Abstract
Prosthetic arteriovenous (AV) graft infection is the principal cause of morbidity related to chronic hemodialysis AV graft fistula. Coxiella burnetii is a known pathogen that causes fever, pneumonia, and intravascular infections with the limitation of negative cultures. Herein, we report the first case of a patient who presented to the emergency department of our hospital with a prosthetic hemodialysis AV graft infection due to Coxiella burnetii. We also performed a literature search with PubMed to identify studies reporting cases of Coxiella burnetii vascular graft infection. Overall, we reviewed 15 cases of vascular graft infection, including ours. We found a high prevalence of male patients (87%); mean age ± standard deviation (SD) of the entire population was 60.4 ± 9.6 years. The dacron infrarenal aortic and the aortobifemoral bypass were the most common involved grafts. The early diagnosis of infection due to Coxiella burnetii was done by serology or with polymerase chain reaction (PCR), in 12 and 3 cases, respectively. All patients underwent partial or complete resection of the infected grafts; the most common antibiotic treatment for this entity was doxycycline and hydroxycloroquine.
Although this is a relatively rare disease, Coxiella burnetii should be included in the differential diagnosis of all patients who present with infection of an endovascular graft of any nature with an inconclusive etiologic diagnosis.
INTRODUCTION
Most patients on chronic hemodialysis have an arteriovenous (AV) graft fistula for vascular access,5 either autologous or prosthetic. Incidence of prosthetic AV graft infection ranges widely, from 3% to 35%, according to the study population and type of prosthetic graft material,27,38 and it is the principal cause of morbidity and the second leading cause of mortality16,35 related to those devices. The most common etiologic agents are Staphylococcus aureus, coagulase-negative staphylococci, Gram-negative rods, and Enterococcus species.25 Fungal infections are uncommon and have seldom been reported.14
Coxiella burnetii is a bacterium that causes fever, pneumonia, and intravascular infections,33 but we were unable to find any documented infections of AV graft fistula due to Coxiella burnetii in patients on chronic hemodialysis.
In this paper we describe the first case, to our knowledge, of a patient with Coxiella burnetii prosthetic AV graft infection, and also review 14 other nonhemodialysis endovascular graft infections caused by Coxiella burnetii.
PATIENTS AND METHODS
Case Report
A 61-year-old man presented to the emergency department (ED) of our institution with a week of nausea, vomiting, and fever following hemodialysis sessions. He was diagnosed with adult polycystic kidney disease in 1981, and underwent kidney transplantation from a deceased donor on 2 occasions but both failed. In 2009 a left humeral-axillary AV fistula was created with an artificial graft of polytetrafluoroethylene (PTFE) for hemodialysis. The post-surgery period was complicated with prosthetic AV graft thrombosis and infection, for which the patient required 3 new prosthetic AV graft implantations, in 2009, 2010, and 2012, respectively, and multiple broad-spectrum antibiotics on several occasions. Since 2012 the prosthetic AV graft had not been used for hemodialysis, and a right jugular perm-cath had been placed for this purpose. At the time of evaluation in the ED, his vital signs were the following: blood pressure 150/80 mm Hg, pulse of 95 beats per minute, respiratory rate of 21 breaths per minute, and temperature of 39°C. The physical examination at admission showed a left arm swelling with a fistula and purulent discharge from the prosthetic AV graft. A computed tomography (CT) scan with tagged white blood cells of the left arm revealed a positive reading in the left humeral prosthetic AV graft with a more localized infection in the distal humeral segment. Blood cultures were sterile and laboratory results showed a white blood cell count (WBC) of 2.6 × 103/μL, serum creatinine of 4.77 mg/dL, urea of 58 mg/dL, no increase in liver enzymes, C-reactive protein (CRP) of 1.9 mg/dL (19 mg/L), and procalcitonin (ProCT) of 2.3 μg/L (normal range, 0–0.5 ng/mL). Empirical daptomycin and piperacillin-tazobactam were initiated and the fever and purulent discharge resolved. A sample of the purulent discharge was collected with a swab and processed only for culture. The Gram stain showed moderate leukocytes and no visible microorganisms, and the culture, after 24 hour incubation, grew Proteus mirabilis. The antibiotic spectrum was narrowed to third-generation cephalosporin IV, and the fever and purulent discharge resolved.
After 2 weeks of admission and intravenous antibiotic therapy the patient was surgically intervened for removal of the infected prosthetic AV grafts. One was completely removed, another was partially explanted, but a third graft was kept in the left humeral trajectory. Multiple intraoperative specimens did not grow any microorganisms in conventional microbiologic cultures for aerobic and anaerobic bacteria. Histopathology showed a segment of elastic artery with an endoprosthetic segment in the inner layer of the arterial wall with fibrosis and a chronic inflammatory infiltrate of lymphocytes, plasma cells and multinuclear giant cells that corresponded with the presence of a foreign body. No microorganisms could be identified using Periodic acid-Schiff and Gram stains.
