~6 emboli.
-
21
Disconnect the PE-50 tubing from the PE-10 one. Draw and flush the clot into and out the PE-10 tubing, respectively, for 10–15 times to wash out the majority of the entrapped RBCs until the clot becomes white-pinkish color (Fig. 1a).
Critical step: To avoid folding and twisting of the clot, always draw the clot from its end with the tubing, and place the clot into a straight line while flushing it back into the Petri dish.
-
22
Cut the washed clot into 40 mm in length and then collect it into the modified PE-50 catheter that is connected to a 100ul Hamilton micro-syringe. The clot is now ready to be used (Fig. 1c). If multiple clots are made, the remaining clots can be stored within a saline filled Petri dish at room temperature for up to 4h and should be discarded thereafter.
Critical step: If the clot contains many RBCs or is relatively fragile, the clot will be broken into segments when it is flushed out of the modified PE-50 catheter. Thus, when it is collected into the modified catheter, the clot should be flushed out the catheter once to make sure the clot remains intact. Only intact single clots can be used to block the MCA. During MCAO, the modified portion (tip) of the delivery catheter could be compressed by forceps that may cause damage to the contained clot. Therefore, in addition to avoiding unnecessary pressure while handling the catheter, the clot should be positioned ~22 mm distal from the tip of the catheter which can eliminate potential damage to the clots (Figure 2c). MCAO:
-
23
Anesthetize a rat using the anesthetic procedure described in step 1, and prepare the incision site with standard aseptic procedure as described in steps 2–3.
-
24
Place the rat in supine position, and hold both forelimbs laterally with surgical tapes to expose the ventral neck area.
-
25
Make a 20–30mm midline incision from jaw to shoulder, gently split the left and right sternohyoid muscles along midline with blunt dissection, and retract the right sternohyoid laterally to expose the trachea.
-
26
Under an operating microscope and with careful blunt dissection, expose the right common carotid artery (CCA), which is situated deeply beneath the sternohyoid muscles and sits laterally to the trachea.
-
27
Bluntly dissect the carotid sheath along the CCA rostrally until the ECA and internal carotid artery (ICA) bifurcation is exposed without harming the vagal nerve which also lies within the carotid sheath.
Critical step: Care must be taken to avoid injury to the vagal nerve during dissection. Do not excessively compress or tug arteries with forceps, which could cause swelling or even rupture the arteries.
-
28
Ligate the ECA along with occipital artery (the first branch of ECA) 2–3mm distal from the bifurcation with 2 4-0 silk suture. Cut the ECA between the two ligations, and retract the proximal portion of the ECA stump laterally and caudally to align the ECA with the ICA (Fig. 2a, arrow).
Critical step: It is critical that the ECA stump should be positioned parallel to the CCA in order to advance the catheter into the intracranial segment of the ICA.
-
29
Temporarily clamp the CCA and the ICA with an aneurysm clip, and apply a 4-0 silk suture loosely around the trunk of the ECA near the bifurcation.
-
30
Create a partial arteriotomy on the ECA, and then insert the tip of the clot filled modified PE-50 catheter into the arteriotomy. Advance the catheter rostrally towards the ICA until it reaches the clamp site. Tighten the 4-0 silk suture around the ECA trunk to secure the tubing.
-
31
Release the clip slowly and further advance the catheter within the ICA rostrally to enter the intracranial segment of the ICA until resistance is felt. The total length of the catheter that has been advanced is approximately 19–22 mm from the ECA arteriotomy site.
Critical step: The entire catheter along with the connected syringe should be aligned with the natural position of the ICA while advancing the catheter. This is essential for the catheter to successfully enter the intra-cranial segment of the ICA via bypassing the pterygopalatine artery, which branches from the extra-cranial segment of the ICA dorsally and medially (Fig. 2a).
-
32
Retract the catheter 1–2 mm, and slowly inject the clot with 5–10 μl of saline at a rate of 10 μl/min.
Critical step: Slow injection is critical because this permits the clot to form a ball-like embolus large enough to obstruct the origin of the MCA. However, forced injection pushes the clot into a thread-like embolus that is unable to block the origin of the MCA (Fig. 1b), and will likely be washed away by blood flow when the catheter is withdrawn.
