Abstract
This study highlights the clinical features, treatments, and outcomes of the rare myocarditis in adult-onset Still disease (AOSD). Among a case series of 57 patients fulfilling either Yamaguchi or Fautrel AOSD criteria and seen between 1998 and 2010, we identified 4 cases of myocarditis. From a comprehensive literature review, we collected 20 additional cases of myocarditis-complicated AOSD. The characteristics of patients with myocarditis were compared with those of AOSD patients without myocarditis.
In these 24 myocarditis-complicated AOSD cases, myocarditis occurred early and was present at AOSD onset in 54% of the cases. Myocarditis was often symptomatic (96% of patients) with nonspecific electrocardiographic abnormalities (79% of patients) and a left ventricle ejection fraction ≤50% (67% of patients). Cardiac magnetic resonance imaging and endomyocardial biopsies showed features consistent with myocarditis in 4 patients and a mononuclear interstitial inflammatory infiltrate in 4 others. Steroids alone were effective in 50% of patients with myocarditis. Intravenous immunoglobulins, methotrexate, and tumor necrosis factor-α-blockers were also prescribed and often found effective. Only 1 patient died from cardiogenic shock. Patients with myocarditis-complicated AOSD were younger and more frequently male than patients with AOSD alone. Pericarditis was more frequent in the myocarditis group; white blood cell count, polymorphonuclear cell count, and serum ferritin levels were also higher.
Myocarditis is a potentially life-threatening complication of AOSD but responds positively to steroids and other immunomodulatory drugs. Its prognosis remains good (only 1 death occurred), but the condition requires close monitoring of heart function.
INTRODUCTION
First described in 1971 by EG Bywaters, adult-onset Still disease (AOSD) is a rare inflammatory disorder of unknown etiology.6 Its main features are high spiking fever, evanescent rash, sore throat, polyarthralgia or arthritis, serositis, lymphadenopathy, hepatosplenomegaly, leukocytosis, elevated polymorphonuclear neutrophils (PMNs), high erythrocyte sedimentation rate, high serum ferritin (SF), and elevated liver enzymes.
Despite the high diagnostic value attributed to high SF associated with low SF glycosylated fraction (<20%), the diagnosis of AOSD is difficult to establish, and the spectrum of differential diagnoses is wide.19 The clinical course of the disease may follow 1 of 3 patterns: a monocyclic systemic course, an intermittent or polycyclic systemic course, and a chronic course that mimics chronic arthritis.52 The treatment of AOSD remains empirical. It includes nonsteroidal antiinflammatory drugs (NSAIDs), corticosteroids, methotrexate, and intravenous immune globulins (IVIGs).17 Biological agents such as tumor necrosis factor-α (TNF-α) blockers, interleukin-1 (IL-1) receptor antagonists, and IL-6 inhibitors were recently used in refractory cases.41
The most frequent cardiac involvement during AOSD is pericarditis. It occurs in nearly 20% of the patients. Its outcome is most often favorable though some cases involved cardiac tamponade.23 Conversely, myocarditis in AOSD is rare. To the best of our knowledge, none of the major AOSD cohort studies have mentioned myocarditis; only isolated cases have been reported.
We review here the clinical features, treatments, and outcomes of patients with myocarditis in AOSD. Four previously unreported cases are described and the features of 20 other cases from the literature are summarized. The main characteristics are then compared with those of a retrospective cohort of non-myocarditis-complicated AOSD cases.
PATIENTS AND METHODS
Retrospective Cases
From a series of 57 patients identified as having AOSD (database of the Medical Information Department of Hospices Civils de Lyon, 1998–2010) and fulfilling either Yamaguchi53 or Fautrel19 criteria, we extracted all cases with myocarditis.23 The exclusion criteria for AOSD were an onset of the disease before 16 years of age and insufficient medical record data.
Despite the fact that endomyocardial biopsy (EMB) remains the gold standard in the diagnosis of myocarditis,8 recent criteria for acute myocarditis have been proposed without the need for EMB.46 This classification considers 3 levels of diagnostic certainty: 1) Definite myocarditis (histologically proven); 2) Probable acute myocarditis (cardiovascular symptoms plus at least 1 of the following signs: raised biomarkers, suggestive electrocardiogram (ECG) findings, or abnormal cardiac function on transthoracic ultrasonography (TTU) or cardiac magnetic resonance imaging (MRI); and, 3) Possible acute myocarditis (without cardiac symptoms but with at least 1 of the latter signs).
The clinical features, laboratory characteristics, imaging data, therapeutic strategies, and outcomes were collected and analyzed by the same investigator (MGV) using a standardized form. The study was conducted with the approval of the institutional review board.
Control Patients
AOSD patients with myocarditis (AOSD+M) were compared with AOSD patients without myocarditis from our cohort (controls, n = 53).23
Literature Review
We conducted in PubMed (National Library of Medicine, Bethesda, MD) a computer-assisted search of publications in English and French from 1971 (when AOSD was first described) to September 2013, using the terms “Myocarditis” AND “Adult-onset Still disease” OR “Adult Still disease.” The reference lists of all the retrieved articles were also scanned for references not identified in the initial search; duplicate publications were then excluded.
All cases with no better differential diagnosis were included in the AOSD+M patient group. The data were summarized using a standardized form with sex, age, clinical and laboratory features of AOSD, time elapsed since the onset of the disease, clinical and diagnostic tests for myocarditis, treatment, and outcome.
Statistical Analysis
As the number of AOSD+M cases from our cohort was too small (4 patients), cases from the literature were added. A highly reliable statistical analysis could not be performed because of the high publication bias. For illustration only, a few relevant variables were tested using Fisher exact test for categorical variables or Kruskal-Wallis test for continuous variables. A p value < 0.05 was required for significance.
CASE REPORTS
We present here the main data on the 4 new cases of AOSD+M (Cases 1–4 in Table 1 and Table 2 ).
TABLE 1.