Universal 16 S rRNA gene PCR and sequencing (16SrRNA PCR) was performed as previously described19 on 4 intraoperative specimens (2 periprosthetic biopsies and 2 vascular AV grafts) that were received in our microbiology laboratory. In all 4 samples Coxiella burnetii was detected. Consequently, these results were confirmed in all samples by real-time PCR of the regions IS1111 and IS630 of Coxiella burnetii.4 The diagnosis of Coxiella burnetii chronic infection was also confirmed by serologic testing. In a recovered serum obtained 4 months before, the results were phase II antibody (IgG): positive, and phase II antibody (IgM): negative. The actual serum had the following results: phase I antibody titer (IgG): 1/6400, phase I antibody titer (IgM): 1/50, and phase I antibody titer (IgA): negative; phase II antibody titer (IgG): 1/6400, phase II antibody titer (IgM): negative, and phase II antibody titer (IgA): negative. Antibiotic therapy with doxycycline (100 mg bid orally) and hydroxychloroquine (200 mg tid orally) was started. A positron emission tomography scan (PET scan) was performed (Figures 1 and 2) that revealed hypercaptation of left fistula, possibly related to recent surgery, as well as less activity in the left vascular access, up to the middle third of the arm suggesting an inflammatory/infectious process. Four months later the patient attended the infectious disease consult for follow-up. He was asymptomatic with a favorable evolution of his left arm and with a good adherence to the antibiotic treatment. The new serum showed phase I antibody titer (IgG): 1/3200, phase I antibody titer (IgM): 1/50, and phase I antibody titer (IgA): negative; phase II antibody titer (IgG): 1/1600, phase II antibody titer (IgM): negative, and phase II antibody titer (IgA): negative.
FIGURE 1.
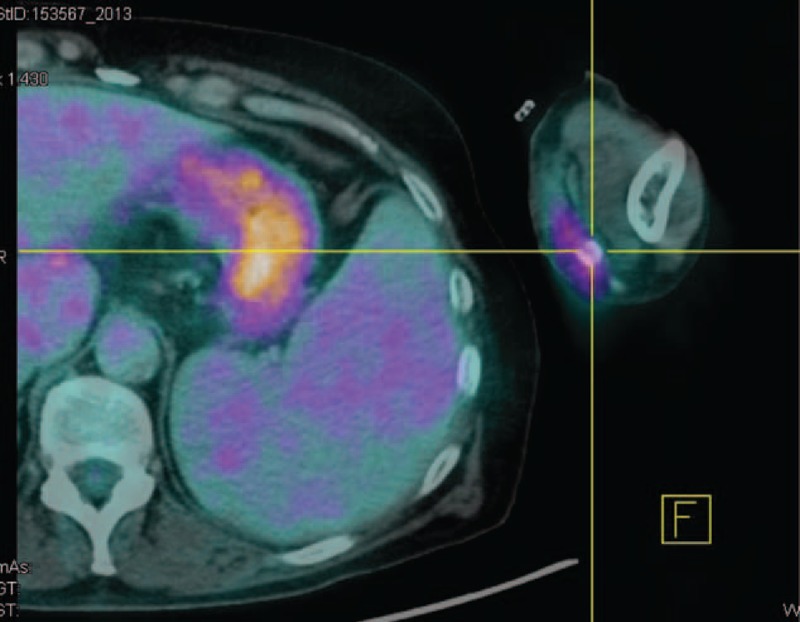
A PET scan revealed an increased radiotracer accumulation of left fistula possibly related to recent surgery.
FIGURE 2.
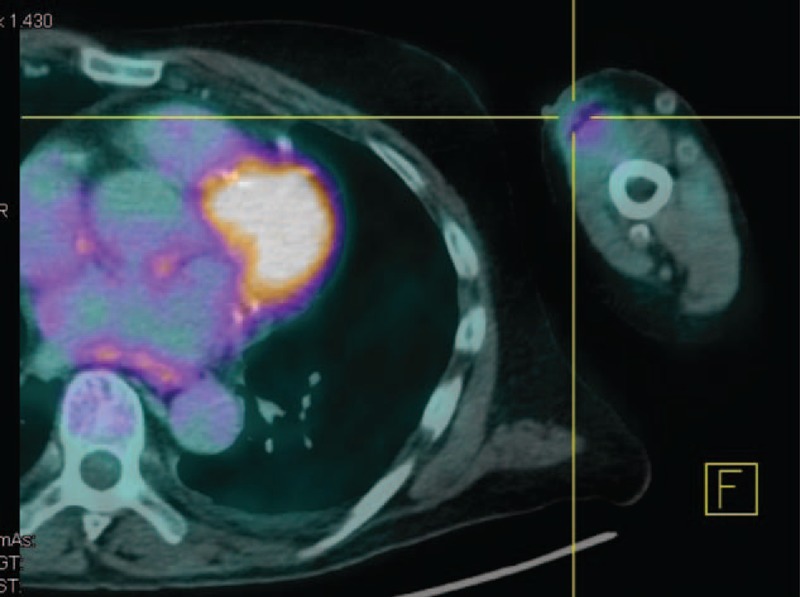
PET scan also revealed less activity in the left vascular access up to the middle third of the arm, suggesting an inflammatory/infectious process.
