Abstract
Obstructive sleep apnea (OSA) is a common disorder, characterized by cyclic cessation of airflow for 10 seconds or more. There is growing awareness that OSA is related to the development and progression of cardiovascular disease. However, only a few studies have associated OSA directly to major cardiovascular events. The aim of this study was to evaluate the relationship between OSA and cardiovascular morbidity in a well defined population of patients.
The electronic database of the central district of a major health management organization was searched for all patients diagnosed with OSA in 2002–2010. For each patient identified, an age- and sex-matched patient was randomly selected from the members of the same health management organization who did not have OSA. Data on demographics, socioeconomic status, and relevant medical parameters were collected as well.
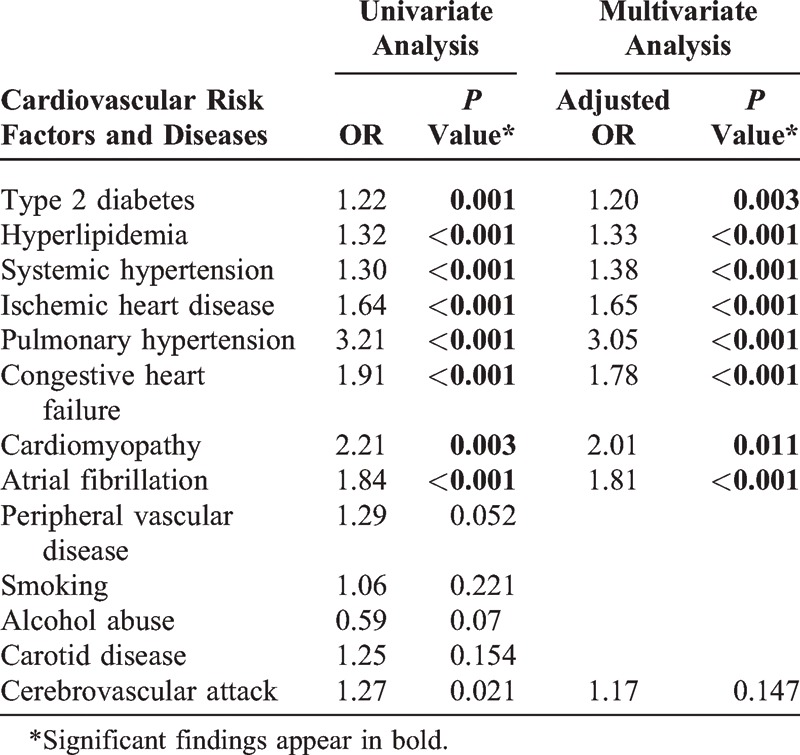
The study population included 2797 patients, average age 58.1, in which 76.6% were males. There was a significant correlation between OSA and the presence of ischemic heart disease (P < 0.001), pulmonary hypertension (P < 0.001), congestive heart failure (P < 0.001), cardiomyopathy (P = 0.003), and arrhythmia (P < 0.001). OSA was also significantly correlated with low socioeconomic status (P < 0.001).
OSA and cardiovascular disease were strongly correlated. As such, early diagnosis and treatment of OSA may change the course of both diseases. We suggest that sleep disordered breathing should be routinely assessed in patients with cardiovascular problems. An ear–nose–throat evaluation may also be important to rule out anatomic disorders that cause upper airway obstruction.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common medical disorder, characterized by cyclic collapse of the upper airway during sleep, leading to partial or complete cessation of airflow for 10 seconds or more. The diagnosis is based on a value of 5 or more per hour on the apnea–hypopnea index, usually accompanied by a 4% decrease in oxygen desaturation.1 OSA affects 4–24% of men and 2–9% of women in the United States. It is estimated that 20% of middle-aged adults have at least mild OSA2 and 80% of cases of OSA remain undiagnosed.3
There is growing awareness that OSA is related to the development and progression of cardiovascular diseases.4 However, despite the wealth of pathophysiologic data linking OSA to cardiovascular risk factors, atrial fibrillation, and hypertension,5–9 only a few studies have associated OSA directly to major cardiovascular events, such as myocardial infarction, stroke, and cardiovascular death, and none of them was population-based. The aim of the present epidemiologic study was to evaluate the relationship between OSA and cardiovascular morbidity in a directly defined population of patients.