Characteristics of AOSD Patients With Myocarditis
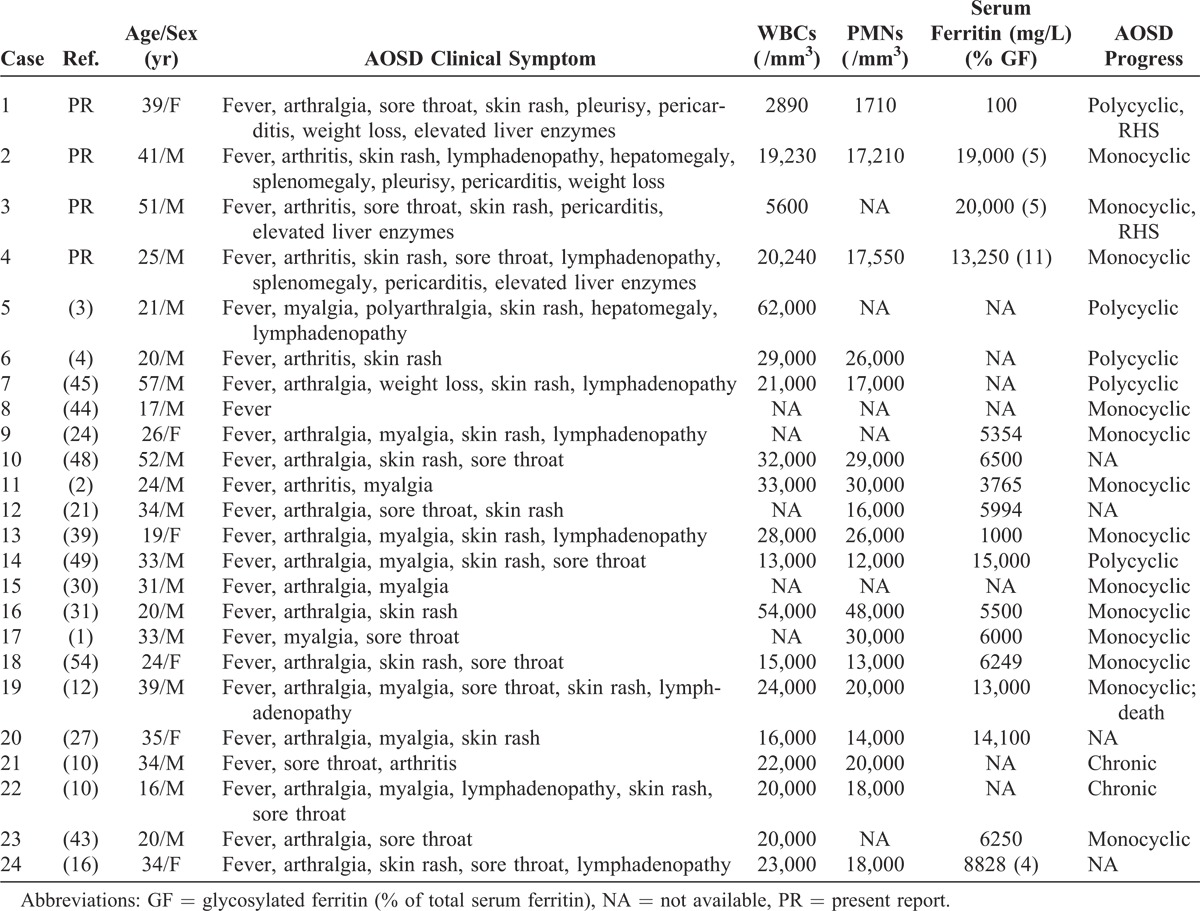
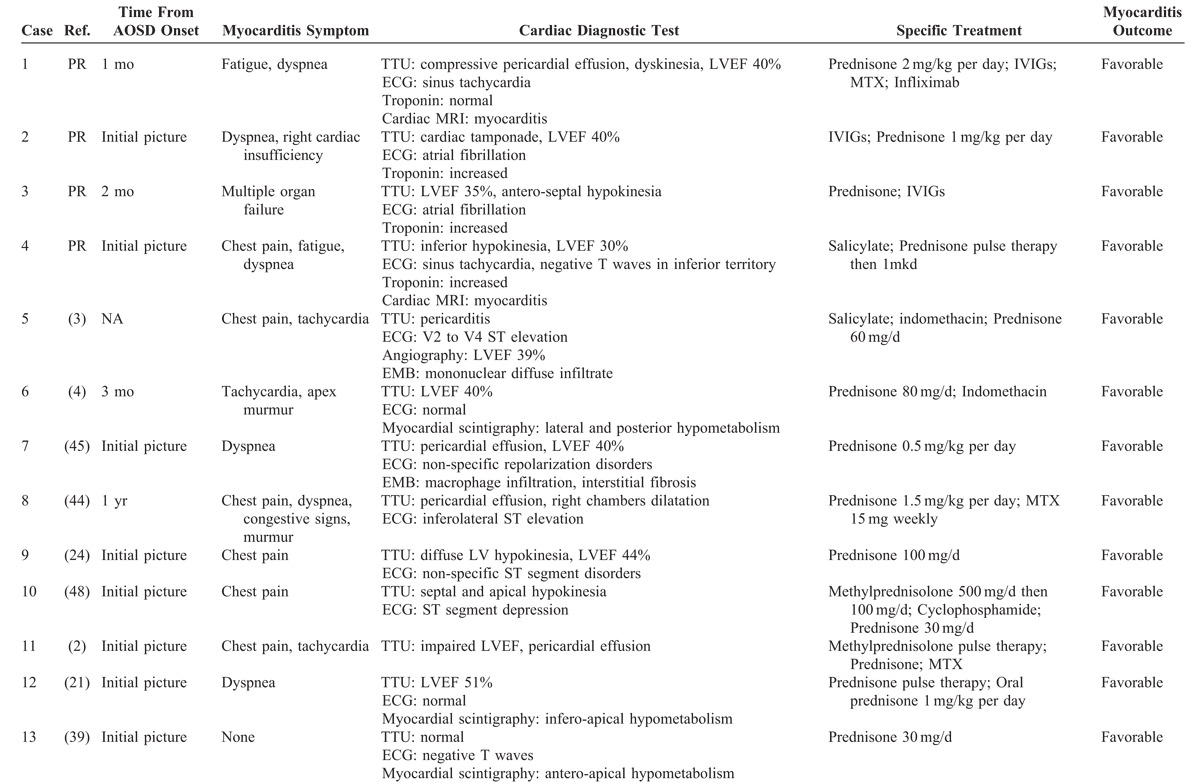
TABLE 2.
Characteristics of Myocarditis in AOSD Patients
Case 1
A 39-year-old woman without medical history was referred to our center in 2006 for recurrent fever of unknown origin, transient pink-salmon skin rash on the trunk, polyarthralgia, 3-kg weight loss, and mild thrombocytopenia (100,000/mm3) for 2 months. The other laboratory data were the following: C-reactive protein (CRP) 24 mg/dL (normal: <0.5 mg/dL), lymphocytes 540/mm3 (normal: 1500–4000/mm3), SF 300 mg/L (normal: <200 mg/L), liver cholestasis with a twofold increase of gamma-glutamyltransferase and alkaline phosphatases.
An infectious disease was ruled out. Rheumatoid factors, antinuclear antibodies, and antineutrophil cytoplasmic antibodies were not detected. Complement factors (C3, C4, and CH50) and serum proteins electrophoresis were normal. There was no abnormality on a thoraco-abdomino-pelvic computed tomography (CT) scan. A bone marrow biopsy was consistent with a reactive hemophagocytic syndrome (RHS).
