Abstract
Multidrug resistance associated with extended-spectrum beta-lactamase (ESBL) and Klebsiella pneumoniae carbapenemase (KPC) among K. pneumoniae is endemic in southern Europe. We retrospectively analyzed the impact of resistance on the appropriateness of empirical therapy and treatment outcomes of K. pneumoniae bloodstream infections (BSIs) during a 2-year period at a 1420-bed tertiary-care teaching hospital in northern Italy. We identified 217 unique patient BSIs, including 92 (42%) KPC-positive, 49 (23%) ESBL-positive, and 1 (0.5%) metallo-beta-lactamase-positive isolates. Adequate empirical therapy was administered in 74% of infections caused by non-ESBL non-KPC strains, versus 33% of ESBL and 23% of KPC cases (p < 0.0001). To clarify the impact of resistance on BSI treatment outcomes, we compared several different models comprised of non-antibiotic treatment-related factors predictive of patients’ 30-day survival status. Acute Physiology and Chronic Health Evaluation (APACHE) II score determined at the time of positive blood culture was superior to other investigated models, correctly predicting survival status in 83% of the study cohort. In multivariate analysis accounting for APACHE II, receipt of inadequate empirical therapy was associated with nearly a twofold higher rate of death (adjusted hazard ratio 1.9, 95% confidence interval 1.1–3.4; p = 0.02). Multidrug-resistant K. pneumoniae accounted for two-thirds of all K. pneumoniae BSIs, high rates of inappropriate empirical therapy, and twofold higher rates of patient death irrespective of underlying illness.
INTRODUCTION
Multidrug resistance among Enterobacteriaceae is a growing public health crisis that threatens to make many health care-associated infections untreatable with current antibiotics.47,57 The widespread use of broad-spectrum cephalosporin and fluoroquinolone antibiotics, in particular, has accelerated the emergence of fluoroquinolone-resistant and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae, which are now endemic in many communities and hospitals worldwide.47,51 Consequently, the diminishing activity of fluoroquinolones and third-generation cephalosporin has led to increasing use of carbapenem antibiotics for common health care-associated infections, creating pressure for the emergence of carbapenem-resistant Enterobacteriaceae (CRE).10,13 Indeed, outbreaks of K. pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae have been reported worldwide, and they are endemic in many hospitals and long-term care facilities in southern Europe, China, South America, and certain regions of North America.47
KPC enzymes efficiently hydrolyze all cephalosporins, monobactams, carbapenems, and even beta-lactamase inhibitors, leaving few effective treatment options.1,16 Triple drug concentrations consisting of meropenem, tigecycline, and colistin have been associated with improved survival in patients with KPC-K. pneumoniae bacteremia,74 but this combination is rarely administered empirically to patients. Moreover, the utility of continuing meropenem therapy as part of an active combination in the setting of extremely elevated carbapenem minimum inhibitory concentration (MICs) (>32 mg/L) remains unclear.22 Most studies examining outcomes associated with KPC K. pneumoniae bacteremia have focused on unmodifiable risk factors such as older age, severity of underlying illness, dialysis, and solid-organ transplantation as predictors of poor outcome.8,48,55
Relatively few studies have examined the impact of modifiable risk factors (for example, empirical antimicrobial therapy, source control) in the outcome of multidrug-resistant (MDR) K. pneumoniae bloodstream infections (BSIs) while taking into account the severity of underlying patient illness. In Italy, approximately 25%–50% of all K. pneumoniae bloodstream isolates are positive for ESBL production, and 20%–30% of strains produce KPC-2 or KPC-3 carbapenemases.24 To understand the impact of these endemic resistance patterns on patient outcome, we performed a 2-year retrospective observational study of K. pneumoniae BSI in our hospital. Our specific objective was to determine if isolation of ESBL or KPC-producing-K. pneumoniae from the bloodstream was associated with higher rates of inadequate empirical antibiotic prescription, which we hypothesized to be an independent risk factor for patient death within 30 days of a positive blood culture. We also performed a literature review to provide a worldwide perspective on epidemiology, risk factors, and microbiologic and treatment issues of BSI due to MDR K. pneumoniae.
PATIENTS AND METHODS
Patient Population
We performed a retrospective analysis of all K. pneumoniae BSIs at our institution from July 2010 to August 2012. The study site was S. Orsola-Malpighi Hospital, University of Bologna, a tertiary 1420-bed hospital with approximately 72,000 yearly inpatient admissions. Cases were eligible for analysis if they had a positive blood culture for K. pneumoniae and sufficient documentation in the medical record to assess treatment and outcomes within 30 days of the positive blood culture.
Study Design
Eligible patients were identified retrospectively from the institutional microbiology surveillance database and medical records. Data were extracted using standardized data collection tools, and the accuracy was confirmed by systematic reconciliation of case records using the original patient electronic medical record. Treatment outcomes associated with the BSI, including clinical response to antibacterial treatment, other intercurrent infections or medical complications, need for intensive care unit (ICU) admission, hospital discharge, or death were analyzed up to 30 days after the positive blood culture. Only the first positive culture (infection episode) per patient was included in the analysis.
Definitions
Bloodstream infections and systemic inflammatory response syndrome (SIRS) were defined according to the United States Centers for Disease Control and Prevention (CDC) criteria.33 Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated for all case patients on the day of their initial blood culture positive for K. pneumoniae.39 Neutropenia was defined as a peripheral absolute neutrophil count of <500 cells/mL. Immunosuppressive corticosteroid therapy was defined as ≥100 mg of prednisone-equivalent therapy administered within 30 days of positive blood culture. Chemo-radiation therapy included receipt of cytotoxic antineoplastic drugs or ionizing radiation for cancer curative intent or palliation within 30 days before the blood culture. Acute renal failure was defined according to the RIFLE criteria.34 Cirrhosis was identified by patient history (with diagnostic liver biopsy) or a probable diagnosis (with clinical and analytical data suggesting chronic liver disease, hepatocellular dysfunction, and portal hypertension). Intercurrent complications were recorded in the 30-day period after a positive blood culture if the patient required blood transfusion for severe hemorrhage or anemia, developed other hospital-acquired infections or pressure ulcers, or required surgical treatment during the hospital stay.
All-cause mortality was assessed in patients within 30 days of the positive blood culture. Empirical therapy consisted of all antibiotics administered to treat a suspected infection before final pathogen identification and availability of in vitro susceptibility results. Definitive therapy was defined as antimicrobial therapy given after the susceptibility testing results were available to the clinician. Antibiotic treatment was considered appropriate for susceptible, ESBL-producing, or KPC-producing K. pneumoniae BSI if at least 1 antibiotic was administered with documented sensitivity according to the breakpoints established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). In the case of KPC-K. pneumoniae, appropriate therapy was defined as the administration of at least 1 agent proven sensitive (colistin [using as standard dosage a loading dose of 9 MU, then 4.5 MU every 12 h intravenously] or tigecycline or gentamicin) with either imipenem, meropenem, or tigecycline. For purposes of our analysis, tigecycline, doxycycline, or rifampin monotherapy was considered inappropriate therapy for KPC-K. pneumoniae BSI. Only regimens continued for at least 48 hours were included in the analysis.