Diagnostic Procedures
Serologic procedures: phase II immunoglobulin G (IgG) and M (IgM) was performed in our laboratory using enzyme-linked immunosorbent assay (ELISA). Titers of phase I and phase II IgG, IgM, and IgA antibodies were performed in a reference laboratory (Instituto de Salud Carlos III, Majadahonda) using the indirect immunofluorescent antibody test.
Molecular detection: 16SrRNA PCR was performed in sonicated AV grafts and biopsies as previously described.19 Specific detection of IS1111 and IS630 of Coxiella burnetii was made using 2 different real-time PCR reactions with TaqMan probes as described by Brouqui and colleagues.4
Bibliographic Research
We performed a literature search with PubMed (National Library of Medicine, Bethesda, MD) (http://www.nlm.ncbi.nih.gov/pubmed/) to identify studies written in English reporting adult cases of Coxiella burnetii vascular graft infection. We used the terms [(Coxiella burnetii) OR (Q fever)] AND [(vascular graft infection) OR (aortic aneurysm) OR (prosthesis) OR (infection of hemodialysis access) OR (dacron graft) OR (blood vessels prosthesis)].
RESULTS
The PubMed search yielded 29 cases of Coxiella burnetii endovascular graft infection, all of them involving large vascular grafts.2,3,7,10,11,15,18,22,26,28,32,36,42,43 However, 15 of these cases, all described in a single study,3 could not be included because the patients’ treatment and clinical course were not individually described.
Overall, we reviewed 15 cases of vascular graft infection, including ours. We found evidence of a high prevalence of male patients (87%) greatly exceeding that of female patients (13%). Mean age ± standard deviation (SD) of the entire population was 60.4 ± 9.6 years. In almost half of the cases (6 patients), a rural environment could be related to the patient’s background. Only in our case the patient referred no previous contact with a rural environment (Table 1).
TABLE 1.
Clinical and Laboratory Characteristics of Coxiella burnetii Infection in Hemodialysis and Other Vascular Grafts, Previous and Present Reports
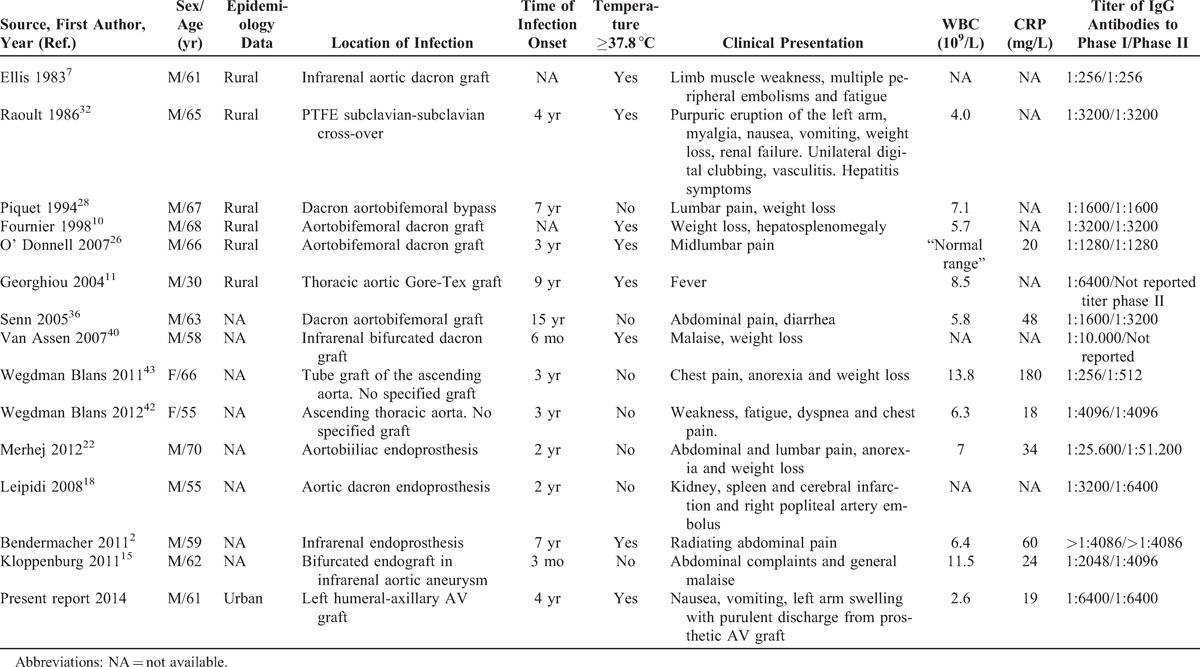
The mean time onset of chronic Q fever after graft placement was 4.6 years (range, 3 mo-15 yr) and in all previous cases the disease affected great vessels. The dacron aortobifemoral bypass10,26,28,36 and infrarenal aortic graft2,7,15,40 were the most common involved grafts (4 patients each), followed by thoracic aortic graft (3 patients),11,42,43 PTFE subclavian-subclavian cross-over (1 patient),32 aortobiiliac endoprosthesis (1 patient),22 aortic endoprosthesis (1 patient),18 and left humeral-axillary AV PTFE graft in our