The patient’s condition worsened when NYHA (New York Heart Association) class III dyspnea and sinus tachycardia occurred. The ECG and serum troponin were normal. A TTU showed a diffuse cardiomyopathy with left ventricle ejection fraction (LVEF) decreased to 40% and a compressive pericardial effusion. The patient was then transferred to an intensive care unit where 400 mL of exudative bloody fluid was drained, which contained no malignant cells. Finally, cardiac MRI findings were consistent with myocarditis.
Despite a mild increase in SF, the patient fulfilled the Yamaguchi and Fautrel classification criteria; thus, RHS and AOSD+M were diagnosed. Treatment with 2 mg/kg per day methylprednisolone resulted in important improvement. The patient was then switched to oral prednisone (1 mg/kg per day), and an angiotensin-converting-enzyme inhibitor (ACE-I) was added. By early 2007, the pericardial effusion regressed and the LVEF increased to 55%. Unfortunately, 3 other flares (pericarditis without myocarditis) occurred in 2007 and 2008 despite high doses of prednisone. Four treatments of IVIGs and methotrexate (30 mg weekly) were then used as steroid-sparing treatment and the TNF-α blocker infliximab was added; the patient had no other flares. On the last TTU, only mild dilated cardiomyopathy without impaired left ventricle function persisted.
Case 2
The patient was a 42-year-old man, a former heroin user receiving substitution treatment, with a history of cured hepatitis C and left ulnar osteitis diagnosed in 2003. He presented in June 2006 with recurrent fever (39°C), polyarthralgia, myalgia, and 10-kg weight loss. The laboratory findings were the following: CRP 25 mg/dL, WBC count 19,230/mm3 with 89% PMNs (that is, 17,210/mm3), elevated liver enzymes up to twice the upper normal limits, and SF 19,000 mg/L. The glycosylated fraction of ferritin was lower than 5%.
A thoraco-abdomino-pelvic CT scan showed small mediastinal lymph nodes (<1 cm in diameter). The bone marrow biopsy was consistent with an inflammatory reactive pattern without hemophagocytosis or lymphomatous infiltrate. An empirical antibiotic therapy did not improve the symptoms; many viral and bacterial infectious diseases were finally ruled out. The immunologic tests showed no signs of autoimmune disease. Because of increasing dyspnea and atrial fibrillation on ECG, a TTU was performed which found a right-chamber-compressing circumferential pericardial effusion. The patient underwent quickly a surgical pericardial drainage that removed 700 mL of a nonpurulent exudative and neutrophilic fluid with neither evidence of malignant cells nor tuberculosis.
A pericardial biopsy showed a slightly thickened pericardium with inflammatory infiltrates made of neutrophils, lymphocytes, and plasma cells suggesting a fibrinous pericarditis in its initial stage. Despite this and a broad-spectrum antibiotic therapy, the patient remained febrile. Findings on TTU worsened with the appearance of diffuse cardiomyopathy, and LVEF decreased to 40%. Increased troponin and creatine kinase levels led to a muscle biopsy that showed no myositis.
Finally, AOSD+M was diagnosed. Intravenous steroid pulse therapy was started together with a unique IVIGs cure. The patient became afebrile within 2 days and felt a significant clinical improvement, which persisted after shifting to oral steroids. ACE-I and metoprolol were also prescribed. After 18 months of follow-up, the progression was favorable despite tapering steroids dosage. The patient was then lost to follow-up. At last news, TTU showed persisting wall-motion abnormalities and impaired LVEF (50%) without evidence of either dilated cardiomyopathy or heart failure.
Case 3
A 51-year-old man with past alcohol abuse felt ill in November 2006 with sore throat, recurrent fever, sweat, fatigue, and 10-kg weight loss. At referral to hospital, he had polyarthritis (ankles, knees, elbows, and shoulders), spiking fever (39°C), bilateral conjunctivitis, and skin rash on the trunk and the limbs. Laboratory tests showed the following: WBC count 5600/mm3, anemia (hemoglobin 80 g/L), thrombocytopenia (platelets 50,000/mm3), elevated liver enzymes up to tenfold the upper normal limits, high erythrocyte sedimentation rate (85 mm/h), CRP (9 mg/dL), fibrinogen (6.56 g/L), and SF (8232 mg/L). Antinuclear antibodies were transiently positive but the Farr test and the search for rheumatoid factors were negative. The patient never fulfilled the diagnostic criteria for systemic lupus erythematous.
As in the previous 2 cases, all infectious causes were ruled out. The patient’s condition worsened in early January 2007. The platelet count decreased to 20,000/mm3 and a bone marrow aspiration showed hemophagocytosis. Then, severe RHS occurred including multiple organ failure with cardiogenic shock, acute respiratory failure, acute renal failure with interstitial nephritis, and lactic acidosis. Antibiotics (piperacillin-tazobactam and amikacin) were administered in the intensive care unit without improvement.
The TTU was consistent with myocarditis presenting with anterior and septal wall-motion abnormalities, as well as impaired LVEF (35%–40%). Cardiac troponin was increased to 15 nmol/mL. At that time, the laboratory findings were the following: serum creatinine 300 mmol/L, SF 20,000 mg/L, glycosylated ferritin 5%, CRP 28.7 mg/dL, proteinuria 0.60 g/24 h. Ultimately, the diagnosis of AOSD complicated by multiple organ failure was made and treatment with methylprednisolone and IVIGs was initiated, which resulted in important improvement. Over 7 years of follow-up, the patient did not have heart failure or dilated cardiomyopathy.
Case 4
The fourth patient was a 25-year-old man who presented to the emergency unit in 2011 with acute chest pain, high fever (40°C), and sore throat. His past medical history included Kawasaki disease in childhood. The initial clinical examination showed pharyngitis and crackles in the lung bases but no signs of cardiogenic shock. The ECG revealed sinus tachycardia with negative T waves in the inferior territory.
The first laboratory findings were as follows: WBC count 21,300/mm3, CRP 42.2 mg/dL, troponin I 9.5 mg/L (normal: <0.2 mg/L), and brain natriuretic peptide 208 ng/L (normal: <100 ng/L). TTU showed diffuse cardiomyopathy with LVEF decreased to 30% but a normal cardiac index. A cardiac MRI showed a high pericardial signal uptake. As viral myopericarditis was considered, the treatment included acetylsalicylic acid, colchicine, perindopril, and bisoprolol. The outcome was then favorable and troponin decreased. A few days later, fever reappeared with high spikes (40–41°C) in the evening, sore throat, monoarthritis of the left wrist, cervical lymphadenopathy, and skin rash. A second TTU was performed and was normal (LVEF 55%). Many blood tests were performed in search of an infectious cause for myopericarditis, but none was positive. Tests for antinuclear antibodies, rheumatoid factor, and antineutrophil cytoplasmic antibodies were also negative. A thoraco-abdomino-pelvic CT scan showed no abnormality. At that time, the WBC count was 21,000/mm3, PMNs 18,700/mm3, SF 13,750 mg/L, glycosylated ferritin 11%, and hepatic cytolysis appeared (transaminases increased fourfold). Thus, AOSD was diagnosed and prednisone added (1 mg/kg per day). After 2 years of follow-up and tapered steroid doses, withdrawal of prednisone did not affect the course of heart disease. No flare occurred during the follow-up. The last TTU was considered normal.