Microbiology
Blood cultures were incubated using the BACTEC FX Automated Blood Culture System (Becton Dickinson, Franklin Lakes, NJ). Identification and susceptibility testing of K. pneumoniae strains isolated from blood samples were performed with the Vitek 2 automated system (bioMerieux, Marcy l’Etoile, France). A positive ESBL test from the automated system was confirmed with disc diffusion tests according to United States Clinical and Laboratory Standards Institute (CLSI) guidelines.21 Enterobacteriaceae MICs were interpreted using EUCAST clinical breakpoints27 for all tested antimicrobials. Meropenem MICs were also confirmed by Etest using methods recommended by the manufacturer (bioMerieux). Phenotypic confirmation of carbapenemase production was performed for isolates with reduced susceptibility to carbapenems (MIC ≥ 0.5 mg/L for ertapenem, imipenem, or meropenem) using the modified Hodge test,4 and a meropenem-based disc-diffusion synergy test (Rosco Diagnostica, Taastrup, Denmark). The presence of the blaKPC gene was confirmed by polymerase chain reaction (PCR) using previously described methods.32
Statistical Analysis
Categorical variables were analyzed as absolute numbers and their relative frequencies. Continuous variables were analyzed as mean and standard deviation if normally distributed, or as median and interquartile range if non-normally distributed. In the univariate analysis of risk factors for all-cause 30-day mortality, categorical variables were compared using the chi-square test, whereas continuous variables were compared using the Mann-Whitney U or 2-tailed Student t-test, where appropriate.
The primary study hypothesis was to determine if ESBL and KPC-producing Enterobacteriaceae would be associated with significantly higher rates of inadequate empirical antibiotic therapy, which would be an independent risk factor for 30-day crude mortality. To account for non-antibiotic therapy covariates impacting 30-day crude mortality, patient APACHE II scores calculated on the day of positive blood culture were entered in a Cox proportional hazards regression model as a continuous variable along with a limited number (<5) of clinically plausible mortality covariates identified in univariate analysis (p < 0.1). This survival model was then used to evaluate the impact of inappropriate empirical or definitive therapy on patient survival, use of carbapenem- or colistin-containing regimens for ESBL-producing isolates, and the impact of combination therapy for KPC-producing-K. pneumoniae.
Model goodness-of-fit was determined by calibration plots using a modified Hosmer-Lemeshow test as previously described.53 Discrimination of the model was assessed by area under receiver operator curves (aROC). Residual plots of excluded and included covariates were used to screen for incorrect covariate inclusion, exclusion, or temporal effects as described by Bradburn et al.9 All analysis was carried out using SPSS 20.0 (SPSS, Chicago, IL) and MedCalc 12.7 (MedCalc Software, bvba, Ostend, Belgium).
Ethics Statement
The study was conducted in accordance with study principles outlined by the Declaration of Helsinki, following review by the institutional ethics committee. Full protocol review was waived because of the noninterventional, observational nature of the study.
Literature Review
We reviewed published studies on K. pneumoniae BSIs in the MEDLINE database (PubMed, National Library of Medicine, Bethesda, MD) from 1977 to 2012 using as keywords “Klebsiella pneumoniae” and “bloodstream infection,” and as a limit “English language.” We focused our literature review on ESBLs and carbapenemase-producing strains, as these are currently the main mechanisms of antibiotic resistance in K. pneumoniae.
RESULTS
During the 2-year study period, we identified 251 episodes of K. pneumoniae BSI. Among these cases, 34 (13.5%) were excluded from further analysis because of insufficient documentation in the medical record or because the bloodstream isolate represented a recurrence in the same patient, resulting in 217 individual cases eligible for analysis.
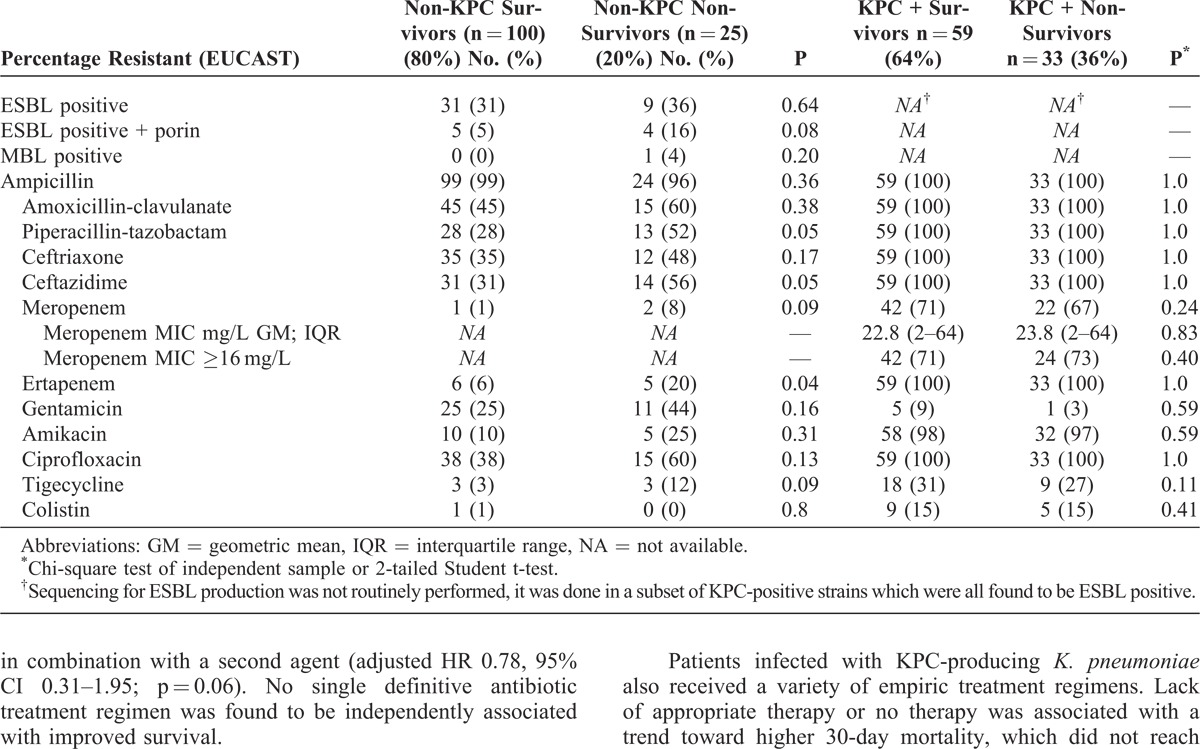
KPC-producing K. pneumoniae accounted for 7% (104/1403) of all BSIs in our hospital during the 2-year study period, and 42% (92/217) of all evaluable BSI episodes. KPC-producing strains were confirmed to be 8% blaKPC-2 and 92% blaKPC-3 by PCR. Non-KPC, ESBL-producing K. pneumoniae confirmed by phenotypic testing were responsible for a further 23% (49/217) of BSIs, with concomitant porin loss detected phenotypically in 4% (9/217) of the isolates based on an MIC superior to the epidemiologic cutoff for 1 or more carbapenems together with a negative confirmatory test for carbapenemase production. Only 1 isolate of K. pneumoniae (0.5%) during the study period was positive for metallo-beta-lactamase (MBL) production and was defined as VIM-1 (Verona integron-encoded MBL-1) producer by PCR assay. Characteristics and susceptibility patterns of the infecting isolates are summarized in Table 1.