case. Fever and local pain were reported in 8 cases respectively, weight loss was reported in 4 cases, embolic phenomena in 3 cases, gastrointestinal symptoms (nausea, vomiting, and diarrhea) and respiratory symptoms (dyspnea and fatigue) in 2 cases, respectively, and other cases reported new symptoms like renal failure, hepatosplenomegaly, and purulent discharge, as was our case (see Table 1). The association of a vascular graft infection with endocarditis was not found in any of the published studies. WBC was reported in 11 studies (mean value, 7.1 × 106/L), while CRP was reported in 8 (mean value, 48 mg/L) (see Table 1).
Phase I and II IgG antibodies varied according to each study. In 7 of them, both phases had the same value, all of them ≥1:800. Of the remaining 8, 6 had phase I IgG antibody higher than 1:800, while 2 presented with phase I IgG of 1:256. Intra operative specimens were cultured for Coxiella burnetii in 9 cases, being isolated in 5 studies of extracted grafts. PCR was performed on intra operative samples in 14 cases, and of them, 12 were positive for Coxiella burnetii. With this available data, the early diagnosis of infection due to Coxiella burnetii was done by serology and with PCR, in 12 and 3 studies respectively. Eleven studies reported imaging techniques: CT scan and PET scan were performed in 5 cases each and in the remaining case a transesophageal echocardiography was performed. In all of the studies, the imaging techniques, when performed, showed abnormalities which raised the suspicion of infection (Table 2).
TABLE 2.
Treatment and Outcome of Coxiella burnetii Infection in Hemodialysis and Other Vascular Grafts, Previous and Present Reports
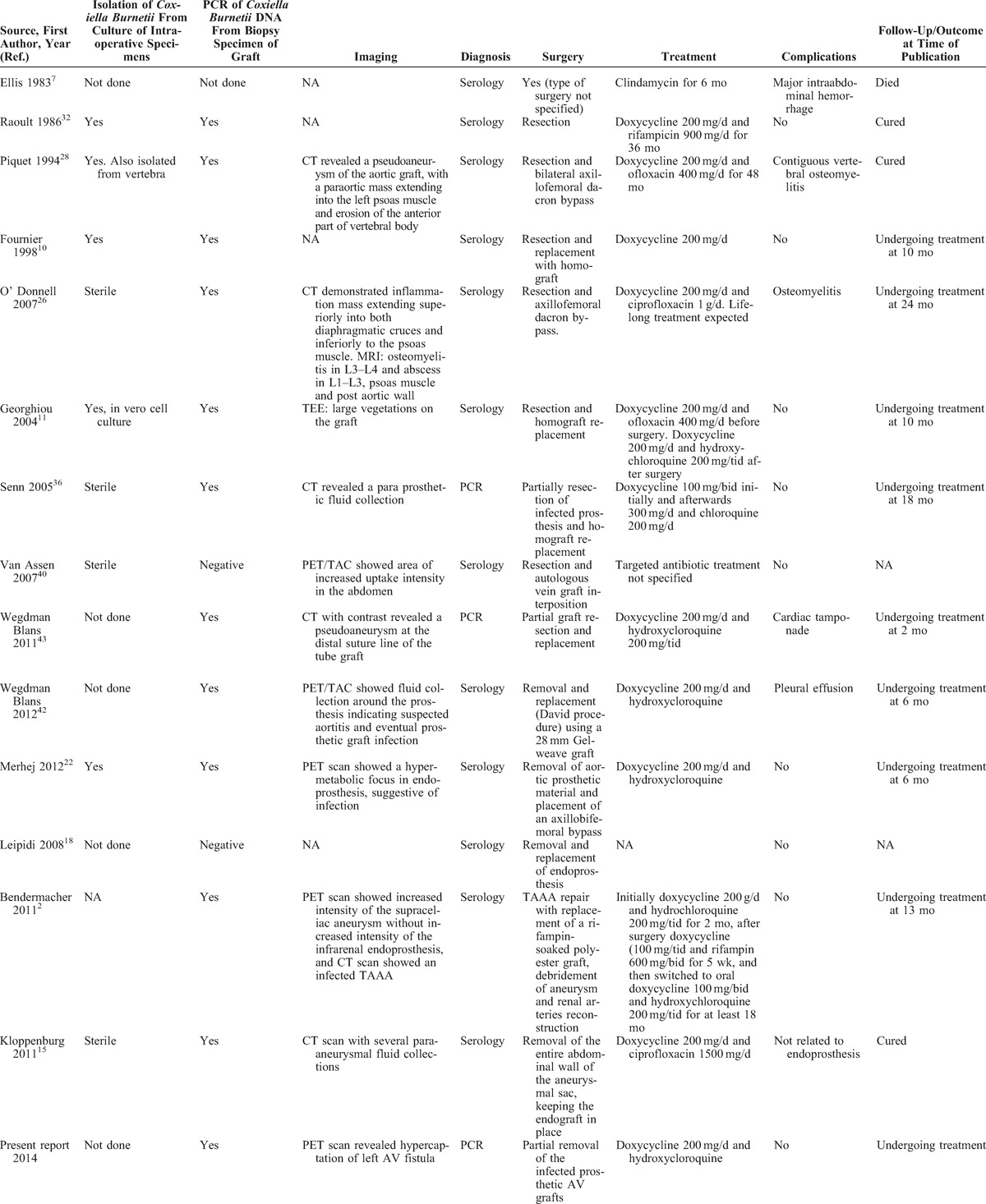
Regarding surgical treatment, all patients underwent partial or complete resection of the infected grafts.2,7,10,11,15,18,22,26,28,32,36,42,43 At follow-up, 12 patients were either cured or still on treatment with a favorable evolution, 1 patient, in which the surgical procedure was not described, died at 9 months after the diagnosis7 and in 2 cases the evolution was not available in the study. The most common antibiotic treatment for this entity was doxycycline and hydroxycloroquine (see Table 2).
DISCUSSION
To the best of our knowledge, this is the first case of a prosthetic hemodialysis AV graft infection caused by Coxiella burnetii reported in the literature. Our review shows that Coxiella burnetii may be occasionally a cause of endovascular infection in other central or peripheral vascular prosthesis.