MATERIALS AND METHODS
The Clalit Health Services is the largest of 4 health maintenance organizations in Israel (4.2 million insured members nationwide). It maintains a chronic-disease registry that includes information from a variety of sources: primary-care physicians, pharmacy claims, laboratory tests, hospitals, and outpatient clinics. In the present study, we searched the electronic database of the Central District of Clalit Health Services for all patients diagnosed with OSA from 2002 to 2010. Patients were identified by crossing data of having a polysomnography test and then receiving the diagnosis of OSA by the caring physician. For each patient identified, we randomly selected another patient matched for age and sex from among the members of the same health management organization during the same period who did not have OSA. The individual files were further reviewed for body mass index and socioeconomic status (SES), and risk factors for cardiovascular disease, and for the presence of other cardiovascular morbidities. Low SES was defined as an exemption from paying social security tax.
The main outcome measure was the prevalence of cardiovascular disorders in patients with OSA compared to controls. The study was approved by the institutional review board.
Statistical Analysis
Student t-test was used to compare continuous variables between groups, and χ2 test was used for proportions (STATA 8.0 statistical software, Stata Corp., College Station, TX). To account for repeated testing, only probabilities of <0.01 (1%) were considered statistically significant. A logistic regression analysis was performed to adjust for the simultaneous effects of the various OSA risk factors for diseases that were significant in the univariate analysis. Adjustments were made for age, sex, and SES.
RESULTS
The study population included 2797 patients diagnosed with OSA during the study period and 2791 sex- and age-matched controls. Table 1 summarizes the demographic characteristics of the 2 groups. A significant association was found between low SES and OSA (P < 0.001). Table 2 shows the prevalence rates and odds ratios for various systemic conditions in the 2 groups. There was a significant correlation between OSA and cardiovascular risk factors such as systemic hypertension (P < 0.001), hyperlipidemia (P < 0.001), and type 2 diabetes mellitus (P = 0.001), and between OSA and several cardiovascular disorders, namely, ischemic heart disease (P < 0.001), pulmonary hypertension (P < 0.001), congestive heart failure (P < 0.001), cardiomyopathy (P = 0.011), and atrial fibrillation (P < 0.001).
TABLE 1.
Demographics in Patients With OSA and Controls∗
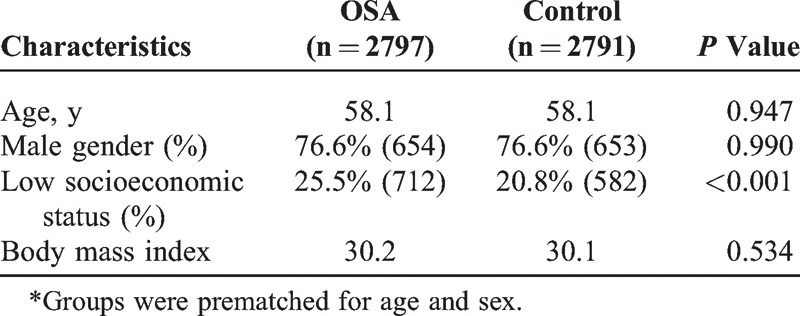
TABLE 2.
Odds Ratios and Adjusted Odds Ratio for Cardiovascular Risk Factors and Diseases Between Patients With OSA and Controls
DISCUSSION
The typical patient with OSA may experience anywhere from 5 to a few dozen episodes of apnea or hypopnea per hour of sleep. The recurrent respiratory events produce an intermittent hypoxia and hypercapnia, often accompanied by loud snoring, which cause brief arousals and marked sleep fragmentation, with diminished amounts of slow-wave and rapid-eye-movement sleep.10 Patients are usually unaware of these disruptions, but the changes in sleep architecture contribute significantly to prominent and chronic daytime sleepiness. There may be no detectable respiratory abnormality during wakefulness.11,12
The underlying pathophysiology of OSA is complex and not fully understood. It is generally accepted that the stability and patency of the upper airway are dependent upon the action of the oropharyngeal dilator and abductor muscles, which are normally activated in a rhythmic fashion during each inspiration.13 The upper airway is subject to collapse when the force produced by these muscles, for a given cross-sectional area, is exceeded by the negative airway pressure generated by the inspiratory activity of the diaphragm and intercostal muscles. Upper airway obstruction can occur if the suction pressure is too high or the counteracting forces of the muscles dilating the upper airway are too weak for any given suction pressure.14 Factors that contribute to or promote upper airway obstruction include anatomical narrowing of the upper airway, excessive loss of upper-airway muscle tone, and defective upper-airway protective reflexes. Accordingly, the relatively high rate of OSA in obese individuals15 may be attributable to fat deposits in the pharyngeal walls. The peripharyngeal soft tissue may increase the external pressure on the pharyngeal walls and enhance their collapsibility.
