Abstract
In patients with venous thromboembolism (VTE), the outcome during the course of anticoagulant therapy may differ according to the patient’s sex. We used the RIETE (Registro Informatizado Enfermedad TromboEmbólica) database to compare the rate of VTE recurrences, major bleeding, and mortality due to these events according to sex.
As of August 2013, 47,499 patients were enrolled in RIETE, of whom 24,280 (51%) were women. Women were older, more likely presented with pulmonary embolism (PE), and were more likely to have recent immobilization but less likely to have cancer than men. During the course of anticoagulation (mean duration: 253 d), 659 patients developed recurrent deep vein thrombosis (DVT), 576 recurrent PE, 1368 bled, and 4506 died. Compared with men, women had a lower rate of DVT recurrences (hazard ratio [HR]: 0.78; 95% confidence interval [CI]: 0.67–0.91), a similar rate of PE recurrences (HR: 0.98; 95% CI: 0.83–1.15), a higher rate of major bleeding (HR: 1.21; 95% CI: 1.09–1.35), and higher mortality due to PE (HR: 1.24; 95% CI: 1.04–1.47). On multivariable analysis, any influence of sex on the risk for recurrent DVT (HR: 0.88; 95% CI: 0.75–1.03), major bleeding (HR: 1.10; 95% CI: 0.98–1.24), or fatal PE (HR: 1.01; 95% CI: 0.84–1.22) was no longer statistically significant.
In conclusion, women had fewer DVT recurrences and more bleeds than men during the course of anticoagulation. These differences were not due to sex, but very likely to other patient characteristics more common in female patients and differences in treatment choice.
INTRODUCTION
Venous thromboembolism (VTE) is a commonly diagnosed condition with significant morbidity and mortality. Current guidelines of anticoagulant therapy recommend patients with VTE to be initially treated with low-molecular-weight heparin (LMWH), fondaparinux or unfractionated heparin, followed by long-term anticoagulation, which is usually accomplished with vitamin K antagonists (VKA).13 The need for long-term therapy of VTE and the preferred intensity of anticoagulation have been established by a number of randomized clinical trials,2,3,11,14,17,18,23,25,28 but the ideal duration of therapy is not well defined. Most clinical trials were underpowered to assess fatal VTE or fatal bleeding events, and recommendations are mainly based on the rates of recurrent VTE, major bleeding and all-cause mortality.
After discontinuing anticoagulation, a number of studies reported a lower rate of VTE recurrences in women than in men.4,5,16,19,31,33 However, there is scarce evidence on the existence of sex differences during anticoagulation, probably because the rate of recurrences during therapy is rather low. Kuijer et al found women with VTE to be at increased risk for major bleeding during the course of therapy,15 and recent clinical trials comparing the efficacy and safety of the new anticoagulants with standard anticoagulation also revealed an increased rate of major bleeding events in women.1,6,7,9 The lower rate of recurrences and the higher rate of bleeding in women might lead to suggest that a less aggressive (or a shorter duration of) anticoagulant strategy would reduce bleeding more than it would increase recurrent VTE.
The RIETE (Registro Informatizado de Enfermedad TromboEmbólica) Registry is an ongoing, multicenter, international (Spain, Italy, France, Israel, Portugal, Germany, Switzerland, Czech Republic, Macedonia, United States, Canada, Brazil, and Ecuador), observational registry of consecutive patients with symptomatic, objectively confirmed, acute VTE. It started in Spain in 2001, and 6 years later the database was translated into English, with the aim to expand the Registry to other countries, ultimately allowing physicians worldwide to use the database to select the most appropriate therapy for their patients. Data from this registry have been used to evaluate outcomes after acute VTE, such as the frequency of recurrent VTE, major bleeding and mortality, and risk factors for these outcomes.21,22,26,27,32 The aim of the current study was to compare the rate of VTE recurrences, major bleeding events, and their impact on mortality during the course of anticoagulation according to sex.
PATIENTS AND METHODS
Consecutive patients with symptomatic, acute deep vein thrombosis (DVT) or pulmonary embolism (PE), confirmed by objective tests (contrast venography or ultrasonography for suspected DVT; pulmonary angiography, lung scintigraphy, or helical computed tomography scan for suspected PE), were enrolled in RIETE. Patients were excluded if they were currently participating in a therapeutic clinical trial with a blinded therapy. All patients (or their relatives) provided written or oral consent to their participation in the registry, in accordance with local Ethics Committee requirements. This analysis was approved by the Ethics Committee of the Hospital Universitari Germans Trias i Pujol (Badalona, Spain) and by the Institutional Review Board (IRB) of NorthShore University HealthSystem (Evanston, IL).
In the RIETE registry, participating physicians ensured that eligible patients were consecutively enrolled. Data were recorded using a computer-based case report form at each participating hospital and submitted to a centralized coordinating center through a secure website. The study coordinating center assigned patients a unique identification number to maintain patient confidentiality and was responsible for all data management. Data quality was regularly monitored electronically, including checks to detect inconsistencies or errors, which were resolved by the local coordinators. Data quality was also monitored by periodic visits to participating hospitals by contract research organizations that compared medical records with the submitted data.
Study Design and Definitions
The major outcome was the development of VTE recurrences, major bleeding events or death during the course of anticoagulation. Secondary outcomes were the rate of fatal PE and fatal bleeding. Major bleeding was defined as an overt bleed that required a transfusion of 2 or more units of blood, was retroperitoneal, spinal or intracranial, or was fatal. Fatal PE, in the absence of autopsy, was defined as any death occurring within 10 days after PE diagnosis, in the absence of any alternative cause of death. Fatal bleeding was defined as any death occurring within 10 days of a major bleeding episode, in the absence of an alternative cause of death.