RESULTS
Main Characteristics of the Retrospective Cohort
Among the 57 AOSD patients of our series (27 men and 30 women), 43 (75%) were followed-up in an internal medicine department and 14 (25%) in a rheumatology department. The mean follow-up period was 8.4 years (median, 6 yr). The median age at AOSD diagnosis was 36 years (range, 16–75 yr). The initial symptoms at disease onset were fever (95%), weight loss (44%), arthralgia (95%), arthritis (46%), rash (77%), sore throat or pharyngitis (53%), lymphadenopathy (60%), splenomegaly (30%), hepatomegaly (21%), pleurisy (18%), pericarditis (19%), and myalgia (44%).
The abnormal laboratory data were leukocytosis with ≥80% PMNs (78%), increased CRP (98%), increased SF (82%), and abnormal liver function tests (66%). Glycosylated ferritin was ≤20% in 28 of 37 tested patients.
The clinical courses were monocyclic in 17 patients (30%), polycyclic in 25 (44%), and chronic in the remaining 15 (26%). The treatments prescribed were NSAIDs (28 patients, 21% of adverse events [AE]), steroids (51 patients, 75% AE and 45% steroid-dependence), IVIGs (23 patients, 4% AE), methotrexate (33 patients, 33% AE), TNF-α blockers (17 patients, 6% AE), anakinra (6 patients, no AE), and other treatments (10 patients). 23
Among these 57 patients, 4 had myocarditis. Thus, the prevalence of AOSD+M in our series was 7%.
Characteristics of Myocarditis in AOSD
The 9 main case series on AOSD totaled 713 AOSD patients but none described the occurrence of myocarditis.9,11,13,19,28,29,40,42,57 Twenty-three AOSD+M cases were reported in the literature, of which 20 were included in the present analysis. Three cases6,50,56 were excluded from our study because of insufficient data.
The main characteristics of the 20 included cases are displayed in Table 1 and Table 2 (Cases 5–24).1–4,10,16,21,24,27,30,31,39,43–45,48,49,54 These cases were 15 men (75%) and 5 women (25%). The mean age at myocarditis onset was 29 years (range, 16–57 yr). Regarding diagnostic certainty, they were 4 definite cases, 15 probable cases, and 1 possible case of myocarditis.
Pooling these 20 cases with the 4 above-described probable acute myocarditis cases totaled 24 cases from which we could extract some characteristics of myocarditis occurring during AOSD.
Myocarditis seems to be an early complication: in all but 5 cases (that is, 80%), it occurred during the first year of the disease course. Thirteen patients (54%) had myocarditis at the onset of the disease. Twenty-two patients (92%) were symptomatic; 14 had chest pain (58%), the remaining symptomatic patients presented with dyspnea and 7 (29%) had heart failure.
In 17 patients (71%), the ECG was abnormal: ST-segment or T-wave abnormalities in 13 and atrial fibrillation in 3. Troponin level was increased in 6 of 7 patients in whom it was assessed. TTU showed an impaired LVEF (≤50%) with localized or diffuse wall-motion abnormalities in 16 patients (67%). Cardiac MRI was performed in 4 patients; it showed features consistent with myocarditis. Finally, EMB was performed in 4 other patients; it showed interstitial inflammatory infiltrate with mononuclear cells and interstitial fibrosis in 2 patients.
The long-term prognosis, and specifically the development of dilated cardiomyopathy, is unknown. After a median of 5.5 years of follow-up (range, 1–7 yr), none of the 4 aforementioned patients with myocarditis developed clinically apparent heart failure, although Case 1 did develop a mildly dilated left ventricle.
Comparison Between AOSD+M and Control Patients
The comparison between the 24 AOSD+M patients (current study cohort and literature cases) and the 53 AOSD control patients (current study cohort) is shown in Table 3. First, AOSD+M patients were younger than the controls and AOSD occurred 5.2 years (median) earlier in the former group. Most AOSD+M patients were male (18/24, 75%) with a male to female sex ratio of 3. The symptoms at the clinical onset of AOSD were not different; AOSD+M patients exhibited fever (100%), joint symptoms (96%), skin rash (75%), and sore throat (54%). However, splenomegaly (8% vs 28%) and arthritis (25% vs 42%) were less frequent in the AOSD+M group than in controls. As expected, pericarditis or pericardial effusion on TTU were much more prevalent in the AOSD+M group than in the controls (54% vs 13%).
TABLE 2 (Continued).
Characteristics of Myocarditis in AOSD Patients
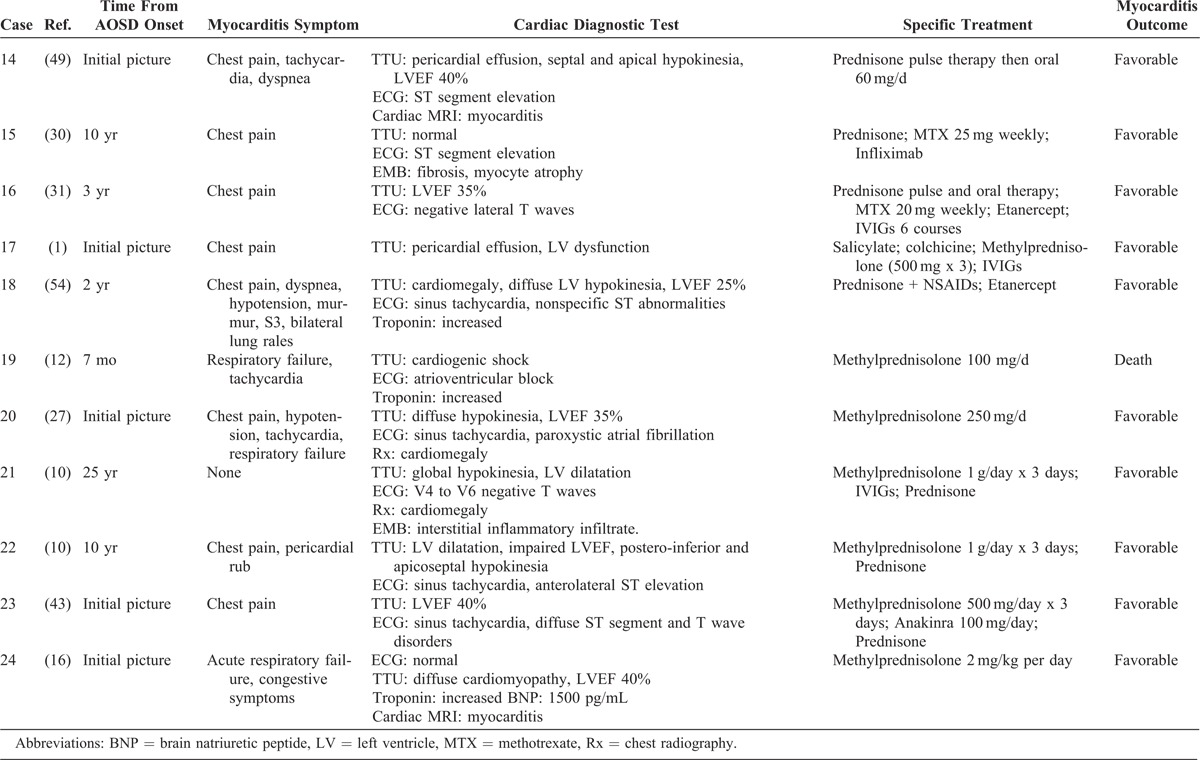
TABLE 3.