TABLE 1.
Klebsiella Pneumoniae Bloodstream Isolate Susceptibility Characteristics by 30-Day Survival Status
Patient Population
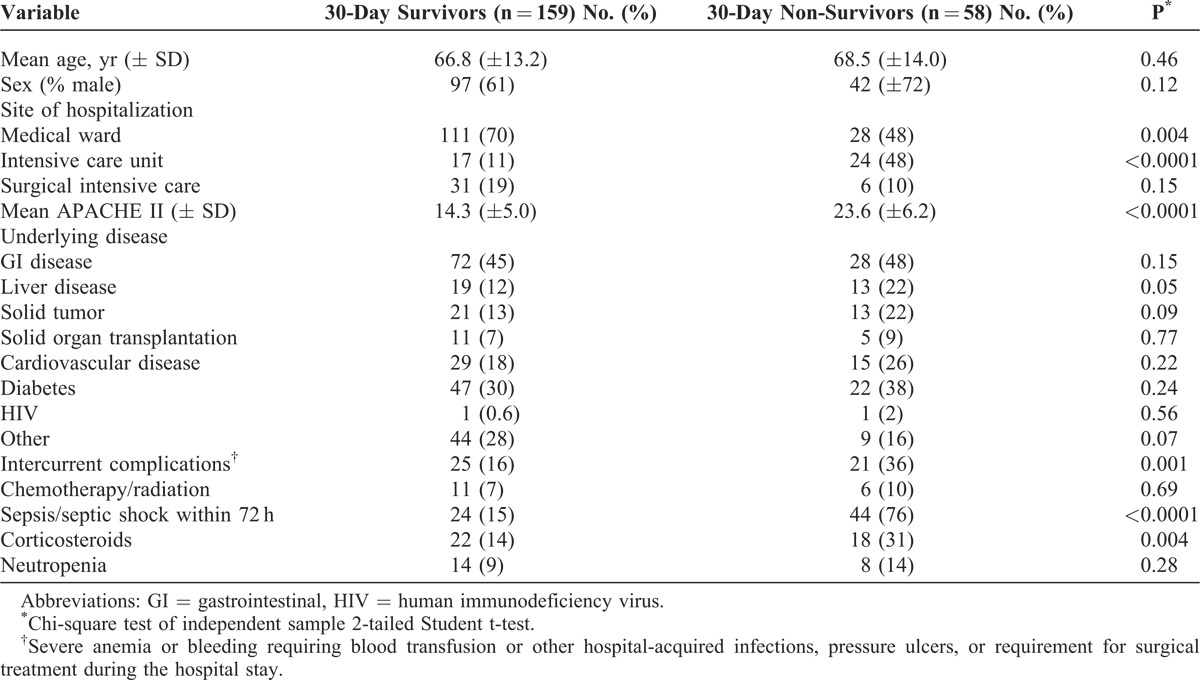
Baseline patient demographic characteristics are presented in Table 2. Compared to 30-day survivors, patients who died were more likely to be in the ICU (48% vs 11%, p < 0.0001), have higher median baseline APACHE II scores (24 vs 14, p < 0.0001), were more likely to develop intercurrent medical complications (36% vs 16%, p = 0.001) and have received immunosuppressive corticosteroid therapy (31% vs 14%, p = 0.004) or develop sepsis or have persistent septic shock in the first 72 hours (76% vs 15%, p < 0.0001). Although primarily validated as a prognostic index for ICU patients, APACHE II scores calculated on the day of positive blood cultures could discriminate non-surviving versus surviving patients at 30-days after the positive blood culture (aROC of 0.88; 0.83–0.92, p < 0.0001); and correctly predicted survival status in 83% of the total study cohort with good calibration (Hosmer-Lemeshow chi-square = 5.4, degree freedom = 8, p = 0.71).
TABLE 2.
Patient Demographic Characteristics
The inclusion of additional candidate clinical covariates for the survival model (cirrhosis, solid-organ transplant, hematologic malignancy, diabetes, corticosteroids, intercurrent complications) did not significantly improve the discrimination of the survival model over APACHE II alone (aROC 0.88 vs 0.89, p = 0.96) even though underlying hematologic malignancy (hazard ratio [HR] 2.5, 95% confidence interval [CI] 1.1–5.7; p = 0.03) and cirrhosis (HR 2.4, 95% CI 1.2–5.1; p = 0.02) were retained in a final multivariate risk model with APACHE II score (HR 1.2 per score point, 95% CI 1.1–1.2; p < 0.0001). Therefore, antibiotic treatment variables were analyzed using only APACHE II score as the underlying survival risk covariate. An APACHE II score >17 was found to be the optimal cutoff for defining patients at high (48/80 patients, 60%) versus lower (10/137 patients, 7%) cumulative 30-day mortality risk.
Antibiotic Treatment and Outcome
Compared to non-MDR strains, BSIs caused by ESBL- or KPC-positive K. pneumoniae were associated with a significantly higher frequency of inadequate empirical antimicrobial therapy (Figure 1). Initial empiric treatment was judged to be adequate in 74% of patients infected with non-ESBL or non-KPC producing K. pneumoniae who received at least 1 active antimicrobial agent, versus only 32% and 22% of patients with ESBL- and KPC-positive strains, respectively (p < 0.0001). In total, only 93/217 patients (43%) of patients with K. pneumoniae BSI received effective empiric therapy. When evaluated by Cox-hazard proportional regression accounting for underlying illness severity, receipt of inadequate empirical therapy was associated with an estimated twofold higher rate of death within 30 days (adjusted HR 1.9; 95% CI 1.1–3.4; p = 0.02).
FIGURE 1.
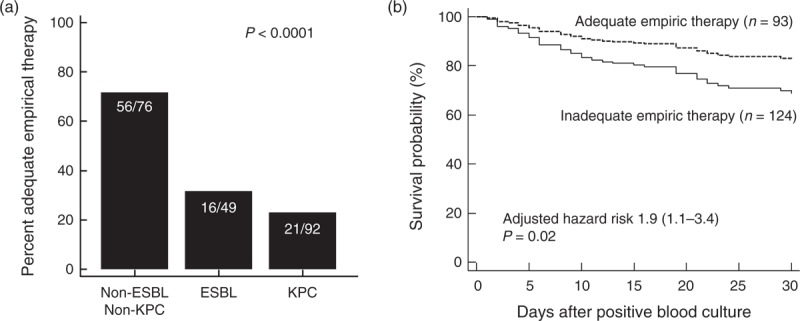
Probability of adequate empirical appropriate antibiotic therapy for K. pneumoniae bloodstream infection by (a) resistance mechanism and (b) impact on survival. P values were determined by (a) chi-square or (b) differences in survival probability was estimated using Cox-proportional hazard regression adjusted for patient APACHE II score calculated at the time of positive blood culture.