Study Variables
The following parameters were recorded: patient’s baseline characteristics, risk factors for VTE, clinical status including any coexisting or underlying conditions, diagnostic tests, the type and dose of treatment received upon VTE diagnosis and the outcome during at least the first 3 months. Immobilized patients were defined in this analysis as nonsurgical patients who had been immobilized (total bed rest with bathroom privileges) for ≥4 days in the 2-month period prior to VTE diagnosis. Surgical patients were defined as those who had undergone an operation in the 2 months prior to VTE diagnosis. Active cancer was defined as newly diagnosed cancer, metastatic cancer, or cancer that was being treated (with surgery, chemotherapy, radiotherapy, support therapy, or combined treatments). Patients with recent surgery, recent immobilization ≥4 days, estrogen use, pregnancy, puerperium or recent travel >6 hours were considered to have transient risk factors for VTE. Those with no cancer nor transient risk factors were considered to have unprovoked VTE.
Treatment and Follow-Up
Patients were managed according to the clinical practice of each participating hospital (there was no standardization of treatment). The type, dose and duration of anticoagulant therapy, as was the insertion of an inferior vena cava filter, were recorded. After discharge, all patients were followed-up for in the outpatient clinic. During each visit, any signs or symptoms suggesting VTE recurrences or bleeding complications were noted. Each episode of clinically suspected recurrent DVT or PE was investigated by repeat ultrasonography, venography, lung scanning, helical-CT scan or pulmonary angiography as appropriate. Most outcomes were classified as reported by the clinical centers. However, if staff at the coordinating center were uncertain how to classify a reported outcome, that event was reviewed by a central adjudicating committee (less than 10% of events). Patients who had recurrent VTE or major bleeding events within 3 months of enrolment remained under surveillance until 3 months of follow-up was completed.
Statistical Analysis
