Abstract
Renalase, a recently discovered enzyme released by the kidneys, breaks down blood-borne catecholamines and may thus regulate blood pressure (BP). Animal studies have suggested that high levels of dietary salt might reduce blood and kidney renalase levels. We conducted a randomized trial to assess the effects of altered salt and potassium intake on serum renalase levels and the relationship between serum renalase levels and BP in humans.
Forty-two subjects (28–65 years of age) were selected from a rural community of northern China. All subjects were sequentially maintained on a low-salt diet for 7 days (3.0 g/day of NaCl), a high-salt diet for additional 7 days (18.0 g/day of NaCl), and a high-salt diet with potassium supplementation for final 7 days (18.0 g/day of NaCl + 4.5 g/day of KCl).
Serum renalase levels were significantly higher than baseline levels during the low-salt diet intervention period. Renalase levels decreased with the change from the low-salt to high-salt diet, whereas dietary potassium prevented the decrease in serum renalase induced by the high-salt diet. There was a significant inverse correlation between the serum renalase level and 24-h urinary sodium excretion. No significant correlation was found between the renalase level and BP among the different dietary interventions.
The present study indicates that variations in dietary salt intake and potassium supplementation affect the serum renalase concentration in Chinese subjects.
INTRODUCTION
Excess dietary salt is strongly correlated with cardiovascular disease, morbidity, and mortality and is considered to be a major contributing factor to the pathogenesis of hypertension.1,2 Similarly, reduced dietary salt has been shown to decrease blood pressure (BP).1 Several mechanisms contribute to the hypertensive effect of dietary salt, including water and salt retention, vascular abnormalities, and/or neurogenically mediated increases in peripheral resistance.2 In both normal and spontaneously hypertensive rats, salt intake was reportedly associated with enhanced sympathetic activity and resultant increases in vascular resistance and systemic BP.3,4
Renalase, a recently discovered flavoprotein, which is strongly expressed in the kidney and heart, effectively metabolizes catecholamines.5,6 Circulating renalase is inactive at baseline and is rapidly activated by epinephrine. Once activated, renalase, in turn, has been found to metabolize epinephrine and norepinephrine. The kidney releases this protein into the bloodstream to regulate BP.7 Renalase-treated animals exhibit a large reduction in BP accompanied by a decreased concentration of circulating catecholamines.5 Renalase lowers BP in vivo by decreasing both cardiac contractility and the heart rate and by degrading circulating adrenaline.5,8 High dietary salt has been shown to downregulate blood and kidney renalase levels in both rats subjected to subtotal nephrectomy and Dahl salt-sensitive rats.9 However, the relationship between circulating renalase levels and dietary salt intake in humans has not been elucidated.
Potassium intake helps to downregulate BP by increasing renal salt excretion.10,11 Our previous studies demonstrated that increased potassium intake in normotensive subjects remarkably alleviated high dietary salt-induced increases in BP.12,13 Clinical trials have also shown that long-term potassium supplementation may lower the risk of cardiovascular disease.14 Furthermore, high potassium intake may have beneficial effects on arterial compliance and stiffness.15 However, data on the link between potassium supplementation and serum renalase levels are sparse.
The present study was designed to examine the effects of salt intake and potassium supplementation on serum renalase levels in normotensive and mildly hypertensive subjects. The correlation between renalase levels and BP was also investigated.
MATERIALS AND METHODS
Subjects
Forty-two subjects with similar dietary habits from a rural community of northern China were enrolled in the present study. Data on demographic characteristics (age, sex, education, ethnicity, occupation, physical activity, cardiovascular disease-related history, and physical examination findings) were collected using a standard questionnaire. Hypertension was defined as a mean systolic BP (SBP) of ≥140 mmHg and/or a mean diastolic BP (DBP) of ≥90 mmHg. The exclusion criteria were stage 2 hypertension; secondary hypertension; a history of clinical cardiovascular disease, chronic kidney disease, or diabetes; use of antihypertensive medication; pregnancy; high alcohol intake; and a current low-salt diet.