In Europe, the number of patients with chronic kidney disease who are currently on regular extracorporeal hemodialysis is estimated to be about 300,000 cases, and similar figures have been described for the United States.6 Although there are several differences between countries, the reported prevalence of AV grafts as vascular access ranges from 10% to 58%8 among patients undergoing dialysis.
Infection is the main cause of morbidity and the second leading cause of mortality in patients with a prosthetic AV graft,16,35 which is mainly caused by Gram-positive, followed by Gram-negative bacteria.25 Until now, to the best of our knowledge, no attention has been focused on infected AV grafts with negative microbiologic results and the search for difficult to culture pathogens such as Coxiella burnetii is not usually performed in most microbiologic laboratories.
Coxiella burnetii is an obligate intracellular Gram-negative bacteria that causes Q fever, a worldwide distributed zoonosis that predominates in rural areas, but also recently, in urban areas as well.15 Humans are incidental hosts in which infection usually occurs by inhalation of contaminated aerosols, especially from infected livestock.39 Transmission through other routes like ingestion of contaminated milk, transplacental, intradermal and blood transfusions have also been described.29,37 Clinical presentation of the disease is extremely variable and the infection may be acute or chronic.33 Acute Q fever is asymptomatic in up to 60% of the cases.21 In symptomatic patients, clinical presentation is mostly nonspecific and may include malaise, fever, atypical pneumonia, hepatitis31 or, less frequently, myopericarditis or meningoencephalitis.33 When symptomatic, the acute disease is usually mild and recovery is spontaneous in most cases. However, 1%–5% of patients with acute Q fever progress to chronic disease which may become evident within 1 year or up to 20 years after the initial infection. The 3 groups at highest risk for chronic Q fever are pregnant women, immunosuppressed persons and patients with pre-existing heart valve defects or vascular abnormalities.31 Endocarditis, with negative blood cultures, has been reported as the main clinical manifestation of chronic Q fever, accounting for 80% of the cases. It is followed by infection of aneurysms or vascular grafts, that have been found in 9% of the cases.31 The high affinity of Coxiella burnetii for the human cardiovascular system is strictly related to its ability to persist in host macrophages that are typically present in vascular thrombus or damaged cardiac valves.12 Interestingly, the association of a vascular graft infection with endocarditis has not been found in any of the published studies.