ANOVA analysis and nonparametric tests were used to compare means and medians of continuous variables. Categorical variables were compared using the chi-square test (2-sided) and Fisher exact test (2-sided). The significance of a number of variables on the risk for VTE recurrences, major bleeding or death was tested by a Chi-Square test for categorical variables. Candidate variables were selected from clinical variables based on published literature and on expert opinion. In order to measure predictors of outcome and the influence of sex, a multivariate analysis was carried out using a Cox proportional hazard regression analysis, and the corresponding Kaplan Meier survival curves were constructed. Variables entering in the multivariate analyses were: age, sex, body weight, chronic lung disease, chronic heart failure, abnormal creatinine levels, recent major bleeding, risk factors fro VTE, prior VTE, and initial therapy with LMWH (vs. UFH). Incidence rates of recurrent VTE, major bleeding and death were calculated as cumulative incidence (events/100 patient-years). Cutoffs for age and body weight were chosen arbitrarily. We compared the cumulative incidence of these events according to sex using the Kaplan-Meier technique. A SPSS software (version 15, SPSS Inc., Chicago, Illinois) was used for the statistical management of the data, and a 2-sided p <0.05 was considered to be statistically significant.
RESULTS
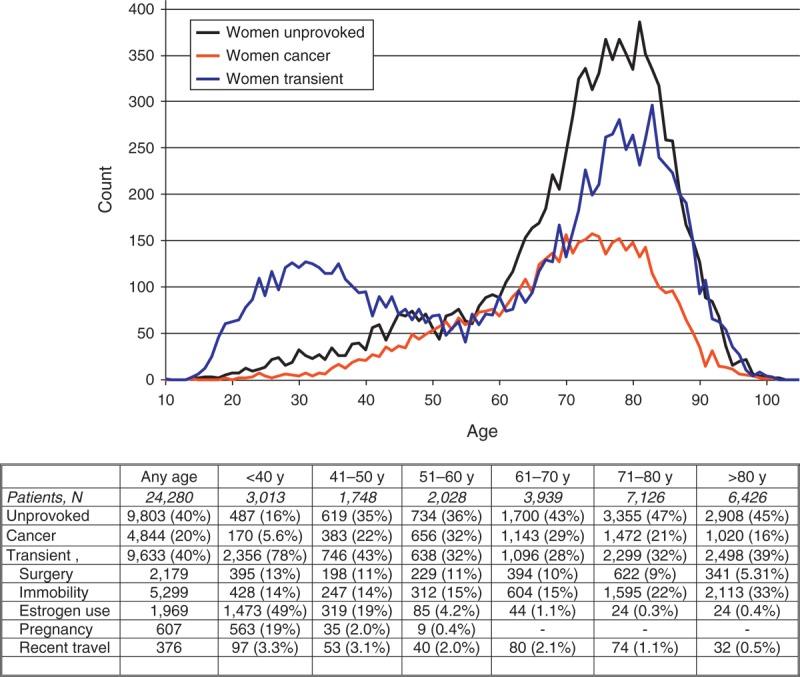
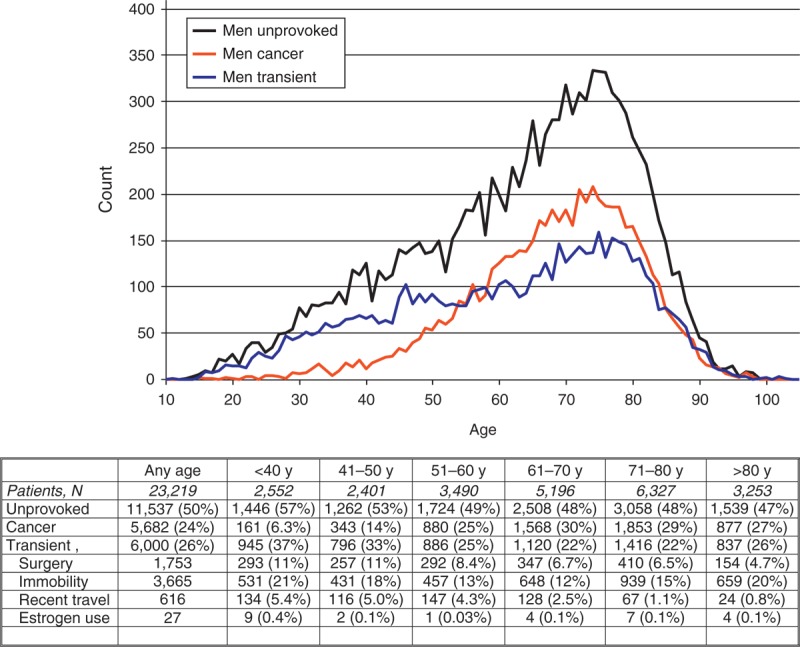
As of August 2013, 47,499 patients were enrolled in RIETE, of whom 24,280 (51%) were women. Compared with men, women were older, more likely presented with PE (53% vs. 47%), were more likely to have chronic heart failure and less likely to have chronic lung disease or renal insufficiency (Table 1). Moreover, women were more likely to have recent immobilization and less likely to have cancer, unprovoked VTE or prior VTE. Most (78%) women aged <40 years and 1 in every 3 (39%) women aged >80 years had transient risk factors for VTE. This was mostly due to the use of hormonal therapy (n = 1491) or pregnancy (n = 566) under the age of 40, and to the presence of immobility (n = 2382) in those aged >80 years (Figure 1). On the other hand, 1 in every 2 men (50%) had unprovoked VTE, irrespective of age (Figure 2). Among patients initially presenting with PE, women were more likely to have systolic blood pressure levels <100 mm Hg, hypoxemia, atrial fibrillation or echocardiographic abnormalities suggesting right heart overload than men (see Table 1).
TABLE 1.
Clinical Characteristics and Diagnostic Tests in 47,499 Patients Presenting With Acute VTE, According to Sex and Initial VTE Presentation
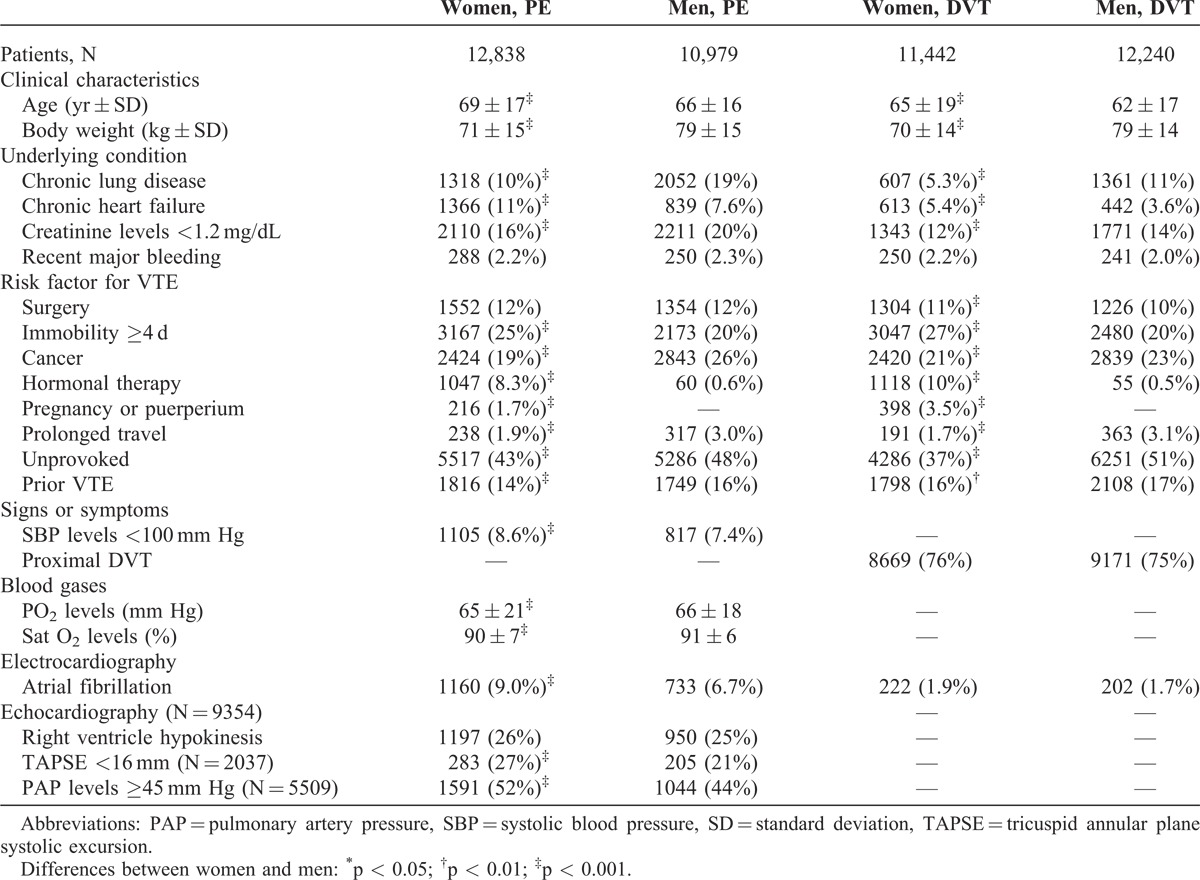
FIGURE 1.