Studies suggest that the respiratory effort and negative intrathoracic pressure typical of OSA may cause a reduction in venous return. There also appears to be resemblance between intermittent hypoxia and the ischemic reperfusion state in terms of the oxidative stress to the heart.16 Thus, OSA may constitute a significant risk factor for the development of cardiovascular disease, even in otherwise healthy individuals, or for the progression of preexisting cardiovascular disease.16 The mechanical, autonomic, and oxidative stresses imposed by sleep apnea can aggravate myocardial ischemia, cause remodeling of the heart, and produce arrhythmias.
The relationship between heart failure and OSA may also be bidirectional. A high rate of OSA has been noted in nonobese patients with heart failure and stroke, relative to the general population.17,18 Peripharyngeal and nasal fluid accumulation may cause narrowing of the upper airway and increased upper-airway resistance.19
The present large epidemiologic cohort study examined the relationship of OSA with cardiovascular disease. The results revealed a strong correlation between OSA and such cardiovascular risk factors as type 2 diabetes, hyperlipidemia, and systemic hypertension, and between OSA and several cardiovascular disorders, namely, ischemic heart disease, pulmonary hypertension, congestive heart failure, cardiomyopathy, and arrhythmia (Table 2).
An independent relationship among type 2 diabetes, hyperlipidemia, and systemic hypertension has not yet been proven. However, systemic hypertension has been reported in approximately 35% of patients with OSA, and several studies found that drug-resistant hypertension is often caused by OSA and may be partly alleviated by treatment of OSA. Furthermore, in experimental studies, the induction of intermittent hypoxia or OSA caused daytime hypertension in rats and dogs.
The association of OSA with ischemic heart disease probably involves hypoxia-induced stimulation of the carotid chemoreceptors, leading to sympathetic nerve activation. This is followed by an increase in sympathetic outflow, with a surge in blood pressure, vasoconstriction, platelet activation, increased fibrinogen level, decreased fibrinolytic activity, and hypercoagulable state—all factors that may cause or aggravate ischemic heart disease. Accordingly, Shahar et al demonstrated a relationship between sleep-disordered breathing and coronary artery disease.
Pathological changes such as medial hypertrophy and tunica intima proliferation have been reported in the distal pulmonary arteries of patients with OSA. These changes cause major increments in pulmonary artery resistance and considerably impede blood flow through the lungs. The characteristics of these pathological processes include hypoxic vasoconstriction, capillary loss, and inflammation. Compensatory changes in the right ventricle lead to ventricular remodeling and hypertrophy. Left untreated, pulmonary hypertension may lead to death within 3 years of onset.
Studies found that the presence of OSA was independently associated with a 2.38-fold increased risk of heart failure and that the severity of OSA was a significant predictor of heart failure in men. OSA has been associated with increased mortality in patients with ischemic heart failure, which was mainly attributable to an excess rate of fatal arrhythmias. At the same time, fluid retention induced by heart failure may contribute to tissue bulking and exacerbate sleep apnea.
OSA may cause or aggravate arrhythmias for several reasons. During each episode of apnea, there is a rise in intrathoracic pressure, which places elevated transmural forces on the heart atria and contributes to atrial chamber enlargement and fibrosis (“atrial remodeling”). This process is considered to be the anatomical substrate for atrial fibrillation. Additionally, the OSA-induced autonomic imbalance during sleep may decrease baroreflex sensitivity and impair the parasympathetic components of the heart rate variability system. Systemic hypertension has been strongly correlated to atrial fibrillation, and pulmonary hypertension is related to atrial and ventricular distortion. Studies have shown an increased risk of fatal and nonfatal cardiovascular events in patients with severe OSA.