As previously reported,42 the current review has confirmed male sex and advanced age as risk factors for Q fever infection. Furthermore, we report the first case of Coxiella burnetii infection of an AV graft, showing that virtually every type of vascular graft may be a potential reservoir for chronic Q fever. The clinical signs and symptoms of chronic Q fever in our patient were not specific; this also proves to be the same in the cases reviewed in the literature, and probably explains why in the majority of the patients, the disease is frequently not suspected.9 Moreover, the diagnosis of Coxiella burnetii vascular graft infection is further complicated by the fact that the majority of ill patients are unaware of a previous exposure to this microorganism and also because the microbiologic workup is complex and still not universally standardized,41 Q fever can be diagnosed through cell culture, serology and molecular methods. Culture in general is not used in routine because it is difficult, time consuming and dangerous.24 The culture requires biosafety level 3 laboratories because of a high risk of transmission to laboratory workers.1 In our review, there are 9 cases in which the culture was performed,10,11,15,22,26,28,32,36,40 half of them being negative, and in none of these the culture was ahead in the diagnosis of infection due to Coxiella burnetii before serology or molecular methods.
Serology is the primary technique used to diagnose this entity in the majority of the published literature.9,17,21 In 12 of the 15 cases we studied, the initial diagnosis of Coxiella burnetii was done with serology. The different antigenic phases to which humans develop antibodies play an important role in the diagnoses. In contrast to acute Q fever infection, chronic infection is associated with increasing phase I IgG titers (typically ≥1:800) by immunofluorescent antibody tests.34 Houpikian and colleagues13 in their study of blood culture-negative endocarditis, demonstrate how serology has been included in the modified Duke criteria, considered a simple and non invasive useful tool for diagnosis.
In recent years, 16SrRNA PCR and specific PCR have demonstrated to be helpful in detecting fastidious microorganisms directly from clinical samples13 and have been proposed as a new Duke criteria for the diagnosis of infective endocarditis.23 16SrRNA is done in our laboratory routinely in explanted vascular grafts and in periprosthetic biopsies.19 This PCR method has demonstrated high sensitivity and specificity in the diagnosis of infective endocarditis.20 In 3 of the 15 patients,36,42 including ours, it was crucial for the diagnosis because there was no clinical suspicion of Coxiella burnetii infection. Considering that serology may be difficult to interpret and culture is only done in reference laboratories as we have stated above, molecular-based methods seem a reasonable approach for the diagnosis of this infection, especially when this entity is not suspected.
If left untreated, chronic Q fever leads to severe complications and to a considerable mortality rate reported up to 33.3%.3 Surgical and long-term medical treatment is actually considered the cornerstone for the correct management of such infection. In our case series, information regarding antibiotic treatment was available for 12 patients. The majority of patients received a combination of doxycycline with hydroxycloroquine (7 patients), rifampicin (2 patients), ofloxacin (2 patients), and ciprofloxacin (2 patients). Doxycycline was administered alone in 1 patient whereas another 1 received clindamycin as monotherapy. The duration of antibiotic treatment ranged between 6 to 48 months. The high variability of antibiotic regimens adopted could be related to the lack of clinical guidelines for Coxiella burnetii vascular graft infection. Extrapolating information from treatment experience of Coxiella burnetii infective endocarditis, doxycycline plus hydroxycloroquine should be the antibiotics of choice for such infection due to a reported lower relapse rate.30 As mentioned, surgery is considered the other essential step in the management of Coxiella burnetii vascular infection. Indeed, Botelho-Nevers et al3 showed that vascular surgery performed shortly after diagnosis of chronic Q fever was significantly associated with a better outcome. In the reviewed literature, surgery consisted of resection of the vascular graft in all patients, with a partial resection in 1 patient,43 and vascular reconstruction in all patients (information regarding surgery was not available in 1 case9). In our patient, only 1 of the 3 infected prosthetic grafts was completely removed because we did not expect an infection due to Coxiella burnetii. Moreover, we did not perform a surgical re-intervention as we had no macroscopic evidence of infection in the other prosthetic AV grafts, nor clear pathologic activity on subsequent PET scan. We have chosen to treat our patient in a conservative way; we will use clinical and serologic follow-up in order to guide our decisions regarding the antibiotic regimen and eventual new surgical treatment to explant the remaining grafts.