The institutional ethics committee of Xi’an Jiaotong University Medical School approved the study protocol, and each subject provided written informed consent to participate. This study adhered to the principles of the Declaration of Helsinki, and all study procedures were performed in accordance with institutional guidelines.
Dietary Intervention
The chronic salt intake and potassium supplementation intervention protocol was performed as previously described.12,13 The protocol comprised a questionnaire survey and physical examination (height, weight, waist circumference, and BP measurements) during a 3-day baseline observation period, a low-salt diet for 7 days (3 g of salt or 51.3 mmol of sodium per day), a high-salt diet for 7 days (18 g of salt or 307.8 mmol of sodium per day), and a high-salt diet with potassium supplementation for 7 days (18 g of salt or 307.8 mmol of sodium + 60 mmol of potassium per day). During the baseline period, each subject was given detailed dietary instructions to avoid table salt, cooking salt, and high-salt foods for the 21-day study duration. To ensure compliance of study participants with the intervention program, they were required to have their breakfast, lunch, and dinner at the study kitchen under supervision of the study staff during the entire study period. All foods were cooked without salt. Onsite study staff members added prepackaged salt to the meals of individual subjects as indicated by the study protocol.
BP Measurement
BP was measured by 3 trained staff members using a standard mercury sphygmomanometer with the subjects in sitting position after a ≥5-min rest. BP was measured 3 times at 1-min intervals during the 3-day baseline observation period as well as on days 6 and 7 of each of the 3 7-day intervention periods. BP observers were blinded to the dietary interventions of participants. The subjects were instructed to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 min prior to their BP measurement. SBP and DBP were determined as the first and fifth Korotkoff sounds, respectively. The pulse pressure was calculated as SBP−DBP. The mean arterial pressure (MAP) was calculated as DBP + (1/3 × pulse pressure). The BP at baseline and during the intervention was calculated as the mean of 6 measurements from 2 clinical visits during the 3-day baseline observation period and the mean of the measurements on days 6 and 7 of each of the 3 7-day intervention periods, respectively.
Biochemical Analyses
Blood samples were obtained by peripheral venous puncture, immediately centrifuged at 3000 × g for 10 min, and stored at –80 °C until analyzed. The total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum creatinine, and fasting plasma blood glucose levels were measured using an automatic biochemical analyzer (model 7600; Hitachi, Ltd., Tokyo, Japan). Serum renalase levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Uscn Life Science, Inc., Wuhan, China).
hour Urinary Salt and Potassium Determination
Twenty-four-hour urine samples were collected at baseline and on day 7 of each intervention period. The samples were kept frozen at –40 °C until analysis. Urinary concentrations of salt and potassium were determined using ion-selective electrodes (Hitachi, Ltd., Tokyo, Japan). The 24-h urinary excretion of sodium and potassium was calculated by multiplying the concentration of sodium and potassium, respectively, by the 24-h total urine volume.
Statistical Analyses
Continuous data are presented as mean ± standard error. Categorical data are expressed as frequency with percentage. Differences in repeated measures were analyzed by repeated measures analysis of variance. Correlations were determined with Pearson’s correlation coefficient if the residuals were normally distributed and with Spearman’s correlation coefficient otherwise. Statistical analyses were performed with SPSS for Windows, Version 16.0 (SPSS Inc., Chicago, IL). A 2-tailed P-value of <0.05 was considered statistically significant.