Prevalence of VTE in women for different age groups (horizontal axis is age in years).
FIGURE 2.
Prevalence of VTE in men for different age groups (horizontal axis is age in years).
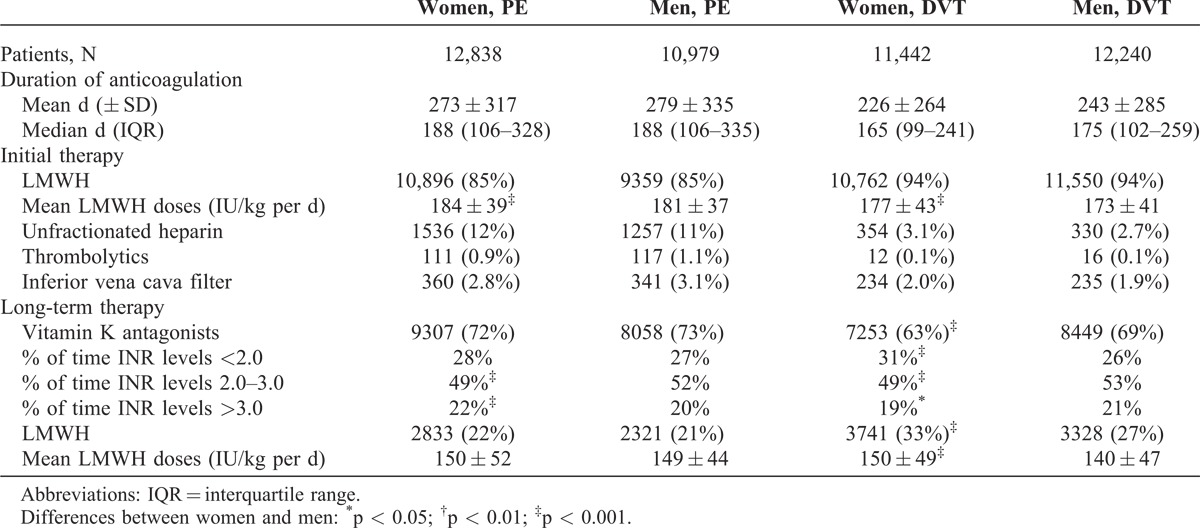
There were no sex differences in the proportion of patients initially treated with LMWH or unfractionated heparin, but women with DVT were more likely to receive long-term therapy also with LMWH (instead of VKA) (Table 2). Interestingly, women received slightly higher LMWH doses per body weight (for initial- and for long-term therapy), and among those receiving VKA there were slight sex differences in terms of proportion of time with INR levels <2.0, 2.0 to 3.0, or >3.0.
TABLE 2.
Therapeutic Strategies, According to Sex and Initial VTE Presentation
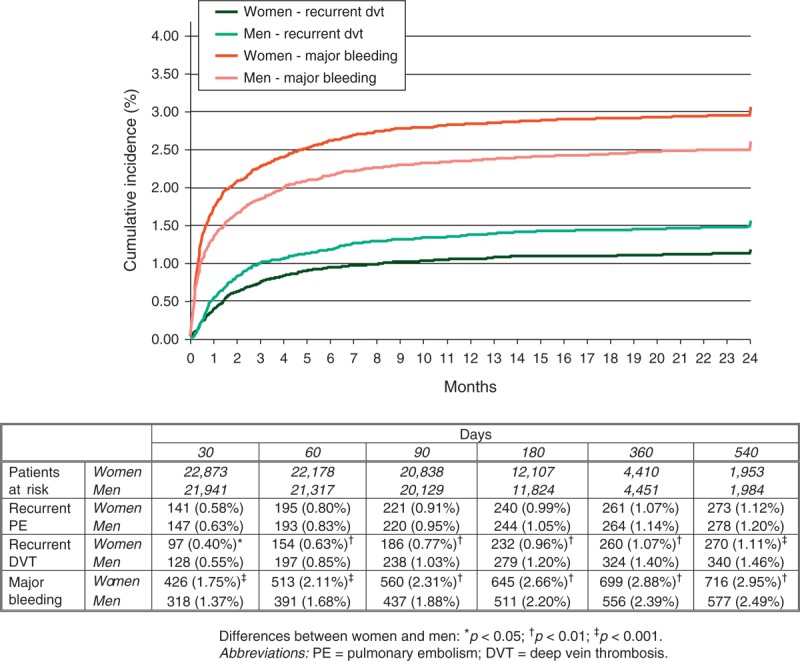
During the course of anticoagulation (mean duration: 253 days; median: 176 days), 659 patients developed recurrent DVT, 576 had recurrent PE (97 were fatal), 1368 major bleeding (236 were fatal) and 4506 died (Table 3). Compared with men, women had a lower rate of DVT recurrences (hazard ratio [HR]: 0.78; 95% confidence interval [CI]: 0.67–0.91), a similar rate of PE recurrences (HR: 0.98; 95% CI: 0.83–1.15) and a higher rate of major bleeding events (HR: 1.21; 95% CI: 1.09–1.35). The lower rate of DVT recurrences and the higher rate of major bleeding in women started early in the course of therapy (Figure 3). There were no sex differences in the rate of gastrointestinal, intracranial or retroperitoneal bleeding, but women had a higher rate of hematoma and a lower rate of urinary bleeding compared with men (see Table 3). The lower rate of DVT recurrences in women was limited to patients aged <40 years (1.4% vs. 2.7%), and the different rates of urinary bleeding or hematoma mostly appeared in patients aged over 60 years. The all cause mortality rate was similar (HR: 1.03; 95% CI: 0.97–1.09), but women had a higher rate of fatal PE (HR: 1.24; 95% CI: 1.04–1.47) and a similar rate of fatal bleeding (HR: 1.18; 95% CI: 0.91–1.52) (Figure 4).