A number of monotherapy and combination therapy regimens were used to treat K. pneumoniae BSI in the study cohort. Among patients with non-KPC producing strains (39% of which were ESBL positive) lack of appropriate antibiotic therapy or no therapy in the first 48 hours after culture was associated with increased adjusted risk of death within 30 days (adjusted HR 2.6, 95% CI 1.1–6.1; p = 0.03). However, empiric therapy with a carbapenem-based regimen was associated with improved 30-day survival (adjusted HR 0.24, 95% CI 0.06–0.9, p = 0.04). A similar benefit was not evident with beta-lactam-based regimens; that is, ampicillin-sulbactam, piperacillin-tazobactam, or cephalosporin alone or in combination with a second agent (adjusted HR 0.78, 95% CI 0.31–1.95; p = 0.06). No single definitive antibiotic treatment regimen was found to be independently associated with improved survival.
Patients infected with KPC-producing K. pneumoniae also received a variety of empiric treatment regimens. Lack of appropriate therapy or no therapy was associated with a trend toward higher 30-day mortality, which did not reach statistical significance when adjusted for severity of underlying illness (adjusted HR 1.51, 95% CI 0.76–3.12; p = 0.22). Because of the treatment heterogeneity, we were also unable to identify a single empiric or definitive treatment regimen associated with improved survival in patients with KPC K. pneumoniae BSI. Nevertheless, the lowest risk of 30-day mortality appeared to be associated with patients who received colistin + meropenem + tigecycline combination therapy administered empirically (adjusted HR 0.48, 95% CI 0.11–2.01; p = 0.30) or as definitive therapy (adjusted HR 0.38, 95% CI 0.06–1.6; p = 0.31).
LITERATURE REVIEW
Epidemiology, Risk Factors, and Outcome of BSIs Due to Multidrug-Resistant K. Pneumoniae
Klebsiella pneumoniae is one of the most important causes of health care- and ICU-acquired infections. It has been reported as the second overall cause of Gram-negative BSI behind Escherichia coli.76 In a population-based surveillance study conducted in the Calgary Health Region (population 1.2 million) from 2000 to 2007, a total of 640 episodes of K. pneumoniae BSIs were identified for an overall annual population incidence of 7.1 per 100,000. Two-thirds of episodes were classified as health care-associated, and rates of resistance to antibiotics increased significantly during the study period.46 Another elegant surveillance study performed at 4 hospitals located on 4 continents showed that environmental factors may lead to an increase in the incidence ratio of K. pneumoniae BSI during the warmest months of the year.3 Unfortunately, no data about the prevalence and trend of antibiotic resistance were provided in that report.
Multidrug resistance in K. pneumoniae is most frequently linked with the production of ESBL. ESBLs were first detected in Germany in K. pneumoniae and Serratia marcescens in 1983,40 and consist of plasmid-mediated enzymes that hydrolyze oxyimino-beta-lactam agents, including third-generation cephalosporins and aztreonam.60,61 These plasmids also carry resistance genes to other antibiotics including aminoglycosides, chloramphenicol, sulfonamides, trimethoprim, and tetracycline. Over 100 different ESBL enzymes have been identified, each with a preferential substrate.61 Most ESBLs can be divided into 3 groups: TEM, SHV, and CTX-M types. Organisms that produce CTX-M enzymes have become the most prevalent type of ESBLs mainly in E. coli, particularly from certain European and South American countries, with potential to spread beyond the hospital environment.14 TEM-derived and SHV-derived ESBLs are mostly limited to nosocomial infections caused by Klebsiella spp.61 The rate of ESBL production is variable worldwide. The SENTRY antimicrobial surveillance program65 found that in 2009–2012, ESBL phenotype rates among Klebsiella spp. were higher in the European Mediterranean regions (35.1%) than in the United States (19.5%).
Epidemiologic studies suggest that widespread use of third-generation cephalosporins is a major risk factor for selection of ESBL-producing K. pneumoniae.36,37,43,73 Several additional risk factors for colonization and infection with ESBL-producing organisms have been reported, including the following: arterial and central venous catheterization, gastrointestinal tract colonization with ESBL-producing organisms, prolonged length of stay in an ICU, low birth weight in preterm infants, prior antibiotic use, and mechanical ventilation.43,58 Carriage of this organism increases dramatically among hospitalized patients, as colonization rates increase in direct proportion to the length of stay.
Clinical outcome in patients with BSIs caused by ESBL-producing bacteria is influenced by differences in epidemiology, type of ESBL enzymes expressed, co-resistance phenotypes, and virulence determinants among Enterobacteriaceae species.64 The mortality rates for patients with BSIs caused by ESBL-producing K. pneumoniae ranged from 14.1% to 43.3%.2,38,56 Delay in initiation of effective antimicrobial therapy has been reported as one of the most important treatment-related prognostic factors in patients with ESBL-producing Enterobacteriaceae BSI, and has been associated with a fivefold increase in BSI mortality.66
Increasing carbapenem used, necessitated by the growing prevalence of ESBL, has contributed significantly to the emergence and rapid dissemination of carbapenem-resistant K. pneumoniae (CR-KP) strains.42 Over the past 15 years, the rate of CR-KP rose dramatically worldwide.59 The CDC reports a significant increase in health care-associated infections from <1% in 2000 to 8% in 2007 in the United States, while European data from the EARS-Net database showed that, during the year 2010, the rates of CR-KP varied from 0.2% (Germany) to 15.8% (Italy) and 59.5% (Greece), with higher rates generally observed in southern European countries.59 The main mechanisms of carbapenem resistance in K. pneumoniae are 1) overproduction of AmpC beta-lactamases (class C beta-lactamases) or ESBLs combined with porin loss; and 2) production of several types of carbapenemases.75 Carbapenemase enzymes are able to hydrolyze nearly all beta-lactam antibiotics and are frequently carried by transferable plasmids that permit a rapid spread from a strain to another, or among different bacterial species, even within in the same patient.68 Carbapenemases are classified into different classes (A, B, and D) based on amino acid sequence homology; the class A K. pneumoniae carbapenemase (KPC) and the class B MBL, including VIM, IMP-type beta-lactamase (active on imipenem), and New Delhi MBL, have been the most implicated in the spread of carbapenem resistance among K. pneumoniae organisms.13,31,59
The main predictors for CR-KP isolation are poor functional status, high severity of underlying conditions, ICU stay, and prior exposure to antimicrobials. As for the latter risk factor, different classes of antibiotics have been associated with CR-KP infection, including carbapenems, fluoroquinolones and cephalosporins.7,29 Among the most common underlying conditions, patients who undergo solid-organ transplantation appear to be at uniquely high risk for CR-KP infection. Patel et al demonstrated in a large case-control matched-cohort study of K. pneumoniae infection that solid-organ transplantation was associated with a fourfold higher risk of CR-KP acquisition.54 This association was confirmed by other investigators for virtually all types of solid-organ transplantation.31
Crude mortality rates among patients with isolation of CR-KP vary from 20% to 70%, depending on the population studied and the type of infection.8,15,35,67,74,80 The crude mortality rates among patients with BSI are higher than those observed for patients with other types of infections, being generally about 45%.15,48,50,62,74,80 Notably, mortality is generally higher for bacteremia secondary to a pulmonary source than for primary episodes or BSI secondary to other sources,62 probably due to the limited pulmonary penetration of the available drugs and the lack of opportunity to remove the infectious source.