-
33
Retract the catheter 5 min after clot delivery until its tip reaches the ECA/ICA bifurcation. Re-apply an aneurysm clip to temporarily clamp the CCA and the ICA, and then withdraw the catheter from the arteriotomy. Tighten the 4-0 silk suture around the ECA trunk to ligate the arteriotomy. Remove the aneurysm clip.
-
34
Close the incision site and terminate the anesthesia. Then, place the rat in the recovery area for further observation. Generally, the rat is awake within 5 min.
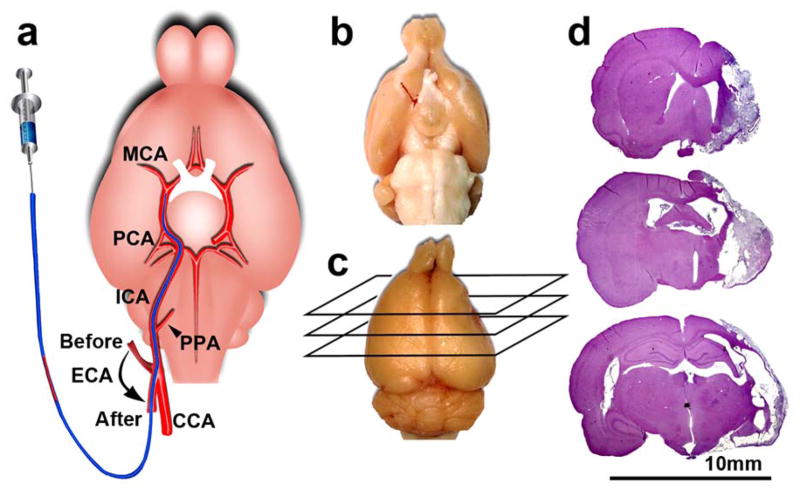
Figure 2. MCAO and ischemic lesion.
(a) A schematic representation of MCAO showing the locations of the ECA stump before and after insertion of a modified PE50 catheter. Note, the stump is positioned parallel to the CCA and aligns toward the ICA after insertion of the catheter rostrally via the arteriotomy; (b) A ventral view of a representative rat brain showing an embolus lodged at the origin of the MCA and intracranial segment of the ICA 2h after delivery of the clot; (c) Dorsal view of a representative rat brain with right hemisphere atrophy 4 weeks after MCAO; (d) H&E stained coronal sections from (c) show infarction within right MCA territory. All experimental procedures were approved by the IACUC of Henry Ford Hospital.
Timing
Steps 1–13. Femoral arterial catheterization and blood collection: 20–30 min
Step 14.Clot incubation time: 24h
Steps 15–18. Preparation of endovascular cathethers: 10–20 min
Steps 19–22. Preparation of embolus: 10–20 min
Steps 23–34. MCA occlusion: 20–30 min
Troubleshooting
See Table 2 for troubleshooting guidance.
Table 2.
Troubleshooting
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 16 | The tubing breaks before stretching | Tubing too close to the fire | Reduce the gas level to lower the heat, or adjust the distance between the tubing and flame. |
| 17 | The catheter OD is greater than 0.3mm | Tubing hardens before stretching | Stretch the tubing immediately while it is still flexible. |
| 21 | Clot breaks | Unevenly formed clot due to agitation | Plan a new experiment or use an extra set of clots collected from another donor rat. |
| 29 | The catheter is stuck at the ECA/ICA bifurcation | The tip of the catheter went into the trunk of occipital artery or CCA | Retract the catheter to the arteriotomy site, and place it rostrally towards the ICA. |
| 30 | The catheter is stuck at half way distance | The catheter went into the pterygopalatine artery | Retract the catheter to the ECA/ICA bifurcation site, align and advance it along the nature position of the ICA. |
| 32 | High resistance is felt during clot injection | Clot tangles and blocks the narrow part (tip) of the catheter | Retract the catheter, and replace it with a new clot. Do not perform a forced injection, which will likely break the single clot into multiple segments. |
Anticipated results
At 24 h after clot injection, a single embolus should be lodged at the intracranial segment of the ICA at the origin of the MCA and/or at the M1of the MCA without obstruction of the PCA (Fig. 2a–b). Histological analysis should show an ischemic lesion in the territory of the right hemisphere supplied by the MCA (Fig. 2c–d). The overall success rate of this model is over 90% (469 of 500 rats) based on the histological analysis of the ischemic lesions. The mortality rate within 7 d post stroke is less than 10% (7 of 85 rats). Sham-operated control rats do not show any ischemic lesions.