TABLE 3.
Clinical Outcome During the Course of Anticoagulation, According to Sex
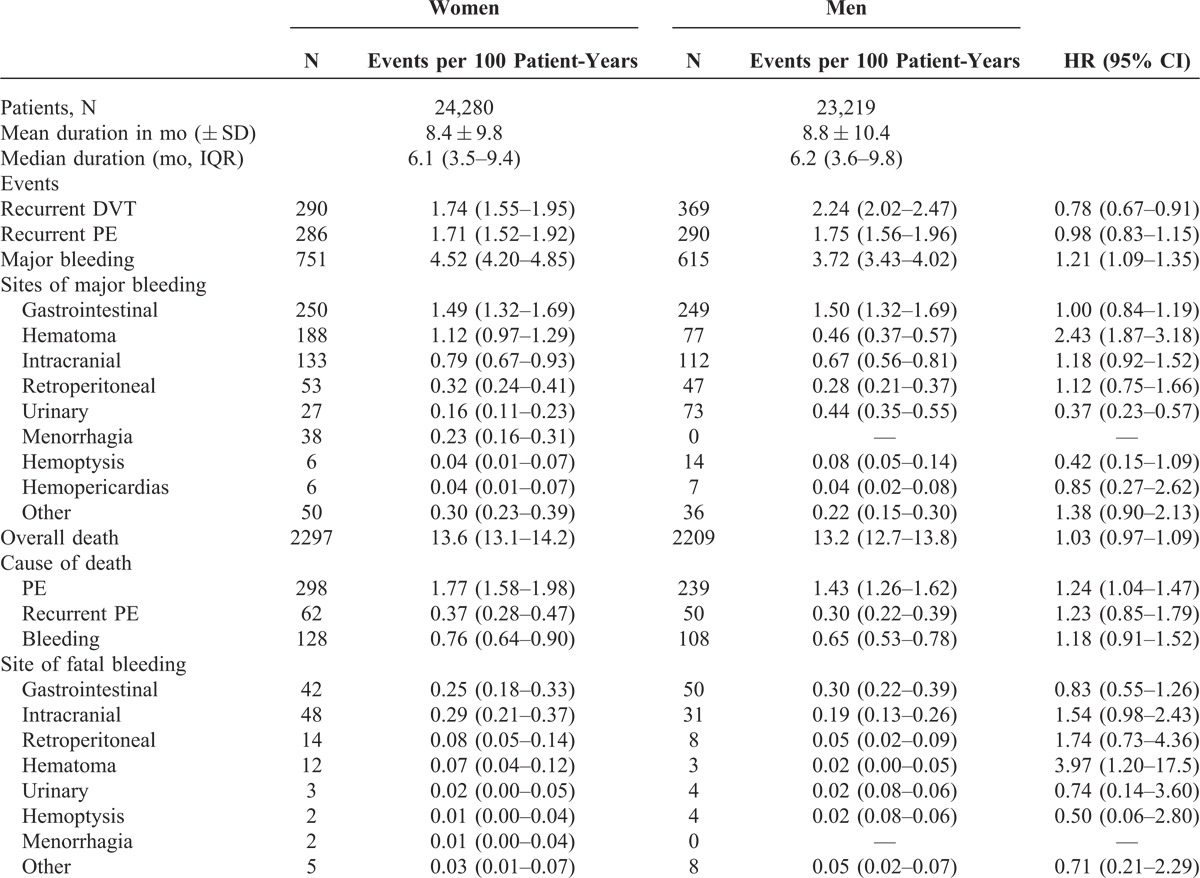
FIGURE 3.
Cumulative rates of major bleeding and recurrent DVT during the course of anticoagulation, according to sex.
FIGURE 4.
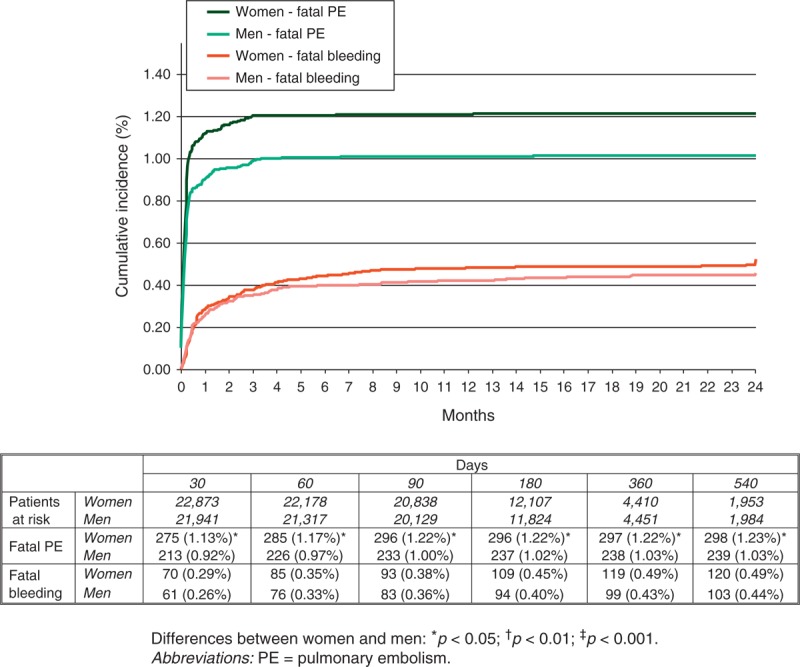
Cumulative rates of fatal PE and fatal bleeding during the course of anticoagulation, according to sex.