Diagnostic Detection
The isolation of the microorganism from blood cultures is still the gold standard for diagnosing K. pneumoniae BSI. Molecular methods such as PCR on blood samples have been proposed as a rapid diagnostic test to identify microorganisms causing BSI, especially among patients already on antimicrobial therapy,41 but they are not considered cost effective compared with standard blood cultures.44 Recently, the matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry assay has been shown to be a very accurate diagnostic technique to identify in a few hours the microorganism growing in the blood culture.20 In the near future, this technique could also be able to detect the pattern of drug resistance of the identified microorganism.12,17 MALDI-TOF currently provides only rapid identification of the pathogen in most laboratories, thus the culture method seems to be the standard for BSI diagnosis so far.
Methods for detection of drug resistance in K. pneumoniae can be broadly divided into 2 groups: phenotypic methods that use non-molecular techniques, which detect the ability of the ESBL or carbapenemase enzymes to hydrolyze different cephalosporins or carbapenems; and genotypic methods, which use molecular techniques to detect the gene responsible for the enzyme production.61 Clinical diagnostic laboratories primarily use phenotypic methods because these tests are easy to perform, cost effective, and are easily incorporated into the workflow of automated susceptibility systems used in most clinical microbiology laboratories, making them widely accessible. However, phenotypic methods are not able to distinguish between the specific enzymes responsible for resistance. Several research or reference laboratories use genotypic methods for the identification of the specific gene responsible for the production of the ESBL or carbapenemases, which have the additional ability to detect low-level resistance (which can be missed by phenotypic methods). Furthermore, molecular assays have the potential to be performed directly on clinical specimens without culturing the bacteria, with subsequent reduction of detection time. The detection of ESBL-producing bacteria remains a contentious issue.61 A study from the United States reported that only 8% of clinical laboratories from rural hospitals routinely screened for ESBL-producing organisms,71 whereas a performance survey of 60 Italian laboratories misidentified up to 50% of known ESBL-producing isolates.45 The United States CLSI and the United Kingdom Health Protection Agency (HPA) have published guidelines for ESBL detection in Enterobacteriaceae specifically for E. coli, Klebsiella spp., and Proteus spp.5,6 These guidelines are based on the principle that most ESBLs hydrolyze third-generation cephalosporins although they are inhibited by clavulanate, and recommend initial screening with either 8 mg/L (CLSI) or 1 mg/L (HPA) of cefpodoxime, 1 mg/L each of cefotaxime, ceftazidime, ceftriaxone, or aztreonam, followed by confirmatory tests (including the E-test ESBL strips) with both cefotaxime and ceftazidime in combination with clavulanate at a concentration of 4 mg/mL. Automated systems that use similar detection principles are widely used in clinical laboratories, especially in North American and European countries, reporting high rates of sensitivity of up to 94% and specificity of 98% for detecting ESBLs in E. coli, Klebsiella spp., and Proteus spp.70,79
In terms of genotypic detection, PCR amplification followed by nucleotide sequencing remains the gold standard for the identification of specific point mutation of blaTEM or blaSHV ESBL genes.61 However, this is not always straightforward and cost effective because clinical isolates often have multiple copies of ESBL genes. The PCR amplification of CTX-M-specific products without sequencing, in an isolate that produces an ESBL, usually provides sufficient evidence that a blaCTX-M gene is responsible for this phenotype.61
Detection of isolates with KPC enzymes or other carbapenemases is challenging because resistance may be low level, and low level carbapenem resistance can also arise through combinations of impermeability and activity of AmpC or ESBL. Carbapenemase production should be suspected when Enterobacteriaceae have resistance or reduced susceptibility to carbapenems that is increased when isolates do not show strong the cephalosporin-clavulanate and cephalosporin-cloxacillin synergies typical of strains with ESBL or high-level AmpC. Carbapenemase activity can be confirmed by the Hodge test (also known as the cloverleaf test) and by acidimetric tests with carbapenems as the substrate, despite their low sensitivity and specificity.47 The inclusion of inhibitors (boronic acid for KPC and either ethylenediaminetetraacetic acid [EDTA] or dipicolinic acid for metalloenzymes) can help discrimination between carbapenemase types, and cloxacillin can be used to inhibit the interfering activity of AmpC.47 Definitive identification of carbapenemases in clinical isolates is best achieved by molecular methods.52 PCR and sequencing are currently considered the gold standard to identify isolates harboring blaKPC genes, but this approach is also time-consuming and requires considerable technical expertise.
Carbapenemase-detection by real-time PCR assay has several advantages. First, results can be obtained within 2 hours (from the initiation of the procedure), which may help clinicians to provide more adequate therapy during the early period of clinical illness and facilitates rapid decisions regarding isolation of patients to prevent dissemination. Second, it is simple and easy to perform and requires minimal training. Third, its theoretical processing capacity (up to 48 samples in 24 hours) makes it suitable for testing even large collections of isolates (for example, in the context of surveillance programs in outbreak situations).69 Furthermore, as novel blaKPC variants continue to emerge, multiplex-PCR assay, using molecular beacons, can be modified in response to epidemiologic developments.19 As previously mentioned, using a hydrolysis reaction to identify beta-lactamases and carbapenemases, MALDI-TOF is able to accurately report the presence of carbapenemases.12 PCR followed by electrospray ionization mass spectrometry (PCR/ESI-MS) quickly identifies Gram-negative bacteria and can target the identification of common resistance genes such as gyrA, parC, and blaKPC with >96% sensitivity and specificity.26 Among methods that are currently available, nucleic acid microarray technologies are proving to be important in the characterization of resistance genes and molecular epidemiology.25 The clinical experience, to date, shows that this technology correctly identifies the presence of resistance genes with 90%–100% accuracy when applied directly from single bacterial colonies grown on agar plates.25 In addition, the nucleic acid microarray technology Check-KPC/ESBL could be employed with DNA extracted directly from blood cultures positive for Gram-negative bacteria, before species identification, reducing the notification time by as much as 18–20 hours.28 All these applications are awaiting large-scale trials in the clinical arena.