Characteristics at Time of Diagnosis of AOSD With and Without Myocarditis
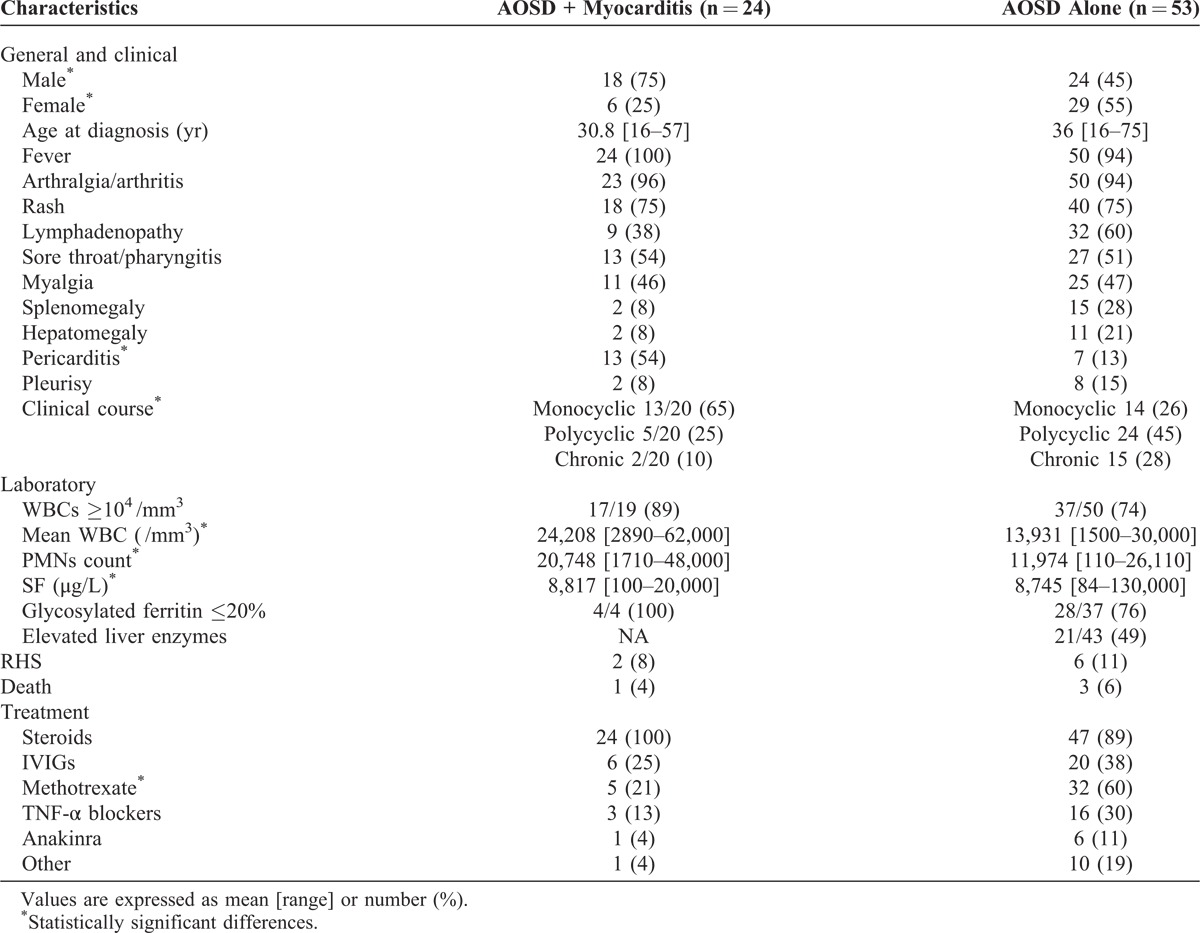
Regarding laboratory tests, WBC and PMN counts were higher in the AOSD+M group than in the controls. The difference between the SF levels was not clinically relevant. In the 4 subjects in whom it was assessed, glycosylated ferritin was <20% of total SF. Data on liver involvement in the literature cases were scarce. In the 4 AOSD+M patients from our series, the liver function tests were disturbed with mild (Case 1) to severe (Case 3) enzyme elevation.
Surprisingly, the medical literature mentioned no other cases of RHS than the 2 we described (except 1 probable case of RHS12). Thus, myocarditis was not significantly more frequent in the context of RHS.
Despite the lack of data on short follow-up periods in the literature cases, most AOSD+M patients (18/20, 90%) had a systemic (monocyclic or polycyclic) pattern compared with only 72% (38/53 patients) in the control group.
The treatment of AOSD+M cases included, in all 24 patients, corticosteroids as first- or second-line therapy. Twelve patients (50%) did not require other supportive drugs than salicylate or nonsteroidal antiinflammatory drugs, which is close to the nonmyocarditis group (22/53 patients, 42% who did not receive a third-line therapy23). IVIGs were prescribed for 6 AOSD+M patients (25%) vs 20 (38%) in the control group. In 4 of these 6 patients, IVIGs were sufficient to control the disease (there was no need for another second-line treatment). Methotrexate was given to 5 AOSD+M patients (21%) vs 32 patients (60%) in the control group but was effective in only 2. TNF-α blockers (infliximab and etanercept) were prescribed to 3 AOSD+M patients (13%) vs 16 patients (30%) in the control group and were effective in all 3. Anakinra was successfully administered in 1 AOSD+M patient (vs 6 control patients, 11%). Cyclophosphamide was also administrated to 1 AOSD+M patient but to none of the controls.
Following this treatment and supportive care, the short-term prognosis of myocarditis was good in all but 1 patient (Case 19), who died from cardiogenic shock the day after the occurrence of a complete atrioventricular block and despite the implantation of a temporary pacemaker.12 Other severe complications (2 cases of RHS and 1 case of multiple organ failure) did not lead to death.