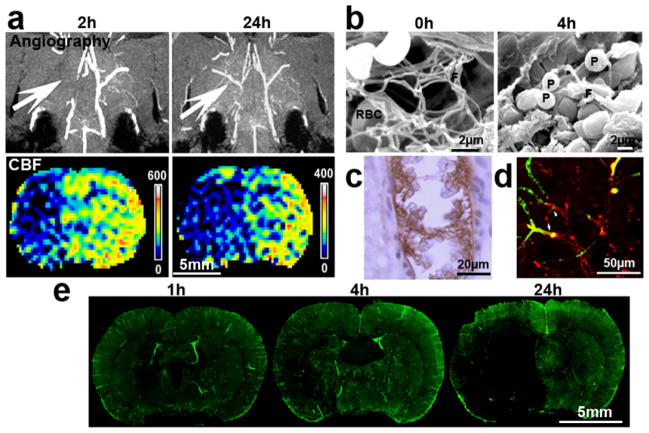
MRI angiogram and perfusion weighted imaging analysis should confirm occlusion of the right MCA immediately after delivery of the clot and substantial reduction of CBF within the territory supplied by the occluded MCA. Although the spontaneous recanalization of MCA occurs in some of the cases 24 hours after MCAO, the hypoperfusion within the MCA territory persists (Fig. 3a).
Figure 3. Angiogram, CBF, and thrombosis.
(a) MRI angiogram and perfusion weighted imaging show occlusion and recanalization of the right MCA 2 and 24h after MCAO, respectively, as well as their corresponding CBF at these two time points. Recanalization of the occluded MCA did not result in an increase of CBF in its downstream territory. The images were acquired from the same representative rat; (b) Scanning electron microscopy images show that a 24h old clot formed ex vivo contains fibrin (F) and a few red blood cells (RBC) prior to injection into the origin of the MCA (Modified from Zhang et al. 2001 Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain Research 912:181–194 with permission). However, when the clot was placed at the origin of the MCA for 4 h, a thrombus mainly containing cluster of platelets (P) and fragments of fibrins was formed; (c) Immunostaining of Fibrin/fibrinogen shows intravascular fibrin immunoreactivity with entrapped RBCs within downstream microvessels of the MCA in a extensively saline perfused rat brain at 1h after MCAO; (d) 3D confocal microscopic image revealed that fibrin immunoreactivity (red) within downstream microvessels blocked plasma perfusion as indicated by the reduced fluorescein isothiocyanate (FITC) dextran (green) signal 1h after MCAO; (e) 3D confocal microscopic images of coronal brain sections from rats subjected to FITC-dextran (green) injection show downstream microvessels blockage revealed by various degrees of filling defect in the MCA area at 1, 4, and 24h after MCAO. All experimental procedures were approved by the IACUC of Henry Ford Hospital.
Ultrastructure and immunohistochemistry analysis shows formation of secondary thrombosis at the occluded origin of the MCA as evident by accumulation of platelets, fibrin, and erythrocytes (Fig. 3b). Moreover, secondary thrombosis is developed in downstream microvessels of the occluded MCA during 24h, which likely contributes to the observed hypoperfusion by perfusion weighted imaging (Fig. 3a,c–d). The occurrence of secondary thrombosis following ischemic stroke is well described in stroke patients35–38.
Neurological deficits are readily detectable immediately after recovery from anesthesia. Rats typically showcontralateral forelimb flexion and circling behaviour, indicating contralateral hemiplegia. Further sophisticated sensorimotor evaluations, such as adhesive removal test, foot-fault test, and modified neurological severity score consistently show long-term neurological impairments in rats after embolic MCAO (Fig. 4.)24,39–43. However, neurological deficits are not detected in sham-operated control rats. Our model has been widely used in evaluating the efficacy of various thrombolytic strategies19,21,20,44,45. Intravenous administration of rtPA within 2h after stroke onset rapidly restores cerebral perfusion and reduces brain infarction. However, delayed treatment with rtPA at 4h leads to an increased risk of hemorrhagic transformation, and fails to restore blood flow and to reduce ischemic brain damage20,44,45. Our model bears a resemblance to the short therapeutic window and hemorrhagic complication observed in thrombolytic therapy in human stroke, thus, could be used for studying the post thrombolytic complications observed in some stroke patients. Indeed, this model has been widely used for the examination of multiple adjuvant pharmacological agents designed to enhance the efficacy and/or to reduce the adverse effects of thrombolytic therapy17,19,23,25,26,46–58.