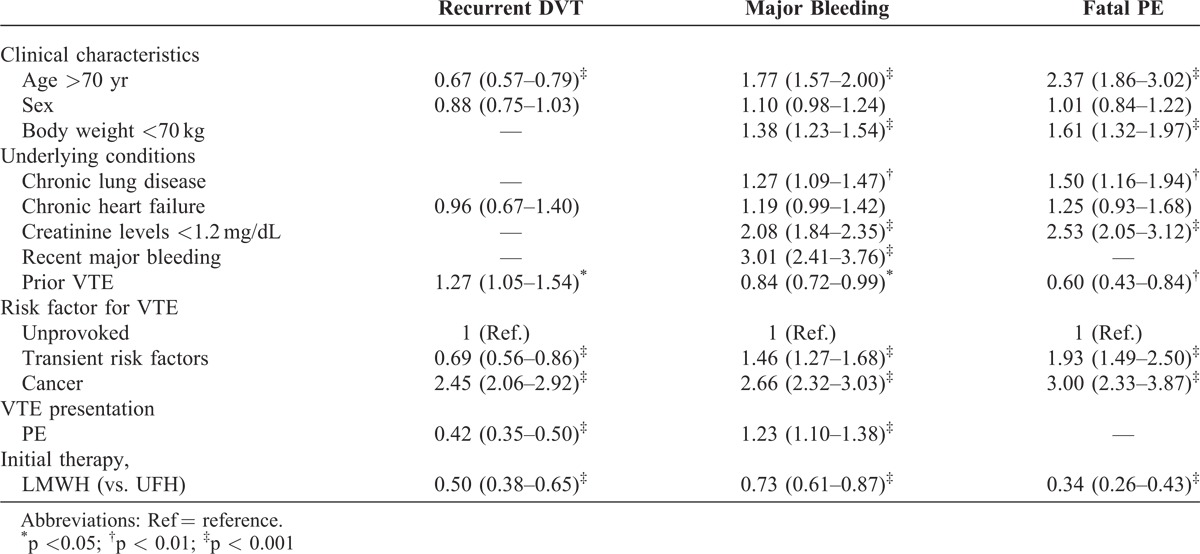
On multivariable analysis, any sex difference in the risk for recurrent DVT (HR: 0.88; 95% CI: 0.75–1.03), major bleeding (HR: 1.10; 95% CI: 0.98–1.24) or fatal PE (HR: 1.01; 95% CI: 0.84–1.22) was no longer statistically significant (Table 4).
TABLE 4.
Multivariate Analysis for Recurrent DVT, Major Bleeding and Fatal PE During Anticoagulation (Data Expressed as HR and 95% CI)
DISCUSSION
In patients with VTE, the duration of anticoagulation should be tailored considering the risk of VTE recurrences and the risk of bleeding. Our data, obtained from a large series of consecutive patients with acute VTE, reveal a number of sex differences in clinical presentation, underlying diseases, treatment and outcome during the course of anticoagulation. Women had a significantly lower rate of DVT recurrences and a higher rate of major bleeding events, but these differences had no impact on mortality. Moreover, any influence of sex on outcome disappeared on multivariable analysis. These data suggest that the duration of anticoagulation should not be decided based on sex alone.
A number of studies previously reported a lower rate of VTE recurrences in women, bust most were focused on the recurrence rate after discontinuing anticoagulant therapy.4,5,16,19,31,33 The major new finding in this analysis is that differences exist also during the course of anticoagulant therapy, but any difference applies only to the rate of DVT recurrences. Since the lower rate of DVT recurrences appeared mostly in women aged <40 years, and half of them were using hormonal therapy, we hypothesize that most women discontinued hormonal therapy after VTE diagnosis, and this might explain their lower recurrence rate. Additionally, the lower rate of DVT recurrences in women might also be explained because their older age (on multivariable analysis, VTE recurrences were less frequent in the elderly), the lower proportion of women with cancer or the higher doses of LMWH used for therapy. However, we failed to find any explanation for the similar rate of PE recurrences.
The current study also confirms an increased rate of major bleeding events in women (although this finding was not confirmed in multivariable analysis). This higher rate has previously been reported in patients receiving anticoagulation for atrial fibrillation.8,10,12,20,24,29,30 In patients with VTE there was less evidence on this association,15 but data in the recent randomized clinical trials comparing the new anticoagulants with standard therapy also revealed an increased rate of bleeding in women, irrespective of the drug used.1,6,7,9 The major new finding in our analysis is that the higher rate of bleeding was limited to the presence of hematoma (particularly frequent in the elderly). There was no difference in the rate of gastrointestinal (the most common site of bleeding in both sexes), intracranial or retroperitoneal bleeding. Since the mortality associated with these hematomas was rather low, the rate of fatal bleeding was similar in both subgroups. In our series, the higher rate of bleeding might be attributed to their older age, lower body weight or the higher LMWH doses used in women.