DISCUSSION
Despite being a life-threatening condition,12 myocarditis is probably the least described visceral involvement in AOSD53; its prevalence is rarely reported even in the largest case series. The present study reports on the first case series of myocarditis among unselected AOSD patients.
The prevalence of AOSD has been estimated at less than 1 case per 100,000 persons,33 and pericarditis is seen in about 20% of AOSD cases.13,19,23,42 According to our data, myocarditis is less common; here, its prevalence in AOSD is about 7%.
The clinical picture of myocarditis in AOSD does not seem to differ from that of myocarditis of other origins. The presence of a majority of male patients has been already noted in myocarditis of different origins7,34 but seems much more marked in AOSD. In comparison with other myocarditis cases, myocarditis in AOSD may be equally asymptomatic (8%) but chest pain seems more frequent (58% vs 32%)25; this is probably due to the high prevalence of associated pericarditis. As in other myocarditis cases, the most common findings on ECG were sinus tachycardia and nonspecific ST-segment and T-wave abnormalities; however, in AOSD+M, 29% of ECGs were normal.14 None of the AOSD patients exhibited severe arrhythmia as reported in other immune myocarditis cases such as giant-cell or sarcoidosis myocarditis.55 Cardiac troponin was assessed in a few literature cases; it cannot thus be reliably analyzed. Nevertheless, Smith et al47 have found that troponin I has a very limited sensitivity (34%) in the diagnosis of myocarditis; it only helps to confirm the diagnosis (specificity, 89%). The findings on TTU were consistent with acute myocarditis but not with fulminant myocarditis because smaller left ventricular chamber size and increased wall thickness were never reported.20 Moreover, TTU was shown to have a prognostic value in non-AOSD myocarditis; the loss of right ventricular function was shown to be the most powerful predictor of adverse outcome.38 As most literature cases of myocarditis are not recent, only 4 patients had cardiac-MRI-confirmed myocarditis. Currently, the place of cardiac MRI is growing because a combination of T2-weighted MRI and post-gadolinium early and late T1-weighted MRI have shown good sensitivity (67%) and specificity (91%) in diagnosing myocarditis22 as well as myocardial involvement in rheumatic and autoimmune diseases.35
As half of myocarditis cases occurred at the onset of AOSD, AOSD diagnosis may be suggested in case of feverish myocarditis or heart failure after ruling out infectious diseases, toxic causes (alcohol, radiation, antineoplastic drugs such as anthracyclines), and immunologic conditions (drug-induced hypersensitivity, eosinophilia, giant-cell myocarditis, sarcoidosis myocarditis, or myocarditis in connective tissue disease).14 In such cases, systemic symptoms, high WBC and PMN counts, high SF with collapsed glycosylated fraction, and steroid sensitivity should enhance the diagnostic probability.
AOSD+M patients were more often male (75% vs 45% in uncomplicated AOSD); here and in other case series, males represented 30%9 to 55%42 of the AOSD population. AOSD+M patients seem younger too and exhibit important local (association with pericarditis) and systemic inflammatory patterns of the disease with higher WBC counts, PMN counts, and serum ferritin levels. Arthritis, at AOSD onset, has been associated with a chronic outcome.23 Here, arthritis was less frequent in the AOSD+M group than in the controls (25% vs 42%); this reinforces the idea that myocarditis is a complication of the systemic patterns of AOSD.
The nonspecific treatment of acute myocarditis in AOSD should not differ from that recommended by the current guidelines.26,32,36 A neurohormonal blockade with an ACE-I or angiotensin-receptor blockers, a β-adrenergic blocker, and refraining from intensive physical activities for a few months after the disease onset are recommended until ventricular recovery has been documented by noninvasive imaging. Life-threatening complications such as ventricular arrhythmias and high-degree heart block might be monitored, and implantable cardiac defibrillator or temporary pacemakers considered early.46
According to our data, steroids constituted the first-line specific treatment in AOSD+M patients since they were very often effective and sufficient (with no need for a next-line treatment) in the acute phase of AOSD+M. Thus, steroids should not be delayed in case of high diagnostic probability. As shown in a previous study, adverse events with steroids are common in AOSD (75%); a steroid-sparing treatment such as methotrexate should be initiated soon after myocarditis control.23
Although IVIGs were comparatively less prescribed in AOSD+M than in AOSD alone, they could be of interest as a second-intention emergency treatment because they were shown effective in acute pediatric lymphocytic myocarditis15 and have led to marked improvements in left ventricular performance in adults.51 Furthermore, the immunosuppressive risk of this treatment is lower than that of other second-line treatments, which is an advantage in acute febrile myocarditis onsets in which an infectious cause has not been definitely ruled out. In fact, in the Intervention for Myocarditis and Acute Cardiomyopathy trial, there were no significant differences between the IVIG group and the placebo group.37 Another restriction is the risk of acute heart failure due to overload induced by IVIGs. However, in our case series, IVIGs were safe in patients with heart disease during AOSD. Thus, IVIGs are probably of interest in selected patients with myocarditis of immunologic origin as in AOSD. Unsurprisingly, methotrexate was less prescribed in AOSD+M than in AOSD alone probably because of its slow onset of action.
TNF-α blockers were effective in the 3 patients in whom they were administrated. According to published data, TNF-α blockers would induce more partial remissions than complete remissions and would be much more valuable in chronic articular AOSD than in systemic AOSD possibly complicated by myocarditis.18,41
Finally, it is probable that recent biological treatments such IL-1 receptor antagonists and IL-6 inhibitors are of interest in refractory AOSD+M occurring in the systemic pattern. IL-1 receptor antagonists would be valuable targeted therapies because of their speed of action and effectiveness in autoinflammatory syndromes,41 but no data currently are available.
According to the last case series,23 the prognosis of AOSD remains good. The occurrence of myocarditis is life-threatening but the risk of death did not seem to be increased in AOSD+M (1 death) compared with AOSD alone (3 deaths). However, the former death was directly attributable to the disease (heart block in a patient who probably had fulminant but not proven RHS despite a 1 mg/kg per day steroid therapy12), whereas the latter 3 deaths were not (1 myocardial infarction, 1 accidental death, and 1 complication of lymphoma treatment). Furthermore, AOSD myocarditis fulfills the criteria of good-prognosis myocarditis as described by Cooper in 2009: acute myocarditis evolving from less than 2 weeks with pathologic correlates consistent with active lymphocytic myocarditis.14 So, as in myocarditis from other causes, the vast majority of patients with myocarditis do not die from myocarditis. The long-term outcomes, especially the risk of chronic heart failure and dilated cardiomyopathy, cannot be estimated on short-term follow-ups such as those of the literature cases.