The optimum duration of the antibiotic therapy has not yet been validated because there is no definite criteria for Coxiella burnetii cure currently available. It has been suggested to perform a clinical and biological evaluation on a monthly basis during antibiotic therapy and that antibiotic treatment should be administered for at least 18 months or until a fourfold reduction of initial phase I Coxiella burnetii antibody titers has been observed.21
In conclusion, although this is a relatively rare disease, molecular techniques play a key role in the diagnosis of vascular graft infection with negative blood culture. Coxiella burnetii should be included in the differential diagnosis in all patients who have infection of an endovascular graft of any nature with an inconclusive etiologic diagnosis.
Footnotes
Abbreviations: AV = arteriovenous, CRP = C-reactive protein, CT = computed tomography, ED = emergency department, PCR = polymerase chain reaction, PET scan = positron emission tomography scan, ProCT = procalcitonin, PTFE = polytetrafluoroethylene, WBC = white blood cell count.
Joint first authors; these authors contributed equally to this work.
Financial support and conflicts of interest: The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Anderson A, Bijlmer H, Fournier PE, et al. Diagnosis and management of Q fever–United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1–30. [PubMed] [Google Scholar]
- 2.Bendermacher BL, Peppelenbosch AG, Daemen JW, et al. Q fever (Coxiella burnetii) causing an infected thoracoabdominal aortic aneurysm. J Vasc Surg. 2011;53:1402–1404. [DOI] [PubMed] [Google Scholar]
- 3.Botelho-Nevers E, Fournier PE, Richet H, et al. Coxiella burnetii infection of aortic aneurysms or vascular grafts: report of 30 new cases and evaluation of outcome. Eur J Clin Microbiol Infect Dis. 2007;26:635–640. [DOI] [PubMed] [Google Scholar]
- 4.Brouqui P, Rolain JM, Foucault C, et al. Short report: Q fever and Plasmodium falciparum malaria co-infection in a patient returning from the Comoros archipelago. Am J Trop Med Hyg. 2005;73:1028–1030. [PubMed] [Google Scholar]
- 5.Chazan JA, London MR, Pono LM. Long-term survival of vascular accesses in a large chronic hemodialysis population. Nephron. 1995;69:228–233. [DOI] [PubMed] [Google Scholar]
- 6.Eknoyan G, Levin NW. Impact of the new K/DOQI guidelines. Blood Purif. 2002;20:103–108. [DOI] [PubMed] [Google Scholar]
- 7.Ellis ME, Smith CC, Moffat MA. Chronic or fatal Q-fever infection: a review of 16 patients seen in North-East Scotland (1967–80). Q J Med. 1983;52:54–66. [PubMed] [Google Scholar]
- 8.Ethier J, Mendelssohn DC, Elder SJ, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2008;23:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier PE, Casalta JP, Piquet P, et al. Coxiella burnetii infection of aneurysms or vascular grafts: report of seven cases and review. Clin Infect Dis. 1998;26:116–121. [DOI] [PubMed] [Google Scholar]
- 10.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georghiou GP, Hirsch R, Vidne BA, Raanani E. Coxiella burnetii infection of an aortic graft: surgical view and a word of caution. Interact Cardiovasc Thorac Surg. 2004;3:333–335. [DOI] [PubMed] [Google Scholar]
- 12.Harris RJ, Storm PA, Lloyd A, et al. Long-term persistence of Coxiella burnetii in the host after primary Q fever. Epidemiol Infect. 2000;124:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore). 2005;84:162–173. [DOI] [PubMed] [Google Scholar]
- 14.Huang HL, Lin CY, Chang YT, et al. Arteriovenous graft infection caused by Candida glabrata: a case report and literature review. J Microbiol Immunol. 2013;pii:S1684-1182:00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Kloppenburg GT, van de Pavoordt ED, de Vries JP. Endograft-preserving therapy of a patient with Coxiella burnetii-infected abdominal aortic aneurysm: a case report. J Med Case Rep. 2011;5:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafrance JP, Rahme E, Lelorier J, et al. Vascular access-related infections: definitions, incidence rates, and risk factors. Am J Kidney Dis. 2008;52:982–993. [DOI] [PubMed] [Google Scholar]
- 17.Lamas CC, Eykyn SJ. Blood culture negative endocarditis: analysis of 63 cases presenting over 25 years. Heart. 2003;89:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lepidi H, Fournier PE, Karcher H, et al. Immunohistochemical detection of Coxiella burnetii in an aortic graft. Clin Microbiol Infect. 2009;15:171–172. [DOI] [PubMed] [Google Scholar]