RESULTS
Profiles of Studied Subjects
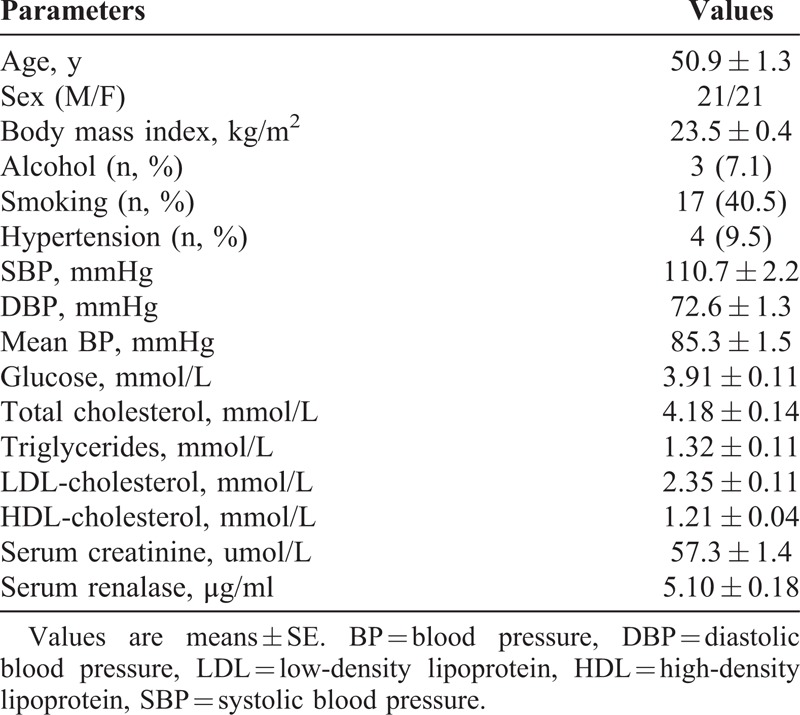
All subjects completed the intervention trials. As shown in Table 1, 4 subjects (9.5%) had hypertension; none of them were taking medication.
TABLE 1.
Baseline Demographic and Clinical Characteristics
Effects of Salt Intake and Potassium Supplementation on BP and 24-h Urinary Sodium and Potassium Excretion
Table 2 shows the BP responses to the low-salt, high-salt, and high-salt + potassium supplementation interventions. The BP significantly increased with the change from the low-salt to high-salt intervention and decreased with the change from the high-salt intervention to the high-salt + potassium supplementation intervention (all P < 0.05).
TABLE 2.
BP Levels (mmHg) and 24-h Urinary Sodium and Potassium Excretions (mmol/d) at Baseline and During Dietary Interventions
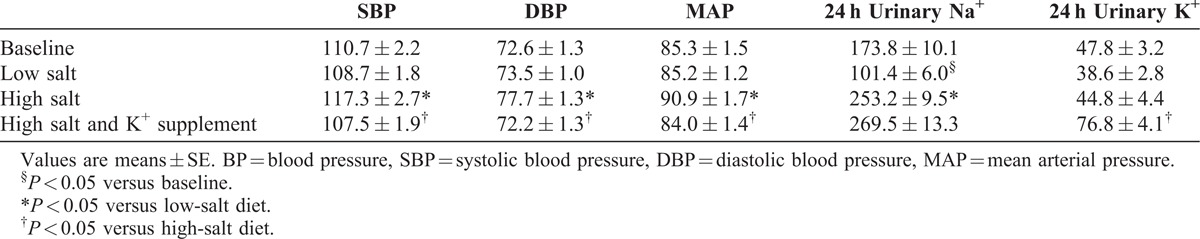
The 24-h sodium and potassium excretions in the urine were calculated at the end of each intervention period to ensure the subjects’ compliance with the study protocol. As shown in Table 2, the urinary sodium excretion significantly decreased with the change from baseline to the low-salt diet, but increased with the change from the low-salt to high-salt diet (all P < 0.05). Potassium supplementation resulted in an increase in urinary potassium excretion and a slight increase in urinary sodium excretion. These results confirmed the subjects’ compliance with the dietary intervention protocol.
Effects of Salt Intake and Potassium Supplementation on Serum Renalase Levels
On one hand, the serum renalase level significantly increased with the change from baseline to the low-salt diet (increased from 5.10 ± 0.18 μg/ml to 8.12 ± 0.42 μg/ml, P < 0.01) and decreased with the change from the low-salt to high-salt diet (decreased from 8.12 ± 0.42 to 5.65 ± 0.17 μg/ml, P < 0.01) (Figure 1). On the other hand, the high-salt diet induced decline in the serum renalase level was abrogated by potassium supplementation (increased from 5.65 ± 0.17 to 8.94 ± 0.74 μg/ml, P < 0.01) (Figure 1).