Figure 4. Neurological outcome.
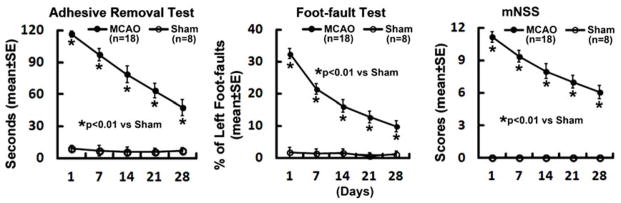
Line graphs show the sensorimotor functional deficits detected by adhesive removal test42, foot-fault test40, and modified neurological severity score391, 7, 14, 21, and 28 days after MCAO. Neurological deficits persist, although there is a spontaneous recovery of neurological function with the time. All experimental procedures were approved by the IACUC of Henry Ford Hospital.
In conclusion, the embolic stroke model presented here enables a controlled delivery of an allogeneic clot to the origin of the MCA, which results in reproducible ischemic brain damage. Our model mimics the pathological features of the common type of ischemic stroke in patients, which inevitably represents a valuable animal model for translation stroke research.
Acknowledgments
This work is supported by NIH grants R01NS079612 (ZGZ) and R01AG037506 (MC).
Footnotes
Competing Interests Statement
The authors declare that they have no competing financial interests.
Author Contributions Statement
RLZ, ZGZ, and MC conceptualized experiments. RLZ, LZ, and ZGZ performed the surgeries and histological analysis. QJ and GD performed MRI experiments and analyzed MRI data. LZ, ZGZ, and MC wrote the manuscripts.
References
- 1.Hacke W, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 4.Fonarow GC, et al. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 5.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke; a journal of cerebral circulation. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 6.Dalal PM, Shah PM, Sheth SC, Deshpande CK. Cerebral Embolism. Angiographic Observations on Spontaneous Clot Lysis. Lancet. 1965;1:61–64. doi: 10.1016/s0140-6736(65)91651-x. [DOI] [PubMed] [Google Scholar]
- 7.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke; a journal of cerebral circulation. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke; a journal of cerebral circulation. 1998;29:2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40:S111–114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuerstein GZ, et al. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 11.O’Collins VE, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- 12.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 13.Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. British journal of pharmacology. 2008;153(Suppl 1):S396–405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR. A rat model of focal embolic cerebral ischemia. Brain research. 1997;766:83–92. doi: 10.1016/s0006-8993(97)00580-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZG, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang ZG, et al. Dynamic platelet accumulation at the site of the occluded middle cerebral artery and in downstream microvessels is associated with loss of microvascular integrity after embolic middle cerebral artery occlusion. Brain research. 2001;912:181–194. doi: 10.1016/s0006-8993(01)02735-4. [DOI] [PubMed] [Google Scholar]
- 17.Ding G, et al. MRI of combination treatment of embolic stroke in rat with rtPA and atorvastatin. Journal of the neurological sciences. 2006;246:139–147. doi: 10.1016/j.jns.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RL, Zhang ZG, Chopp M, Zivin JA. Thrombolysis with tissue plasminogen activator alters adhesion molecule expression in the ischemic rat brain. Stroke; a journal of cerebral circulation. 1999;30:624–629. doi: 10.1161/01.str.30.3.624. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke; a journal of cerebral circulation. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- 20.Zhang RL, Chopp M, Zhang ZG, Divine G. Early (1 h) administration of tissue plasminogen activator reduces infarct volume without increasing hemorrhagic transformation after focal cerebral embolization in rats. Journal of the neurological sciences. 1998;160:1–8. doi: 10.1016/s0022-510x(98)00155-5. [DOI] [PubMed] [Google Scholar]
- 21.Marder VJ, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke; a journal of cerebral circulation. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 22.Singh P, Doostkam S, Reinhard M, Ivanovas V, Taschner CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: does stent-retriever cause intimal damage? Stroke; a journal of cerebral circulation. 2013;44:1720–1722. doi: 10.1161/STROKEAHA.113.000964. [DOI] [PubMed] [Google Scholar]