Treatment Options
To our knowledge there are no randomized controlled studies performed to determine the best therapy for infections caused by ESBL organisms. Carbapenems remain preferable to piperacillin/tazobactam or partially susceptible cephalosporins such as cefepime when a critically ill patients is in need of empirical therapy.49 Paterson et al56 reported that patients who received a carbapenem as monotherapy or combination therapy during the first 5 days after a blood culture positive for ESBL-producing K. pneumoniae had a significantly lower mortality than those who received non-carbapenem antibiotics. Vardakas et al77 recently conducted a systematic review and meta-analysis of the efficacy of carbapenem vs non-carbapenem-based regimens for ESBL-producing Enterobacteriaceae BSI. They found that carbapenems were associated with lower mortality versus non-beta-lactam/beta-lactamase inhibitor combinations (BL/BLIs) for empirical and definitive treatment. However the analysis did not control for the severity of underlying illness. In a post hoc analysis of 192 patients with BSI due to ESBL-producing Enterobacteriaceae, including 6 prospective cohorts carried out in Spain over 2001–2007, mortality in patients treated with an in vitro active BL/BLIs (amoxicillin-clavulanic acid and piperacillin-tazobactam) or carbapenem was compared in 2 cohorts: the empirical therapy cohort and the definitive therapy cohort.63 After adjustment for confounders, no association was found between either empirical therapy with BL/BLIs (adjusted HR 1.14, 95% CI 0.29–4.40; p = 0.84) or definitive therapy (adjusted HR 0.76, 95% CI 0.28–2.07; p = 0.5) and increased mortality. These results suggest that, if active in vitro, amoxicillin-clavulanic acid and piperacillin-tazobactam are suitable alternatives to carbapenems for treating patients with BSI due to ESBL-producing Enterobacteriaceae. In addition, the study suggests that in patients who have reached clinical stability, a step-down regimen from carbapenems to BL/BLIs could be considered.
A recent surveillance study that examined outcome of antibiotic treatment showed that ESBL-producing K. pneumoniae organisms are generally more susceptible to cefepime than piperacillin/tazobactam or other BL/BLIs,49 therefore standard-dose cefepime could be considered a reasonable option for the definitive therapy of invasive infections resulting from ESBL-producing E. coli and Klebsiella spp. when the MIC for the organism is ≤2 mg/L (CLSI) or ≤1 mg/L (EUCAST), although higher doses may be considered for MICs in the 4–8 mg/L range. The authors stated that piperacillin/tazobactam is also a reasonable option when the MIC is ≤16 mg/L.49
No consensus exists regarding the best regimen for treating CR-KP. Several strategies have been proposed based on retrospective analysis and mostly single-center studies of CR-KP BSI, but controlled trials supporting these recommendations are lacking.59,78 The first option consists of administering a first-line antibiotic, mainly carbapenems, at higher doses to overcome resistance. However, some CR-KP isolates exhibit such high MICs to first-line agents that extreme doses with unacceptable toxicity would be required to achieve pharmacokinetic/pharmacodynamic exposures required for efficacy. The second option is to use a second-line antibiotic with Gram-negative activity for which resistance is not yet developed (for example, colistin, tigecycline, gentamicin, fosfomycin). Unfortunately, many second-line agents are more toxic than first-line drugs, or have significant pharmacokinetic deficiencies that limit their activity in anatomical sites where CR-KP are most likely to emerge, including the urine, bloodstream, and lung.59 Moreover, all of the second-line antimicrobials are prone to rapid emergence of resistance during treatment, especially if used as monotherapy.15 A third strategy, now widely regarded as superior to other treatment approaches, is to treat CR-KP infections with a combination of first- and second-line antibiotics with the hope that synergistic interactions between antibiotics will lessen the need for extremely high antibiotic doses, suppress the emergence of resistance, and overcome the pharmacokinetic weaknesses of individual agents.59,75 Some reports showed that patients who received combinations of a carbapenem + colistin, and/or tigecycline experienced lower mortality rates (0–40%) than those on carbapenem, colistin, or tigecycline monotherapy (40%–80%).62,74,80 Tumbarello et al74 found that the overall 30-day mortality rate was 42%, but it was significantly higher among patients who received monotherapy versus combination regimens (54% vs 34%, p = 0.02), and the combination of tigecycline, colistin, and meropenem was associated with the lowest mortality risk.74 A significant benefit in survival among patients with CR-KP BSI treated with carbapenem-containing combination regimens was shown in a recent observational study of 205 patients with CR-KP BSI.23 Among other combination regimens, meropenem or doripenem + ertapenem, and colistin + rifampicin have been proposed.11,72 Although in vitro data are encouraging for both these combinations, clinical data are yet too scant to conclude about their efficacy.18,30
DISCUSSION
To the best of our knowledge, the current retrospective study represents the largest epidemiologic analysis of K. pneumoniae BSIs with accompanying data on antimicrobial resistance patterns and treatment. Over 65% of the isolates recovered in our patient cohort were resistant to third-generation cephalosporins. The rates of inadequate empirical therapy were high as 26%, 67% and 78% for non-ESBL/non-KPC, ESBL,and KPC-positive K. pneumoniae, respectively. Somewhat unexpectedly, we found that APACHE II scores calculated at the time of BSI onset better predicted patient 30-day survival status (correct in 83% of cases) compared with a multivariate risk model developed from variables identified in univariate analysis (see Table 1). When the impact of antimicrobial therapy for K. pneumoniae BSI was evaluated accounting for APACHE II, inadequate empirical antimicrobial therapy was associated with nearly a twofold higher rate of death within 30-days of a positive K. pneumoniae blood culture.
Our analysis is unique from previous studies in that we evaluated the survival impact of various empirical and definitive antimicrobial regimens, accounting for the severity of underlying patient illness.
Treatment of hospital-acquired Enterobacteriaceae is becoming a serious challenge given the increasingly high rates of ESBL-producing strains coinciding with a diminished activity of fluoroquinolones, penicillins and cephalosporins for many common health care-associated infections.10 As a result, the clinical need for carbapenem-based regimens has increased dramatically, particularly in the treatment of BSIs. The widespread carbapenem use has undoubtedly contributed to an explosive increase in KPC carbapenemase-producing K. pneumoniae, which now compromises over 40% of K. pneumoniae BSIs in our hospital.
Unlike treatment of ESBL organisms, for which carbapenems, cefepime and in selected cases BL/BLI are shown to be effective in eradicating infection and were associated with good outcomes,49,56,77 there are no ideal “rescue” treatment options for KPC-K. pneumoniae.59 As discussed in our literature review, limited data from non-randomized case cohort studies suggest that combination therapy is associated with improved survival in patients with KPC-K. pneumoniae BSI.62,74 In the current study cohort, we observed a more modest benefit (4% higher response rate) with combination versus monotherapy regimens for KPC-K. pneumoniae BSI compared to that reported in previous studies.8,25 Mortality rates in our monotherapy-treated patients were substantially lower than those reported by Tumbarello et al (for example, 41% vs 54.3%, respectively), and colistin, tigecycline, or gentamicin monotherapy, now recognized as inferior regimens for KPC-K. pneumoniae BSI,59 was used less frequently during the period of the current study compared to that of Tumbarello et al (11% vs 37%, respectively). Therefore, it is not surprising that margin of benefit observed with combination therapy was less pronounced.