FIGURE 1.
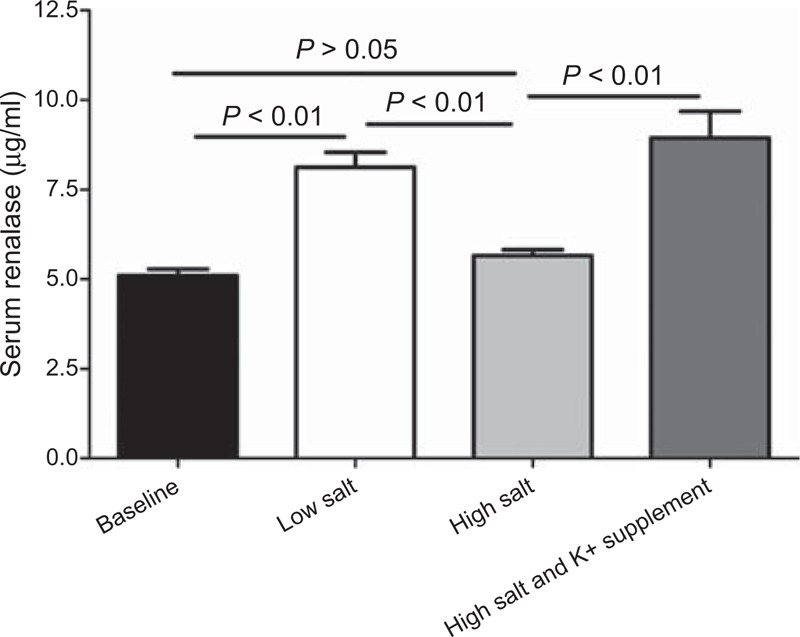
The effect of low-salt and high-salt intakes, and potassium supplementation on serum renalase in all subjects.
Further analyses showed that the serum renalase concentration was inversely correlated with the 24-h urinary sodium excretion during both low-salt and high-salt diet intervention periods (r = –0.459, P < 0.01) (Figure 2), but was not correlated with the 24-h urinary potassium excretion during the high-salt diet + potassium supplementation intervention period (r = –0.153, P = 0.333) (Figure 2). Moreover, no correlation was observed between the serum renalase level and BP in the 3 intervention periods (r = 0.065, P = 0.40) (Figure 3).
FIGURE 2.
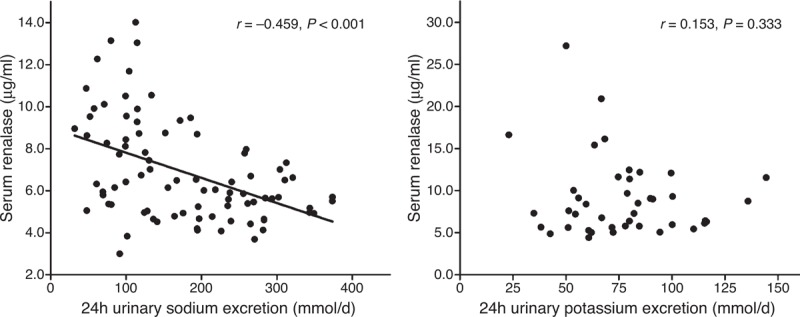
The correlation between serum renalase levels and 24 h urinary sodium and potassium excretions in all subjects on a low-salt diet and on a high-salt diet, or on a high-salt diet with potassium supplementation.
FIGURE 3.