- 23.Cheng T, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nature medicine. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, et al. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke; a journal of cerebral circulation. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, et al. Adjuvant treatment with a glycoprotein IIb/IIIa receptor inhibitor increases the therapeutic window for low-dose tissue plasminogen activator administration in a rat model of embolic stroke. Circulation. 2003;107:2837–2843. doi: 10.1161/01.CIR.0000068374.57764.EB. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, et al. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelm-Schwenkmezger T, et al. Therapeutic application of 20-kHz transcranial ultrasound in an embolic middle cerebral artery occlusion model in rats: safety concerns. Stroke; a journal of cerebral circulation. 2007;38:1031–1035. doi: 10.1161/01.STR.0000257966.32242.0b. [DOI] [PubMed] [Google Scholar]
- 28.Hobohm C, et al. Long-lasting neuronal loss following experimental focal cerebral ischemia is not affected by combined administration of tissue plasminogen activator and hyperbaric oxygen. Brain research. 2011;1417:115–126. doi: 10.1016/j.brainres.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Guluma KZ, Lapchak PA. Comparison of the post-embolization effects of tissue-plasminogen activator and simvastatin on neurological outcome in a clinically relevant rat model of acute ischemic stroke. Brain research. 2010;1354:206–216. doi: 10.1016/j.brainres.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka Y, Marumo T, Omura T, Yoshida S. Serum S100B indicates successful combination treatment with recombinant tissue plasminogen activator and MK-801 in a rat model of embolic stroke. Brain research. 2007;1154:194–199. doi: 10.1016/j.brainres.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZG, et al. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke; a journal of cerebral circulation. 2005;36:2701–2704. doi: 10.1161/01.STR.0000190007.18897.e3. [DOI] [PubMed] [Google Scholar]
- 32.Undas A, Ariens RA. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:e88–99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 33.Wohner N, et al. Lytic resistance of fibrin containing red blood cells. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2306–2313. doi: 10.1161/ATVBAHA.111.229088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17:1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Del Sette M, Angeli S, Stara I, Finocchi C, Gandolfo C. Microembolic signals with serial transcranial Doppler monitoring in acute focal ischemic deficit. A local phenomenon? Stroke; a journal of cerebral circulation. 1997;28:1311–1313. doi: 10.1161/01.str.28.7.1311. [DOI] [PubMed] [Google Scholar]
- 36.Idicula TT, Naess H, Thomassen L. Microemboli-monitoring during the acute phase of ischemic stroke: is it worth the time? BMC neurology. 2010;10:79. doi: 10.1186/1471-2377-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koennecke HC, et al. Frequency and determinants of microembolic signals on transcranial Doppler in unselected patients with acute carotid territory ischemia. A prospective study. Cerebrovascular diseases. 1998;8:107–112. doi: 10.1159/000015827. [DOI] [PubMed] [Google Scholar]
- 38.Poppert H, Sadikovic S, Sander K, Wolf O, Sander D. Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke; a journal of cerebral circulation. 2006;37:2039–2043. doi: 10.1161/01.STR.0000231644.47325.aa. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke; a journal of cerebral circulation. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Experimental neurology. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 41.Bouet V, et al. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nature protocols. 2009;4:1560–1564. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 42.Schallert T, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacology, biochemistry, and behavior. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, et al. Tadalafil, a long-acting type 5 phosphodiesterase isoenzyme inhibitor, improves neurological functional recovery in a rat model of embolic stroke. Brain research. 2006;1118:192–198. doi: 10.1016/j.brainres.