The pathophysiology of myocarditis in AOSD remains unclear. However, 2 mechanisms could be involved: an AOSD-specific myocarditis and a nonspecific myocardial involvement during a cytokine storm. On the one hand, data collected from the 4 EMBs performed were consistent with those of Zhao et al56 showing an active lymphocytic myocarditis at the acute phase, which may lead, in the chronic phase, to irregular fibrosis and atrophy. This argues for an inflammatory mechanism linked to the AOSD. On the other hand, cardiac failure occurring mostly in RHS-complicated AOSD—which is probably underreported in this study—may be secondary to a myocardial stunning in the context of a severe systemic inflammatory response syndrome as described in severe sepsis. MRI assessment of myocardial involvement could lead to a better understanding of these mechanisms.
The present retrospective study has many biases. The major one is the analysis of a group of individually reported AOSD+M cases (a publication bias). However, there are no other unselected myocarditis cases from AOSD retrospective cohorts, and a comparison using only 4 cases would have been impossible; the addition of reported AOSD+M cases was a practical solution but one that did not allow advanced statistical tests.
Conclusion
The current study presents 4 unselected original cases of myocarditis in AOSD and a literature review on this topic. Including these 4 cases, there are now 24 easily accessible reported cases.
Myocarditis was present at AOSD onset in half the cases; given its severity, cardiologists should be aware of its occurrence and risks (normal cardiac enzymes and normal ECG do not rule out myocarditis in the context of AOSD). A TTU should be rapidly considered. Cardiac MRI is probably a useful noninvasive diagnostic tool to confirm myocarditis without resorting to EMB in this condition.
In the current study we found that compared with patients with AOSD alone, AOSD+M patients were younger, more often male, and more often had pericarditis and higher WBC counts, PMN counts, and SF levels.
Specific treatments such as steroids and IVIGs seem effective. As in other myocarditis cases, close cardiac monitoring should be established to detect heart blocks or ventricular arrhythmias and prompt specific management. Further studies are needed to assess the long-term outcomes of myocarditis in AOSD.
Footnotes
Abbreviations: ACE-I = angiotensin-converting enzyme inhibitor, AE = adverse events, AOSD = adult-onset Still disease, AOSD+M = AOSD+MAOSD with myocarditis, CRP = C-reactive protein, CT = computed tomography, ECG = electrocardiogram, EMB = endomyocardial biopsy, IL = interleukin, IVIGs = intravenous immunoglobulins, LVEF = left ventricle ejection fraction, MRI = magnetic resonance imaging, NSAIDs = nonsteroidal antiinflammatory drugs, NYHA = New York Heart Association, PMNs = polymorphonuclear cells, RHS = reactive hemophagocytic syndrome, SF = serum ferritin, TNF-α = TNF-αtumor necrosis factor-alpha, TTU = transthoracic ultrasonography.
Financial support and conflicts of interest: No funding source or sponsor has been involved in this study. PS has received consulting fees in the past from Pfizer, LFB, and GlaxoSmithKline. AH has received consulting fees in the past from LFB and Roche. The other authors have no conflicts of interest to disclose.
References
- 1.Audia S, Vinit J, Leguy V, et al. [Myopericarditis initiating an adult-onset Still’s disease]. Rev Med Interne. 2008;Suppl 1:S125. [Google Scholar]
- 2.Ballard M, Chazerain P, Charpentier J, et al. [Severe myocarditis in an adult-onset Still’s disease]. Rev Med Interne. 2002;23Suppl 5:S653. [Google Scholar]
- 3.Bank I, Marboe CC, Redberg RF, et al. Myocarditis in adult Still’s disease. Arthritis Rheum. 1985;28:452–454. [DOI] [PubMed] [Google Scholar]
- 4.Bergemer AM, Fouquet B, Goupille P, et al. [Myocarditis in Still’s disease in adults]. Rev Rhum Mal Osteo-Articul. 1988;55:945–948. [PubMed] [Google Scholar]
- 5.Bouhour JB, Haddak M, Lefèvre M. [Fatal form of pericardo-myocarditis in Wissler-Fanconi syndrome]. Arch Mal Coeur Vaiss. 1986;79:741–744. [PubMed] [Google Scholar]
- 6.Bywaters EG. Still’s disease in the adult. Ann Rheum Dis. 1971;30:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caforio ALP, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. [DOI] [PubMed] [Google Scholar]
- 8.Caforio AL, Pankuweit S, Arbustini E, et al. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. [DOI] [PubMed] [Google Scholar]
- 9.Cagatay Y, Gul A, Cagatay A, et al. Adult-onset Still’s disease. Int J Clin Pract. 2009;63:1050–1055. [DOI] [PubMed] [Google Scholar]
- 10.Cavallasca JA, Vigliano CA, Perandones CE, et al. Myocarditis as a form of relapse in two patients with adult Still’s disease. Rheumatol Int. 2010;30:1095–1097. [DOI] [PubMed] [Google Scholar]
- 11.Chen PD, Yu SL, Chen S, et al. Retrospective study of 61 patients with adult-onset Still’s disease admitted with fever of unknown origin in China. Clin Rheumatol. 2012;31:175–181. [DOI] [PubMed] [Google Scholar]
- 12.Colina M, Govoni M, Trotta F. Fatal myocarditis in adult-onset Still disease with diffuse intravascular coagulation. Rheumatol Int. 2009;29:1355–1357. [DOI] [PubMed] [Google Scholar]
- 13.Colina M, Zucchini W, Ciancio G, et al. The evolution of adult-onset Still disease: an observational and comparative study in a cohort of 76 Italian patients. Semin. Arthritis Rheum. 2011;41:279–285. [DOI] [PubMed] [Google Scholar]
- 14.Cooper LT Jr. Myocarditis. N Engl J Med Engl Med. 2009;360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drucker NA, Colan SD, Lewis AB, et al. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation. 1994;89:252–257. [DOI] [PubMed] [Google Scholar]
- 16.Duburcq T, Delannoy PY, Sivova N, et al. [Myocarditis revealing an adult-onset Still’s disease]. Ann Fr Anesth Reanimation. 2013;32:50–52. [DOI] [PubMed] [Google Scholar]
- 17.Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still’s disease. Ann Rheum Dis. 2006;65:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fautrel B, Sibilia J, Mariette X, et al. Club Rhumatismes et Inflammation. Tumour necrosis factor alpha blocking agents in refractory adult Still’s disease: an observational study of 20 cases. Ann Rheum Dis. 2005;64:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fautrel B, Zing E, Golmard JL, et al. Proposal for a new set of classification criteria for adult-onset still disease. Medicine (Baltimore). 2002;81:194–200. [DOI] [PubMed] [Google Scholar]
- 20.Felker GM, Boehmer JP, Hruban RH, et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. [DOI] [PubMed] [Google Scholar]