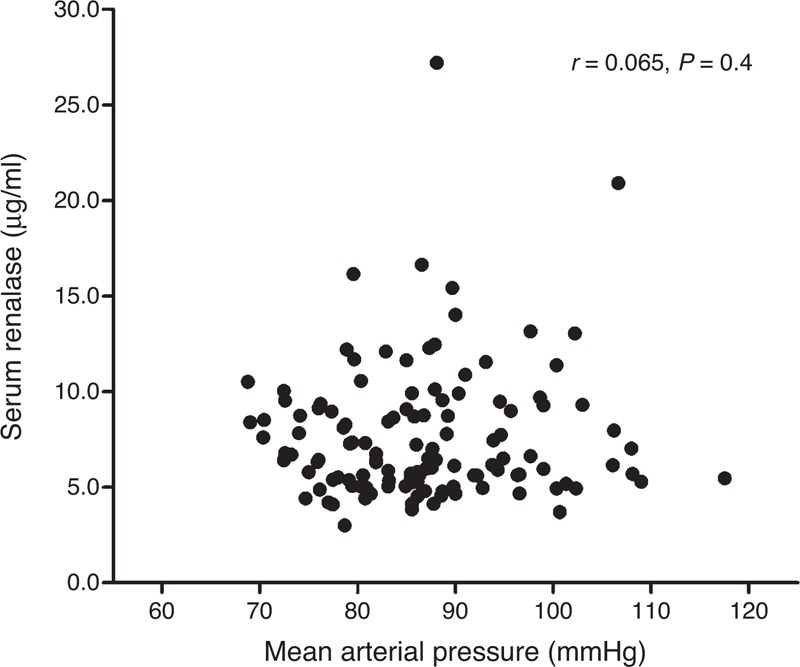
The correlation between serum renalase and MAP in all subjects on a low-salt diet, high-salt diet, and high-salt diet with potassium supplementation.
DISCUSSION
The results of the present study demonstrate that high salt intake decreases serum renalase and BP from the levels of low-salt diet, and low salt intake increases serum renalase from the baseline. In addition, an inverse correlation between the 24-h urinary sodium excretion and serum renalase level was demonstrated in these Chinese subjects. These data indicate that variations in dietary salt intake significantly influence the serum renalase level.
Renalase deficiency is reportedly associated with chronic kidney disease, heart disease, diabetes, stroke, and hypertension.16–19 Renalase knockout mice exhibit higher BP and susceptibility to myocardial ischemia.16 Animal studies have indicated that administration of human recombinant renalase decreases BP.20 This phenomenon has also been observed in humans. Schlaich et al21 found that arterial renalase levels were significantly higher in 4 normotensive control subjects than in 22 patients with resistant hypertension (P < 0.05). An association between renalase levels and hypertension was also demonstrated by Zhao et al,18 who were the first to show that in the Han Chinese population, the renalase-encoding gene is a novel susceptibility gene for essential hypertension and that its genetic variations might influence BP. They found that among 2586 Asian subjects, those bearing G allele frequencies of rs2576178 showed a higher incidence of hypertension.18 Renalase gene polymorphisms have also been shown to be correlated with hypertension in patients on hemodialysis and in patients with type 2 diabetes.17,22 In the present study, we observed no correlation between the serum renalase concentration and BP in 3 intervention periods. This result is consistent with those reported by Przybylowski et al23 and Zbroch et al,24 both of whom reported that the renalase level was not related to BP in heart transplant recipients or patients undergoing peritoneal dialysis. The different study populations, sample sizes, and racial differences among these various studies may be the causes of the discrepant results.