Interestingly, we observed no significant association between OSA and either carotid artery disease or cerebrovascular accident (CVA). This might be due to CVA generally being a phenomenon of old age. The mean age of patients with CVA in the United States is 68.8 years in males46 whereas the reported life expectancy of patients with OSA is 57 years, with a gain of 5.4 years after treatment with continuous positive airway pressure.47 Nevertheless, in an observational control study of 697 patients with OSA, Yaggi et al44 reported that OSA was an independent risk factor for stroke and death from any cause. In addition, there was a significant correlation between the severity of sleep apnea and the risk of stroke and death.45
Very few and confounding data have been reported on the role of treatment of OSA in alleviating cardiovascular disease. Marin et al45 showed a beneficial effect of OSA treatment on cardiovascular outcomes, and several studies noted that treating patients with OSA with continuous positive airway pressure can reverse hypercoagulability48,49 and hemodynamic changes50–54 and even reduce the risk of cardiovascular events.45 However, in one large cohort study, the risk of stroke and death was not changed in patients receiving various therapies for OSA.44 The authors suggested that this finding may be due to long-standing OSA and irreversible cardiovascular changes, or an insufficient follow-up period for a definitive conclusion to be reached.
A limitation of the present study, being a large cohort epidemiologic study, is the fact that OSA was not ruled out in the control group of patients. However, The incidence of OSA in the general population was taken into consideration in the statistical analysis. Another limitation is the observational-retrospective type of the study.
Considering the strong correlation between OSA and cardiovascular disease shown in this study, and supported by previous ones, we suggest that early diagnosis and treatment of OSA may change the course of both diseases. Therefore, we encourage physicians to address the issue of sleep-disordered breathing in the cardiovascular patient. The routine follow-up of patients with cardiovascular disease should include a detailed history and a validated quality-of-life questionnaire such as the Epworth sleepiness scale,55 followed by definitive polysomnography in highly suspect cases. An ear–nose–throat evaluation is also important to rule out anatomic disorders that cause upper airway obstruction.
Footnotes
Abbreviations: CVA = cerebrovascular accident, OSA = obstructive sleep apnea, SES = socioeconomic status.
The authors have no funding and conflicts of interest to disclose.
References
- 1. Sleep related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 2.Sorajja D, Gami AS, Somers VK, et al. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–706. [DOI] [PubMed] [Google Scholar]
- 4.Somers VK, White DP, Amir R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J Am Coll Cardiol. 2008;52:686–717. [DOI] [PubMed] [Google Scholar]
- 5.Caples SM, Somers VK. Sleep disordered breathing and atrial fibrillation. Prog Cardiovasc Dis. 2009;51:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakaris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. J Am Med Assoc. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7.Haenjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence of a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–764. [DOI] [PubMed] [Google Scholar]
- 8.Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. [DOI] [PubMed] [Google Scholar]
- 9.Alajmi M, Mulgrew AT, Fox J, et al. Impact of continuous positive airway pressure therapy on blood pressure in obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung. 2007;185:67–72. [DOI] [PubMed] [Google Scholar]
- 10.Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnea. Eur Respir J. 1995;8:1161–1178. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Tikian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–484. [DOI] [PubMed] [Google Scholar]
- 12.Stadling JR, Philipson EA. Breathing disorders during sleep. Q J Med. 1986;58:3–18. [PubMed] [Google Scholar]
- 13.Reemers JE, DeGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respirat Environ Exerc Physiol. 1978;44:931–938. [DOI] [PubMed] [Google Scholar]
- 14.Hudgel DW, Hendricks C, Hamilton HB. Characteristics of the upper airway pressure-flow relationship during sleep. J Apl Physiol. 1988;64:1930–1935. [DOI] [PubMed] [Google Scholar]
- 15.Gilat H, Shpitzer T, Guttman D, et al. Obstructive sleep apnea after radial forearm free flap reconstruction of the oral tongue. Laryngoscope. 2013;12:3223–3226. [DOI] [PubMed] [Google Scholar]