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Q, et al. Magnetic resonance imaging indexes of therapeutic efficacy of recombinant tissue plasminogen activator treatment of rat at 1 and 4 hours after embolic stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:21–27. doi: 10.1097/00004647-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Jiang Q, et al. Diffusion-, T2-, and perfusion-weighted nuclear magnetic resonance imaging of middle cerebral artery embolic stroke and recombinant tissue plasminogen activator intervention in the rat. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:758–767. doi: 10.1097/00004647-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, et al. Combination treatment with N-acetyl-seryl-aspartyl-lysyl-proline and tissue plasminogen activator provides potent neuroprotection in rats after stroke. Stroke. 2014;45:1108–1114. doi: 10.1161/STROKEAHA.113.004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding G, et al. MRI evaluation of BBB disruption after adjuvant AcSDKP treatment of stroke with tPA in rat. Neuroscience. 2014;271:1–8. doi: 10.1016/j.neuroscience.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012;33:415–422. doi: 10.1016/j.tips.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, et al. Combination treatment with VELCADE and low-dose tissue plasminogen activator provides potent neuroprotection in aged rats after embolic focal ischemia. Stroke. 2010;41:1001–1007. doi: 10.1161/STROKEAHA.109.577288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, et al. Treatment of embolic stroke in rats with bortezomib and recombinant human tissue plasminogen activator. Thromb Haemost. 2006;95:166–173. [PubMed] [Google Scholar]
- 52.Zhang L, et al. Multitargeted effects of statin-enhanced thrombolytic therapy for stroke with recombinant human tissue-type plasminogen activator in the rat. Circulation. 2005;112:3486–3494. doi: 10.1161/CIRCULATIONAHA.104.516757. [DOI] [PubMed] [Google Scholar]
- 53.Ding G, et al. Analysis of combined treatment of embolic stroke in rat with r-tPA and a GPIIb/IIIa inhibitor. J Cereb Blood Flow Metab. 2005;25:87–97. doi: 10.1038/sj.jcbfm.9600010. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke. 2004;35:2890–2895. doi: 10.1161/01.STR.0000147963.68238.da. [DOI] [PubMed] [Google Scholar]
- 55.Jiang Q, et al. MRI evaluation of treatment of embolic stroke in rat with intra-arterial and intravenous rt-PA. J Neurol Sci. 2004;224:57–67. doi: 10.1016/j.jns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Ding G, et al. Multiparametric ISODATA analysis of embolic stroke and rt-PA intervention in rat. J Neurol Sci. 2004;223:135–143. doi: 10.1016/j.jns.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Morris DC, et al. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- 58.Zhang RL, et al. Postischemic intracarotid treatment with TNK-tPA reduces infarct volume and improves neurological deficits in embolic stroke in the unanesthetized rat. Brain Res. 2000;878:64–71. doi: 10.1016/s0006-8993(00)02693-7. [DOI] [PubMed] [Google Scholar]
- 59.Nakayama H, Dietrich WD, Watson BD, Busto R, Ginsberg MD. Photothrombotic occlusion of rat middle cerebral artery: histopathological and hemodynamic sequelae of acute recanalization. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8:357–366. doi: 10.1038/jcbfm.1988.71. [DOI] [PubMed] [Google Scholar]
- 60.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Annals of neurology. 1985;17:497–504. doi: 10.1002/ana.410170513. [DOI] [PubMed] [Google Scholar]
- 61.Zivin JA, et al. A model for quantitative evaluation of embolic stroke therapy. Brain research. 1987;435:305–309. doi: 10.1016/0006-8993(87)91613-1. [DOI] [PubMed] [Google Scholar]
- 62.Gerriets T, et al. The macrosphere model: evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. Journal of neuroscience methods. 2003;122:201–211. doi: 10.1016/s0165-0270(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 63.Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke; a journal of cerebral circulation. 1982;13:505–508. doi: 10.1161/01.str.13.4.505. [DOI] [PubMed] [Google Scholar]
- 64.Overgaard K, et al. A rat model of reproducible cerebral infarction using thrombotic blood clot emboli. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12:484–490. doi: 10.1038/jcbfm.1992.66. [DOI] [PubMed] [Google Scholar]
- 65.Benes V, Zabramski JM, Boston M, Puca A, Spetzler RF. Effect of intra-arterial tissue plasminogen activator and urokinase on autologous arterial emboli in the cerebral circulation of rabbits [corrected] Stroke; a journal of cerebral circulation. 1990;21:1594–1599. doi: 10.1161/01.str.21.11.1594. [DOI] [PubMed] [Google Scholar]