When weighing the risks and benefits of anticoagulation in an individual patient, in addition to considering the absolute risk of thrombosis and major bleeding, the consequences associated with each of these outcomes need to be considered. From a theoretical point of view, the lower rate of DVT recurrences and the higher rate of major bleeding in women might suggest the need for a different therapeutic approach (that is, a shorter duration of anticoagulant therapy in women). However, the similar rate of fatal bleeding and PE recurrences (a condition associated with a higher mortality than recurrent DVT) suggests that any variation in therapy may be dangerous.
Finally, women had a higher rate of fatal PE. At baseline, women with PE were more likely to present with systolic blood pressure levels <100 mm Hg, hypoxemia, atrial fibrillation or echocardiographic abnormalities suggesting right heart overload. Again, multivariable analysis failed to find sex as an independent predictor for fatal PE. Thus, the higher mortality in women might be explained because they were older and more likely to be immobilized. The influence of immobilization on mortality in patients with acute PE has been previously reported.21 Thus, our findings may help to implement the use of VTE prophylaxis in immobilized women.
The RIETE registry provides insights into the natural history of VTE with an unselected patient population, in contrast to the rigorously controlled conditions of randomized clinical studies. It can, therefore, help to identify factors associated with better or worse outcomes, and provide feedback from real-world clinical situations, which may be valuable for those designing new randomized clinical studies. Despite our efforts to control any bias from underlying diseases, it is likely that we were unable to eliminate such bias completely. Thus, the lower rate of recurrent DVT and the higher rate of major bleeding among women may reflect pre-existing, unrecognized disease processes not considered in the RIETE database.
In summary, women had fewer DVT recurrences and more major bleeding events during the course of anticoagulation. However, our data suggest that these differences were not due to sex, but very likely were due to other patient characteristics more common in female patients and differences in treatment choice.
APPENDIX
Coordinator of the RIETE Registry: Manuel Monreal (Spain). RIETE Steering Committee Members: Hervè Decousus (France), Paolo Prandoni (Italy), Benjamin Brenner (Israel). RIETE National Coordinators: Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Sebastian Schellong (Germany), Manolis Papadakis (Greece), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R.Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic). RIETE Registry Coordinating Center: S & H Medical Science Service.
Members of the RIETE Group: Spain: Arcelus JI, Ballaz A, Barba R, Barrón M, Barrón-Andrés B, Bascuñana J, Bedate P, Blanco-Molina A, Bueso T, Casado I, Conget F, del Molino F, del Toro J, Falgá C, Fernández-Capitán C, Fuentes MI, Gallego P, Garcia-Bragado F, Gómez V, González J, González-Bachs E, Grau E, Guil M, Guijarro R, Gutiérrez J, Hernández L, Hernández-Huerta S, Hernando-López E, Jaras MJ, Jiménez D, Lobo JL, López-Jiménez L, López-Reyes R, López-Sáez JB, Lorente MA, Lorenzo A, Luque JM, Madridano O, Maestre A, Marchena PJ, Martín M, Monreal M, Muñoz FJ, Nauffal D, Nieto JA, Núñez MJ, Ogea JL, Pedrajas JM, Peris ML, Porras JA, Raissouni N, Riera-Mestre A, Rivas A, Rodríguez-Dávila MA, Román P, Rosa V, Ruiz-Giménez N, Sahuquillo JC, Samperiz A, Sánchez Muñoz-Torrero JF, Soler S, Suriñach JM, Tiberio G, Tirado R, Tolosa C, Trujillo-Santos J, Uresandi F, Valdés M, Valero B, Valle R, Vela J, Vidal G, Villalobos A, Villalta J, Brazil: Gadelha T, Czech Republic: Malý R, Hirmerova J, Kaletova M, Tomko T, France: Bertoletti L, Bura-Riviere A, Farge-Bancel D, Grange C, Hij A, Mahe I, Merah A, Quere I, Germany: Schellong S, Greece: Babalis D, Papadakis M, Tzinieris I, Israel: Braester A, Brenner B, Tzoran I, Zeltser D, Italy: Bacciottini N, Barillari G, Bucherini E, Cattabiani C, Ciammaichella M, Dalla Valle F, Di Micco P, Duce R, Giorgi-Pierfranceschi M, Gussoni G, Maida R, Pasca S, Piovella C, Poggio R, Prandoni P, Quintavalla R, Rocci A, Rota L, Schenone A, Tiraferri E, Tonello D, Tufano A, Visonà A, Zalunardo B, Portugal: Bastos MS, Brinquinho M, Goņcalves F, Santos M, Sousa MS, Republic of Macedonia: Bosevski M, Kovacevic D, Switzerland: Alatri A, Aujeski D, Bounameaux H, Calanca L, Mazzolai L.
ACKNOWLEDGMENTS
The authors thank Sanofi Spain and Bayer Pharma AG for supporting the Registry. The authors thank the RIETE Registry Coordinating Center, S & H Medical Science Service, for quality control, logistic, and administrative support, and Prof. Salvador Ortiz, Universidad Autónoma de Madrid and Statistical advisor, S & H Medical Science Service, for statistical analysis of the data.
Footnotes
Abbreviations: CI = confidence intervals, DVT = deep vein thrombosis, HR = hazard ratio, LMWH = low-molecular-weight heparin, PE = pulmonary embolism, VKA = vitamin K antagonists, VTE = venous thromboembolism.