- 16.Khayat R, Patt B, Hayes D. Obstructive sleep apnea: the new cardiovascular disease. Part 1: obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev. 2009;14:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arzt M, Kleiman J, Carrington M, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716–1722. [DOI] [PubMed] [Google Scholar]
- 18.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41:e129–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease. A bidirectional relationship. Circulation. 2012;126:1495–1510. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom C, Lindberg E, Elmasry A, et al. Prevalence of sleep apnoea and snoring in hypertensive men: a population-based study. Thorax. 2002;57:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan AG, Peliikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;1:2271–2277. [DOI] [PubMed] [Google Scholar]
- 22.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811–817. [DOI] [PubMed] [Google Scholar]
- 23.Logan AG, Tkacova R, Perlikowski SM, et al. Refractory hypertension and sleep apnea: effect of CPAP on blood pressure and baroreflex. Eur Respir J. 2003;21:241–247. [DOI] [PubMed] [Google Scholar]
- 24.Brooks D, Horner RL, Kozar LF, et al. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher EC, Lesske J, Behm R, et al. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol. 1992;72:1978–1984. [DOI] [PubMed] [Google Scholar]
- 26.Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol. 1993;74:2969–2975. [DOI] [PubMed] [Google Scholar]
- 27.Clavin AD, Somers VK. Obstructive sleep apnea and cardiovascular disease. Curr Opin Cardiol. 2009;24:516–520. [DOI] [PubMed] [Google Scholar]
- 28.Somers VK, Dyken M, Clary M, et al. Sympathetic ceural mechanisms in obstructive sleep apnea. J Clin Investig. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin K, Oji M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. [DOI] [PubMed] [Google Scholar]
- 30.Rangrmark C, Hedner JA, Carlson JT, et al. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–194. [DOI] [PubMed] [Google Scholar]
- 31.von Känel R, Loredo JS, Ancoli-Israel S, et al. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. [DOI] [PubMed] [Google Scholar]
- 32.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 33.Adegunsove A, Ramachandran S. Ethiopathogenic mechanisms of pulmonary hypertension in sleep-related breathing disorders. Pulm Med. 2012;2012:273591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thor Soc. 2008;5:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuder RM, Abman SH, Braun T, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54:s3–s9. [DOI] [PubMed] [Google Scholar]
- 36.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49:1625–1631. [DOI] [PubMed] [Google Scholar]
- 38.Baranchuk A. Sleep apnea, cardiac arrhythmias, and conduction disorders. J Electrocardiol. 2012;45:508–512. [DOI] [PubMed] [Google Scholar]
- 39.Baranchuk A, Simpson CS, Redfearn DP, et al. It’s time to wake up! Sleep apnea and cardiac arrhythmias. Europace. 2008;10:666. [DOI] [PubMed] [Google Scholar]
- 40.Hersi AS. Obstructive sleep apnea and cardiac arrhythmias. Ann Thorac Med. 2010;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep. 2010;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kannel WB, Wolf PA, Benjamin EJ. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N. [DOI] [PubMed] [Google Scholar]
- 43.Atwood CW, McCrory D, Garcia JGN, et al. Pulmonary artery hypertension and sleep-disordered breathing. Chest. 2004;126:72s. [DOI] [PubMed] [Google Scholar]
- 44.Yaggi KH, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. NEJM. 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 45.Marin JM, Carrizo SJ, Vincente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;65:1046–1053. [DOI] [PubMed] [Google Scholar]
- 46.Melville NA. Average age at first stroke decreases in United States but not Italy. 2010. http://www.medscape.com/viewarticle/730149 Accessed July 20, 2014. [Google Scholar]
- 47.Tousignant P, Cosio MG, Levy RD, et al. Quality adjusted life years added by treatment of obstructive sleep apnea. Sleep. 1994;17:52–60. [PubMed] [Google Scholar]
- 48.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. [DOI] [PubMed] [Google Scholar]
- 49.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 50.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. [DOI] [PubMed] [Google Scholar]
- 51.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. [DOI] [PubMed] [Google Scholar]
- 52.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. [DOI] [PubMed] [Google Scholar]
- 53.Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diomedi M, Placidi F, Cupini LM, et al. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998;51:1051–1056. [DOI] [PubMed] [Google Scholar]
- 55.http://www.stanford.edu/∼dement/epworth.html Accessed August 10, 2012. [Google Scholar]