- 21.Fontaine C, Millot O, Reny J, et al. [Dilated cardiomyopathy with normal creatin kinase in an adult-onset Still’s disease]. Rev Med Interne. 2003;24Suppl 1:S116. [Google Scholar]
- 22.Friedrich MG, Sechtem U, Schulz-Menger J, et al. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerfaud-Valentin M, Maucort-Boulch D, Hot A, et al. Adult-onset Still disease: manifestations, treatments, outcome, and prognostic factors in 57 patients. Medicine (Baltimore). 2014;93:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosaka S, Takashina N, Ishikawa A, et al. Adult Still’s disease with myocarditis and peritonitis. Intern Med Tokyo Jpn. 1992;31:812–815. [DOI] [PubMed] [Google Scholar]
- 25.Hufnagel G, Pankuweit S, Richter A, et al. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz. 2000;25:279–285. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology Foundation, and American Heart Association. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. J Am Coll Cardiol. 2009;53:e1-90. [DOI] [PubMed] [Google Scholar]
- 27.Jadhav P, Nanayakkara N. Myocarditis in adult onset Stills disease. Int J Rheum Dis. 2009;12:272–274. [DOI] [PubMed] [Google Scholar]
- 28.Kim YL, Koo BS, Kim YG, et al. Clinical features and prognosis in 82 patients with adult-onset Still’s disease. Clin Exp Rheumatol. 2014;32:28–33. Epub 2013 Sep 18. [PubMed] [Google Scholar]
- 29.Kong XD, Xu D, Zhang W, et al. Clinical features and prognosis in adult-onset Still’s disease: a study of 104 cases. Clin Rheumatol. 2010;29:1015–1019. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen LE, Bartosik I. Myocarditis in adult-onset Still’s disease despite significant immunosuppressive therapy. Scand J Rheumatol. 2006;35:330–331. [DOI] [PubMed] [Google Scholar]
- 31.Kuek A, Weerakoon A, Ahmed K, et al. Adult-onset Still’s disease and myocarditis: successful treatment with intravenous immunoglobulin and maintenance of remission with etanercept. Rheumatology (Oxford). 2007;46:1043–1044. [DOI] [PubMed] [Google Scholar]
- 32.Lindenfeld J, Albert NM, Boehmer JP, et al. Heart Failure Society of America. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–194. [DOI] [PubMed] [Google Scholar]
- 33.Magadur-Joly G, Billaud E, Barrier JH, et al. Epidemiology of adult Still’s disease: estimate of the incidence by a retrospective study in west France. Ann Rheum Dis. 1995;54:587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnani JW, Danik HJS, Dec GW, Jr, et al. Survival in biopsy-proven myocarditis: a long-term retrospective analysis of the histopathologic, clinical, and hemodynamic predictors. Am Heart J. 2006;151:463–470. [DOI] [PubMed] [Google Scholar]
- 35.Mavrogeni S, Vassilopoulos D. Is there a place for cardiovascular magnetic resonance imaging in the evaluation of cardiovascular involvement in rheumatic diseases? Semin. Arthritis Rheum. 2011;41:488–496. [DOI] [PubMed] [Google Scholar]
- 36.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 37.McNamara DM, Holubkov R, Starling RC, et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. [DOI] [PubMed] [Google Scholar]
- 38.Mendes LA, Dec GW, Picard MH, et al. Right ventricular dysfunction: an independent predictor of adverse outcome in patients with myocarditis. Am Heart J. 1994;128:301–307. [DOI] [PubMed] [Google Scholar]
- 39.Nishimagi E, Hirata S, Kawaguchi Y, et al. Myocardial dysfunction in a patient with adult-onset Still’s disease (AOSD). Clin Exp Rheumatol. 2004;22:506–507. [PubMed] [Google Scholar]
- 40.Pay S, Turkcapar N, Kalyoncu M, et al. Ankara Rheumatology Study Group. A multicenter study of patients with adult-onset Still’s disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol. 2006;25:639–644. [DOI] [PubMed] [Google Scholar]
- 41.Pouchot J, Arlet JB. Biological treatment in adult-onset Still’s disease. Best Pract Res. Clin Rheumatol. 2012;26:477–487. [DOI] [PubMed] [Google Scholar]
- 42.Pouchot J, Sampalis JS, Beaudet F, et al. Adult Still’s disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore). 1991;70:118–136. [PubMed] [Google Scholar]
- 43.Raffeiner B, Botsios C, Dinarello C, et al. Adult-onset Still’s disease with myocarditis successfully treated with the interleukin-1 receptor antagonist anakinra. Jt Bone Spine Rev Rhum. 2011;78:100–101. [DOI] [PubMed] [Google Scholar]
- 44.Roblot P, Boiffard O, Allal J, et al. [Myocarditis in adult-onset Still’s disease: one case]. Rev Med Interne. 1990;11Suppl 1:S368. [Google Scholar]
- 45.Sachs RN, Talvard O, Lanfranchi J. Myocarditis in adult Still’s disease. Int J Cardiol. 1990;27:377–380. [DOI] [PubMed] [Google Scholar]
- 46.Sagar S, Liu PP, Cooper LT Jr. Myocarditis. Lancet. 2012;379:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SC, Ladenson JH, Mason JW, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95:163–168. [PubMed] [Google Scholar]
- 48.Ueda T, Mizushige K, Sakamoto S, et al. Adult Still’s disease with myocardial dysfunction induced by microangiopathy. Jpn Circ J. 1997;61:74–77. [DOI] [PubMed] [Google Scholar]
- 49.Vandergheynst F, Gosset J, van de Borne P, et al. Myopericarditis revealing adult-onset Still’s disease. Acta Clin Belg. 2005;60:205–208. [DOI] [PubMed] [Google Scholar]
- 50.Ward SC, Wiselka MJ, Nicholson KG. Still’s disease and myocarditis associated with recent mumps infection. Postgrad Med J. 1988;64:693–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, et al. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. [DOI] [PubMed] [Google Scholar]
- 52.Wouters JM, van de Putte LB. Adult-onset Still’s disease; clinical and laboratory features, treatment and progress of 45 cases. Q J Med. 1986;61:1055–1065. [PubMed] [Google Scholar]
- 53.Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424–430. [PubMed] [Google Scholar]
- 54.Yang DH, Chang DM, Lai JH, et al. Etanercept as a rescue agent in patient with adult onset Still’s disease complicated with congestive heart failure. Rheumatol Int. 2008;29:95–98. [DOI] [PubMed] [Google Scholar]
- 55.Yazaki Y, Isobe M, Hiramitsu S, et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:537–540. [DOI] [PubMed] [Google Scholar]
- 56.Zhao DB, Dai SM, Liu XP, et al. Interstitial inflammation in visceral organs is a pathologic feature of adult-onset Still’s disease. Rheumatol Int. 2011;31:923–927. [DOI] [PubMed] [Google Scholar]
- 57.Zhu G, Liu G, Liu Y, et al. Liver abnormalities in adult onset Still’s disease: a retrospective study of 77 Chinese patients. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis. 2009;15:284–288. [DOI] [PubMed] [Google Scholar]