∗A full list of RIETE investigators is given in the appendix.
Financial support and conflicts of interest: Sanofi Spain supported the Registry with an unrestricted educational grant, and Bayer Pharma AG supported the part of RIETE Registry outside Spain, which accounts for 19.1% of the total patients included in the RIETE Registry.
Contributor Information
Collaborators: RIETE Investigators∗
References
- 1.Agnelli G, Buller HR, Cohen A, et al. For the AMPLIFY Investigators. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139:19–25. [DOI] [PubMed] [Google Scholar]
- 3.Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal Duration Italian Trial Investigators. N Engl J Med. 2001;345:165–169. [DOI] [PubMed] [Google Scholar]
- 4.Baglin T, Luddington R, Brown K, et al. High risk of recurrent venous thromboembolism in men. J Thromb Haemost. 2004;2:2152–2155. [DOI] [PubMed] [Google Scholar]
- 5.Christiansen SC, Lijfering WM, Helmerhorst FM, et al. Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. J Thromb Haemost. 2010;8:2159–2168. [DOI] [PubMed] [Google Scholar]
- 6.EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. [DOI] [PubMed] [Google Scholar]
- 7.EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. [DOI] [PubMed] [Google Scholar]
- 8.Gomberg-Maitland M, Wenger NK, Feyzi J, et al. Anticoagulation in women with non-valvular atrial fibrillation in the stroke prevention using an oral thrombin inhibitor (SPORTIF) trials. Eur Heart J. 2006;27:1947–1953. [DOI] [PubMed] [Google Scholar]
- 9.Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. [DOI] [PubMed] [Google Scholar]
- 10.Hughes M, Lip GY. Guideline Development Group for the NICE national clinical guideline for management of atrial fibrillation in primary and secondary care. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. Q J Med. 2007;100:599–607. [DOI] [PubMed] [Google Scholar]
- 11.Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855–858. [DOI] [PubMed] [Google Scholar]
- 12.Humphries KH, Kerr CR, Connolly SJ, et al. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. [DOI] [PubMed] [Google Scholar]
- 13.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy and prevention of thrombosis (9th Edition): American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141suppl:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–639. [DOI] [PubMed] [Google Scholar]
- 15.Kuijer PM, Hutten BA, Prins MH, et al. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457–460. [DOI] [PubMed] [Google Scholar]
- 16.Kyrle PA, Minar E, Bialonczyk C, et al. The risk of recurrent venous thromboembolism in men and women. N Engl J Med. 2004;350:2558–2563. [DOI] [PubMed] [Google Scholar]
- 17.Lagerstedt CI, Olsson CG, Fagher BO, et al. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. 1985;515–518. [DOI] [PubMed] [Google Scholar]
- 18.Levine MN, Hirsh J, Gent M, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost. 1995;74:606–611. [PubMed] [Google Scholar]
- 19.McRae S, Tran H, Schulman S, et al. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet. 2006;368:371–378. [DOI] [PubMed] [Google Scholar]
- 20.Michelena HI, Powell BD, Brady PA, et al. Gender in atrial fibrillation: ten years later. Gend Med. 2010;7:206–217. [DOI] [PubMed] [Google Scholar]
- 21.Nauffal D, Ballester M, Reyes RL, et al. Influence of recent immobilization and recent surgery on mortality in patients with pulmonary embolism. J Thromb Haemost. 2012;10:1752–1760. [DOI] [PubMed] [Google Scholar]
- 22.Nieto JA, Solano R, Ruiz-Ribo MD, et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE Registry. J Thromb Haemost. 2010;8:1216–1222. [DOI] [PubMed] [Google Scholar]
- 23.Pinede L, Ninet J, Duhaut P, et al. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf vein thrombosis. Circulation. 2001;103:2453–2460. [DOI] [PubMed] [Google Scholar]
- 24.Poli D, Antonucci E, Testa S, et al. Gender differences of bleeding and stroke risk in very Old atrial fibrillation patients on VKA treatment: results of the EPICA study on the behalf of FCSA (Italian Federation of Anticoagulation Clinics). Thromb Res. 2013;131:12. [DOI] [PubMed] [Google Scholar]
- 25.Ridker PM, Goldhaber SZ, Danielson E, et al. PREVENT Investigators. Long-term, low-intensity warfarin therapy for prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348:1425–1434. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:26–31. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez Munoz-Torrero JF, Bounameaux H, Pedrajas JM, et al. Effects of age on the risk of dying from pulmonary embolism or bleeding during treatment of deep vein thrombosis. J Vasc Surg. 2011;54:26S–32S. [DOI] [PubMed] [Google Scholar]
- 28.Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. N Engl J Med. 1995;332:1661–1665. [DOI] [PubMed] [Google Scholar]
- 29.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. [DOI] [PubMed] [Google Scholar]
- 30.Shireman TI, Mahnken JD, Howard PA, et al. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130:1390–1396. [DOI] [PubMed] [Google Scholar]
- 31.Tagalakis V, Kondal D, Ji Y, et al. Men had a higher risk of recurrent venous thromboembolism than women: a large population study. Gend Med. 2012;9:33–43. [DOI] [PubMed] [Google Scholar]
- 32.Trujillo-Santos J, Schellong S, Falga C, et al. Low-molecular-weight or unfractionated heparin in venous thromboembolism: the influence of renal function. Am J Med. 2013;126:425–434. [DOI] [PubMed] [Google Scholar]
- 33.White RH, Dager WE, Zhou H, et al. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96:267–273. [DOI] [PubMed] [Google Scholar]