- 19.Marin M, Garcia-Lechuz JM, Alonso P, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin M, Munoz P, Sanchez M, et al. Molecular diagnosis of infective endocarditis by real-time broad-range polymerase chain reaction (PCR) and sequencing directly from heart valve tissue. Medicine (Baltimore). 2007;86:195–202. [DOI] [PubMed] [Google Scholar]
- 21.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merhej V, Cammilleri S, Piquet P, et al. Relevance of the positron emission tomography in the diagnosis of vascular graft infection with Coxiella burnetii. Comp Immunol Microbiol Infect Dis. 2012;35:45–49. [DOI] [PubMed] [Google Scholar]
- 23.Millar B, Moore J, Mallon P, et al. Molecular diagnosis of infective endocarditis—a new Duke’s criterion. Scand J Infect Dis. 2001;33:673–680. [DOI] [PubMed] [Google Scholar]
- 24.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q-fever patients. J Clin Microbiol. 1995;33:3129–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60:1–13. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell ME, Manshani N, McCaughey C, et al. Coxiella burnetii infection of an aortic graft with multiple vertebral body erosion. J Vasc Surg. 2007;45:399–403. [DOI] [PubMed] [Google Scholar]
- 27.Padberg FT Jr, Lee BC, Curl GR. Hemoaccess site infection. Surg Gynecol Obstet. 1992;174:103–108. [PubMed] [Google Scholar]
- 28.Piquet P, Raoult D, Tranier P, et al. Coxiella burnetii infection of pseudoaneurysm of an aortic bypass graft with contiguous vertebral osteomyelitis. J Vasc Surg. 1994;19:165–168. [DOI] [PubMed] [Google Scholar]
- 29.Raoult D, Fenollar F, Stein A. Q fever during pregnancy: diagnosis, treatment, and follow-up. Arch Intern Med. 2002;162:701–704. [DOI] [PubMed] [Google Scholar]
- 30.Raoult D, Houpikian P, Tissot Dupont H, et al. Treatment of Q fever endocarditis: comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch Intern Med. 1999;159:167–173. [DOI] [PubMed] [Google Scholar]
- 31.Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet. 2005;5:219–226. [DOI] [PubMed] [Google Scholar]
- 32.Raoult D, Piquet P, Gallais H, et al. Coxiella burnetii infection of a vascular prosthesis. N Engl J Med. 1986;315:1358–1359. [PubMed] [Google Scholar]
- 33.Raoult D, Tissot-Dupont H, Foucault C, et al. Q fever 1985–1998. Clinical and epidemiologic features of 1,383 infections. Medicine (Baltimore). 2000;79:109–123. [DOI] [PubMed] [Google Scholar]
- 34.Rolain JM, Lecam C, Raoult D. Simplified serological diagnosis of endocarditis due to Coxiella burnetii and Bartonella. Clin Diagn Lab Immunol. 2003;10:1147–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. [DOI] [PubMed] [Google Scholar]
- 36.Senn L, Franciolli M, Raoult D, et al. Coxiella burnetii vascular graft infection. BMC Infect Dis. 2005;5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Signs KA, Stobierski MG, Gandhi TN. Q fever cluster among raw milk drinkers in Michigan, 2011. Clin Infect Dis. 2012;55:1387–1389. [DOI] [PubMed] [Google Scholar]
- 38.Taylor B, Sigley RD, May KJ. Fate of infected and eroded hemodialysis grafts and autogenous fistulas. Am J Surg. 1993;165:632–636. [DOI] [PubMed] [Google Scholar]
- 39.Tissot Dupont H, Raoult D, Brouqui P, et al. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med. 1992;93:427–434. [DOI] [PubMed] [Google Scholar]
- 40.Van Assen S, Houwerzijl EJ, van den Dungen JJ, et al. Vascular graft infection due to chronic Q fever diagnosed with fusion positron emission tomography/computed tomography. J Vasc Surg. 2007;46:372. [DOI] [PubMed] [Google Scholar]
- 41.Wegdam-Blans MC, Kampschreur LM, Delsing CE, et al. Chronic Q fever: review of the literature and a proposal of new diagnostic criteria. J Infect. 2012;64:247–259. [DOI] [PubMed] [Google Scholar]
- 42.Wegdam-Blans MC, ter Woorst JF, Klompenhouwer EG, et al. David procedure during a reoperation for ongoing chronic Q fever infection of an ascending aortic prosthesis. Eur J Cardiothorac Surg. 2012;42:e19–e20. [DOI] [PubMed] [Google Scholar]
- 43.Wegdam-Blans MC, Vainas T, van Sambeek MR, et al. Vascular complications of Q-fever infections. Eur J Vasc Endovasc Surg. 2011;42:384–392. [DOI] [PubMed] [Google Scholar]