A number of patients (68/251, 27%) in our study cohort did not receive any empiric antibiotic therapy for K. pneumoniae BSI. The majority (49/68, 72%) of these patients were initially managed by source control only (that is, catheter removal, interventional drainage) without an antibiotic, while 6/251 (9%) either died or were discharged prior to the positive culture. Nevertheless, in our analysis accounting for underling severity of disease, lack of empiric antimicrobial therapy was associated with increased mortality risk in patients with non-KPC K. pneumoniae BSI, with a similar (albeit non-significant) trend towards increased mortality in patients with KPC K. pneumoniae BSI.
This study has several limitations. Data were collected retrospectively, which could lead to potential confounding factors and selection bias that cannot be completely eliminated in the analysis. Additionally, this is a monocentric study accounting for a specific local epidemiology for Enterobacteriaceae. Further data including a larger sample size of patients will be necessary to confirm our study results and allow for more robust comparisons of empiric treatment regimens for K. pneumoniae BSI.
Nevertheless, we confirmed that high endemic rates of ESBL and KPC-resistance among K. pneumoniae is associated with increased risk of inappropriate empirical therapy, which was an independent risk factor for patient death. Development of validated clinical risk models, and improved diagnostic and resistance detection methods for earlier detection of ESBL- and carbapenemase-producing Enterobacteriaceae, will be essential tools for improving empiric treatment strategies for these increasingly MDR pathogens.
Footnotes
Abbreviations: AmpC = class C beta-lactamase, APACHE II = Acute Physiology and Chronic Health Evaluation II, aROC = area under receiver operator curves, BL/BLI = beta-lactam/beta-lactamase inhibitor, BSI = bloodstream infection, CDC = United States Centers for Disease Control and Prevention, CLSI = Clinical and Laboratory Standards Institute, CRE = carbapenem-resistant Enterobacteriaceae, CR-KP = carbapenem-resistant Klebsiella pneumoniae, ESBL = extended-spectrum beta-lactamase, EUCAST = European Committee on Antimicrobial Susceptibility Testing, HPA = United Kingdom Health Protection Agency, ICU = intensive care unit, KPC = Klebsiella pneumoniae carbapenemase, MALDITOF = Matrix-Assisted Laser Desorption Ionization Time-of-Flight, MBL = metallo-beta-lactamase, MDR = multidrug resistant, MIC = minimum inhibitory concentration, PCR = polymerase chain reaction, VIM = Verona integron-encoded metallo-beta-lactamase.
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
References
- 1.Akova M, Daikos GL, Tzouvelekis L, et al. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect. 2012;18:439–448. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DJ, Engemann JJ, Harrell LJ, et al. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006;50:1715–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DJ, Richet H, Chen LF, et al. Seasonal variation in Klebsiella pneumoniae bloodstream infection on 4 continents. J Infect Dis. 2008;197:752–756. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007;45:2723–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. Performance standards for antimicrobial susceptibility testing. National Committee for Clinical Laboratory Standards 12th Informational Supplement M100-S12. Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 6.Anonymous. UK Health Protection Agency. Laboratory Detection and Reporting of Bacteria with Extended Spectrum b-Lactamases. National Standard Method QSOP. 2008;51. [Google Scholar]
- 7.Borer A, Saidel-Odes L, Eskira S, et al. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K pneumoniae. Am J Infect Control. 2012;40:421–425. [DOI] [PubMed] [Google Scholar]
- 8.Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30:972–976. [DOI] [PubMed] [Google Scholar]
- 9.Bradburn MJ, Clark TG, Love SB, et al. Survival analysis part III: Multivariate data analysis-choosing a model and assessing its adequacy and fit. Br J. Cancer. 2003;89:605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braykov NP, Eber MR, Klein EY, et al. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999-2010. Infect Control Hosp Epidemiol. 2013;34:259–268. [DOI] [PubMed] [Google Scholar]
- 11.Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011;55:3002–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burckhardt I, Zimmermann S. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol. 2011;49:3321–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canton R, Akova M, Carmeli Y, et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect. 2012;18:413–431. [DOI] [PubMed] [Google Scholar]
- 14.Canton R, Coque TM. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–475. [DOI] [PubMed] [Google Scholar]
- 15.Capone A, Giannella M, Fortini D, et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin Microbiol Infect. 2013;19:E23–E30. [DOI] [PubMed] [Google Scholar]
- 16.Carmeli Y, Akova M, Cornaglia G, et al. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin Microbiol Infect. 2010;16:102–111. [DOI] [PubMed] [Google Scholar]
- 17.Carvalhaes CG, Cayo R, Visconde MF, et al. Detection of carbapenemase activity directly from blood culture vials using MALDI-TOF MS: a quick answer for the right decision. J Antimicrob Chemother. 2014;69:2132–2136. [DOI] [PubMed] [Google Scholar]
- 18.Ceccarelli G, Falcone M, Giordano A, et al. Successful ertapenem-doripenem combination treatment of bacteremic ventilator-associated pneumonia due to colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2900–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Mediavilla JR, Endimiani A, et al. Multiplex real-time PCR assay for detection and classification of Klebsiella pneumoniae carbapenemase gene (bla KPC) variants. J Clin Microbiol. 2011;49:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clerc O, Prod’hom G, Vogne C, et al. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis. 2013;56:1101–1107. [DOI] [PubMed] [Google Scholar]
- 21.Clinical Laboratory Standards Institute. Twenty-first Informational Supplement M 100-S21. Wayne, PA: Clinical Laboratory Standards Insitute; 2011. [Google Scholar]
- 22.Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect. 2011;17:1135–1141. [DOI] [PubMed] [Google Scholar]
- 23.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EARS-NET. Antimicrobial resistance interactive database. European Centre for Disease Prevention and Control. 2013. http://ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/database. Accessed November 19, 2013. [Google Scholar]
- 25.Endimiani A, Hujer AM, Hujer KM, et al. Evaluation of a commercial microarray system for detection of SHV-, TEM-, CTX-M-, and KPC-type beta-lactamase genes in Gram-negative isolates. J Clin Microbiol. 2010;48:2618–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endimiani A, Hujer KM, Hujer AM, et al. Rapid identification of bla KPC-possessing Enterobacteriaceae by PCR/electrospray ionization-mass spectrometry. J Antimicrob Chemother. 2010;65:1833–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.EUCAST. Breakpoint tables for interpretation of MICs and zone diameters, version 3.1. 2013. [Google Scholar]