The evidence available to date indicates that decreased renalase production is associated with a high salt intake. Ghosh et al25 reported that the renalase expression level is regulated by salt intake in Dahl salt-sensitive rats. Blood and kidney tissue renalase levels were significantly lower in Dahl salt-sensitive rats maintained on an 8% salt diet for 3 weeks and became virtually undetectable after 4 weeks of a high-salt diet.9 Additionally, increased dietary salt inhibits renalase protein expression in both rats with normal renal function and those subjected to subtotal nephrectomy.9 More recently, Quelhas-Santos et al26 demonstrated that high salt intake markedly accentuated the decrease in blood and renal tissue renalase levels in 3/4-nephrectomized rats. These observations are in agreement with the results of our study in which salt-loading significantly inhibited serum renalase levels, as well as with the inverse association found between the 24-h urinary sodium and serum renalase concentrations in humans. The present study builds upon these previous findings by also evaluating the effects of low salt intake. We found that salt restriction resulted in a marked increase in circulating renalase levels from baseline. However, the exact mechanism of how sodium regulates serum renalase levels remains unclear. High salt intake stimulates the sympathetic nervous system, facilitates the secretion of epinephrine and norepinephrine, and thus elevates the serum renalase level.3,4,7 Sympathetic activation may also decrease the circulating renalase level as reported by Jiang et al.27 In their study of spontaneously hypertensive rats, renal denervation lowered BP and upregulated the plasma renalase level and renalase expression level in the kidney. In addition, Han et al28 recently showed that the angiotensin-converting enzyme inhibitor lisinopril markedly increased the expression of renalase in kidney tissue in rats with adriamycin-induced nephropathy. Therefore, a high salt load may reduce the serum renalase level secondary to activation of the renal renin–angiotensin system.29 Moreover, high sodium levels increase renal dopamine synthesis and excretion, promote the excretion of renalase, and may thus result in a decline in serum renalase levels.26,30 However, further studies are needed to prove these hypotheses.
Our study is the first to clearly demonstrate that potassium supplementation can reverse the effects of a high-salt diet on low serum renalase levels in the Chinese population. The molecular mechanisms that modulate the serum renalase level in response to potassium supplementation, however, remain elusive. In the current study, potassium supplementation facilitated renal sodium excretion, which may have reduced the effects of high sodium levels on renalase. Furthermore, it is possible that potassium directly impacted the activation, synthesis, and secretion of renalase. However, we found no correlation between renalase levels and urinary potassium excretion during the high-salt + potassium supplementation intervention. This may have been due to the intervention method and individual differences among the subjects in this study.
The present study has some limitations that should be acknowledged. First, the study population was relatively small and restricted to northern Chinese individuals. Therefore, our results will require replication in other cohorts to determine generalizability to other ethnicities and to populations with different backgrounds. In addition, the method used to assess renalase levels has not been fully validated. No validated methods other than ELISA assay are available; however, the use of ELISA for this purpose has been previously discussed.30
In summary, the present study has shown that the circulating renalase level increases with a low-salt diet, decreases with a high-salt diet, and increases with salt loading followed by potassium supplementation in Chinese subjects. These findings may contribute to a better understanding of the roles of salt and potassium in BP regulation and may have potential clinical and public health implications.
ACKNOWLEDGEMENTS
We are indebted to the participants in the study for their outstanding commitment and cooperation.
Footnotes
Abbreviations: BP = blood pressure, SBP = systolic blood pressure, DBP = diastolic blood pressure, MAP = mean arterial pressure, ELISA = enzyme-linked immunosorbent assay.
YW, F-QL, and DW equally contributed to this work.
This work was supported by the grant 2012CB517804 from the National Program on Key Basic Research Project of China (973 Program) and grants 81200512 (to Liu) and 81070218 and 8130357 (to Mu) from the Natural Science Foundation of China.
The authors declare that there is no conflict of interest.