- 28.Fishbain JT, Sinyavskiy O, Riederer K, et al. Detection of extended-spectrum beta-lactamase and Klebsiella pneumoniae carbapenemase genes directly from blood cultures by use of a nucleic acid microarray. J Clin Microbiol. 2012;50:2901–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasink LB, Edelstein PH, Lautenbach E, et al. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta N, Limbago BM, Patel JB, et al. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53:60–67. [DOI] [PubMed] [Google Scholar]
- 32.Hindiyeh M, Smollan G, Grossman Z, et al. Rapid detection of blaKPC carbapenemase genes by internally controlled real-time PCR assay using bactec blood culture bottles. J Clin Microbiol. 2011;49:2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: A modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 34.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalpoe JS, Sonnenberg E, Factor SH, et al. Mortality associated with carbapenem-resistant Klebsiella pneumoniae infections in liver transplant recipients. Liver transplantation. Liver Transplant. 2012;18:468–474. [DOI] [PubMed] [Google Scholar]
- 36.Kang CI, Kim SH, Kim DM, et al. Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2004;25:860–867. [DOI] [PubMed] [Google Scholar]
- 37.Kang CI, Kim SH, Park WB, et al. Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 2004;48:4574–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim BN, Woo JH, Kim MN, et al. Clinical implications of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteraemia. J Hosp Infect. 2002;52:99–106. [DOI] [PubMed] [Google Scholar]
- 39.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 40.Knothe H, Shah P, Krcmery V, et al. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983;11:315–317. [DOI] [PubMed] [Google Scholar]
- 41.Lamoth F, Jaton K, Prod’hom G, et al. Multiplex blood PCR in combination with blood cultures for improvement of microbiological documentation of infection in febrile neutropenia. J Clin Microbiol. 2010;48:3510–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landman D, Bratu S, Kochar S, et al. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, NY. J Antimicrob Chemother. 2007;60:78–82. [DOI] [PubMed] [Google Scholar]
- 43.Lautenbach E, Patel JB, Bilker WB, et al. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–1171. [DOI] [PubMed] [Google Scholar]
- 44.Lupetti A, Barnini S, Dodi C, et al. New rapid methods cannot replace the current method to diagnose bloodstream infections. J Med Microbiol. 2014;63:767–769. [DOI] [PubMed] [Google Scholar]
- 45.Luzzaro F, Gesu G, Endimiani A, et al. Performance in detection and reporting beta-lactam resistance phenotypes in Enterobacteriaceae: a nationwide proficiency study in Italian laboratories. Diagn Microbiol Infect Dis. 2006;55:311–318. [DOI] [PubMed] [Google Scholar]
- 46.Meatherall BL, Gregson D, Ross T, et al. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122:866–873. [DOI] [PubMed] [Google Scholar]
- 47.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen HM, Shier KL, Graber CJ. Determining a clinical framework for use of cefepime and beta-lactam/beta-lactamase inhibitors in the treatment of infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2014;69:871–880. [DOI] [PubMed] [Google Scholar]
- 50.Nguyen M, Eschenauer GA, Bryan M, et al. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis. 2010;67:180–184. [DOI] [PubMed] [Google Scholar]
- 51.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–236. [DOI] [PubMed] [Google Scholar]
- 52.Nordmann P, Poirel L. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013;68:487–489. [DOI] [PubMed] [Google Scholar]
- 53.Parzen M, Lipsitz SR. A global goodness of fit statistic for Cox-Regression models. Biometrics. 1999;55:580–584. [DOI] [PubMed] [Google Scholar]
- 54.Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. [DOI] [PubMed] [Google Scholar]
- 55.Patel N, Harrington S, Dihmess A, et al. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J Antimicrob Chemother. 2011;66:1600–1608. [DOI] [PubMed] [Google Scholar]
- 56.Paterson DL, Ko WC, Von Gottberg A, et al. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin Infect Dis. 2004;39:31–37. [DOI] [PubMed] [Google Scholar]
- 57.Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pena C, Pujol M, Ardanuy C, et al. An outbreak of hospital-acquired Klebsiella pneumoniae bacteraemia, including strains producing extended-spectrum beta-lactamase. J Hosp Infect. 2001;47:53–59. [DOI] [PubMed] [Google Scholar]
- 59.Petrosillo N, Giannella M, Lewis R, et al. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther. 2013;11:159–177. [DOI] [PubMed] [Google Scholar]
- 60.Pfaller MA, Segreti J. Overview of the epidemiological profile and laboratory detection of extended-spectrum beta-lactamases. Clin Infect Dis. 2006;42suppl 4:S153–S163. [DOI] [PubMed] [Google Scholar]
- 61.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. [DOI] [PubMed] [Google Scholar]
- 62.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-Bano J, Navarro MD, Retamar P, et al. Beta-Lactam/beta-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis. 2012;54:167–174. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Bano J, Pascual A. Clinical significance of extended-spectrum beta-lactamases. Expert Rev Anti Infect Ther. 2008;6:671–683. [DOI] [PubMed] [Google Scholar]
- 65.Sader HS, Farrell DJ, Flamm RK, et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int J Antimicrob Agents. 2014;43:328–334. [DOI] [PubMed] [Google Scholar]
- 66.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;60:913–920. [DOI] [PubMed] [Google Scholar]
- 67.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother. 2008;52:1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sidjabat HE, Silveira FP, Potoski BA, et al. Interspecies spread of Klebsiella pneumoniae carbapenemase gene in a single patient. Clin Infect Dis. 2009;49:1736–1738. [DOI] [PubMed] [Google Scholar]
- 69.Spanu T, Fiori B, D’Inzeo T, et al. Evaluation of the New NucliSENS EasyQ KPC test for rapid detection of Klebsiella pneumoniae carbapenemase genes (blaKPC). J Clin Microbiol. 2012;50:2783–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spanu T, Sanguinetti M, Tumbarello M, et al. Evaluation of the new VITEK 2 extended-spectrum beta-lactamase (ESBL) test for rapid detection of ESBL production in Enterobacteriaceae isolates. J Clin Microbiol. 2006;44:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevenson KB, Samore M, Barbera J, et al. Detection of antimicrobial resistance by small rural hospital microbiology laboratories: comparison of survey responses with current NCCLS laboratory standards. Diagn Microbiol Infect Dis. 2003;47:303–311. [DOI] [PubMed] [Google Scholar]
- 72.Tascini C, Tagliaferri E, Giani T, et al. Synergistic activity of colistin plus rifampin against colistin-resistant KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:3990–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tumbarello M, Spanu T, Sanguinetti M, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. [DOI] [PubMed] [Google Scholar]
- 75.Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. [DOI] [PubMed] [Google Scholar]
- 77.Vardakas KZ, Tansarli GS, Rafailidis PI, et al. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother. 2012;67:2793–2803. [DOI] [PubMed] [Google Scholar]
- 78.Viale P, Giannella M, Lewis R, et al. Predictors of mortality in multidrug-resistant Klebsiella pneumoniae bloodstream infections. Expert Rev Anti Infect Ther. 2013;11:1053–1063. [DOI] [PubMed] [Google Scholar]
- 79.Wiegand I, Geiss HK, Mack D, et al. Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J Clin Microbiol. 2007;45:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect. 2011;17:1798–1803. [DOI] [PubMed] [Google Scholar]