References
- 1.Meneton P, Jeunemaitre X, de Wardener HE, et al. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. 2005;85:679–715. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH. Pathogenesis of salt sensitivity of blood pressure. Curr Hypertens Rep. 2006;8:166–170. [DOI] [PubMed] [Google Scholar]
- 3.Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of central sympathetic networks. Physiol Behav. 2010;100:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz R, Schoumig A, Rascher W, et al. Enhanced sympathetic activity caused by salt loading in spontaneously hypertensive rats. Clinical Science. 1980;59:171s–173s. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Li G, Wang P, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Xing T, Li J, et al. Renalase’s expression and distribution in renal tissue and cells. PloS One. 2012;7:e46442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Xu J, Wang P, et al. Catecholamines regulate the activity, secretion, and synthesis of renalase. Circulation. 2008;117:1277–1282. [DOI] [PubMed] [Google Scholar]
- 8.Desir GV, Tang L, Wang P, et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. 2012;1:e002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desir G. Novel insights into the physiology of renalase and its role in hypertension and heart disease. Pediatr Nephrol. 2012;27:719–725. [DOI] [PubMed] [Google Scholar]
- 10.Treasure J, Ploth D. Role of dietary potassium in the treatment of hypertension. Hypertension. 1983;5:864–872. [DOI] [PubMed] [Google Scholar]
- 11.He J, Whelton PK. What is the role of dietary sodium and potassium in hypertension and target organ injury. Am J Med Sci. 1999;317:152–159. [DOI] [PubMed] [Google Scholar]
- 12.Fang Y, Mu JJ, He LC, et al. Salt loading on plasma asymmetrical dimethylarginine and the protective role of potassium supplement in normotensive salt-sensitive Asians. Hypertension. 2006;48:724–729. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, Mu J, Yuan Z, et al. Potassium supplement ameliorates salt-induced haemostatic abnormalities in normotensive subjects. Acta Cardiol. 2011;66:635–639. [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Obarzanek E, Cutler JA, et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He FJ, Marciniak M, Carney C, et al. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension. 2010;55:681–688. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, Xu J, Velazquez H, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79:853–860. [DOI] [PubMed] [Google Scholar]
- 17.Buraczynska M, Zukowski P, Buraczynska K, et al. Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke. Neuromolecular Med. 2011;13:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Q, Fan Z, He J, et al. Renalase gene is a novel susceptibility gene for essential hypertension: a two-stage association study in northern Han Chinese population. J Mol Med (Berl). 2007;85:877–885. [DOI] [PubMed] [Google Scholar]
- 19.Malyszko J, Zbroch E, Malyszko JS, et al. Renalase, a novel regulator of blood pressure, is predicted by kidney function in renal transplant recipients. Transplant Proc. 2011;43:3004–3007. [DOI] [PubMed] [Google Scholar]
- 20.Baraka A. Cardioprotective effect of renalase in 5/6 nephrectomized rats. J Cardiovasc Pharmacol Ther. 2012;17:412–416. [DOI] [PubMed] [Google Scholar]
- 21.Schlaich M, Socratous F, Eikelis N, et al. Renalase plasma levels are associated with systolic blood pressure in patients with resistant hypertension. J Hypertens. 2010;28:e437. [Google Scholar]
- 22.Stec A, Semczuk A, Furmaga J, et al. Polymorphism of the renalase gene in end-stage renal disease patients affected by hypertension. Nephrol Dial Transplant. 2012;27:4162–4166. [DOI] [PubMed] [Google Scholar]
- 23.Przybylowski P, Malyszko J, Kozlowska S, et al. Serum renalase depends on kidney function but not on blood pressure in heart transplant recipients. Transplant Proc. 2011;43:3888–3891. [DOI] [PubMed] [Google Scholar]
- 24.Zbroch E, Malyszko J, Malyszko J, et al. Renalase in peritoneal dialysis patients is not related to blood pressure, but to dialysis vintage. Perit Dial Int. 2012;32:348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh SS, Gehr TWB, Sica DA, et al. Renalase regulates blood pressure in salt sensitive Dahl rats. J Am Soc Nephrol. 2006;17:208A.16221868 [Google Scholar]
- 26.Quelhas-Santos J, Sampaio-Maia B, Simoes-Silva L, et al. Sodium-dependent modulation of systemic and urinary renalase expression and activity in the rat remnant kidney. J Hypertens. 2013;3:543–552. [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Guo Y, Tan L, et al. Impact of renal denervation on renalase expression in adult rats with spontaneous hypertension. Exp Ther Med. 2012;4:493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han P, Sun H, Xu Y, et al. Lisinopril protects against the adriamycin nephropathy and reverses the renalase reduction: potential role of renalase in adriamycin nephropathy. Kidney Blood Press Res. 2013;37:295–304. [DOI] [PubMed] [Google Scholar]
- 29.Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. [DOI] [PubMed] [Google Scholar]
- 30.Desir GV, Peixoto AJ. Renalase in hypertension and kidney disease. Nephrol Dial Transplant. 2014;29:22–28. [DOI] [PubMed] [Google Scholar]


