Abstract
Data on clinical, mycologic characteristics, and outcome of posttraumatic mucormycosis are scarce and often limited to case reports. From the French nationwide “RetroZygo” study, we compared posttraumatic mucormycosis cases with other forms of mucormycosis. We also reviewed reports of posttraumatic mucormycosis in the English-language literature from 1993 to 2013. We included all proven or probable cases for which underlying condition, route of infection, surgical and antifungal treatments, and outcome were detailed. From our cohort, posttraumatic mucormycosis (n = 16) differed significantly from other forms (n = 85) by rarity of underlying disease (31.2% vs 81%, p < 0.0001), frequency of cutaneous localization (87% vs 7%, p < 0.0001), short time before diagnosis (4.5 vs 21 d, p = 0.0002), species involved (Apophysomyces elegans complex and Saksenaea vasiformis), surgical requirement (93.7% vs 47%, p = 0.0006) and better survival (87.5% vs 47.6% at day 90, p = 0.03). We studied 122 cases of posttraumatic mucormycosis through our literature review. Most frequently reported traumas were traffic (37%), domestic accidents (15.1%), or natural disasters (13.4%). Mucormycosis occurred after extensive soft-tissue damage in 47.5% cases, with symptoms occurring a median of 9.5 days after trauma with necrosis being reported in 76.2% cases. Dissemination was found in 9% of patients, and bacterial coinfection in 41%. Nineteen percent of cases occurred in the Middle East or in India where Apophysomyces elegans complex was the predominant species recovered. Awareness of mucormycosis as a cause of posttrauma soft-tissue infection is warranted, especially in cases of soil-contaminated wounds. Survival is higher than in other forms of mucormycosis, but morbidity remains high.
INTRODUCTION
Mucormycosis is a rare emerging angioinvasive infection caused by ubiquitous filamentous fungi. During the past decade, several countries reported an increase in mucormycosis incidence.4,28Mucorales are present in soil and plant debris and responsible for rhinocerebral and pulmonary infections following inhalation in immunocompromised hosts such as those with diabetes mellitus or hematologic malignancy.17,32 By contrast, acquisition through disrupted cutaneous barriers, occurring after traumatic injury, is the major route of infection in immunocompetent hosts. To our knowledge, there has been no definitive, comprehensive review of the literature to date on posttraumatic mucormycosis (PTM) to guide our understanding of the epidemiology and outcome of this entity. Therefore, we took advantage of the French nationwide retrospective “RetroZygo” study to identify the clinical and epidemiologic features of PTM.17 We also reviewed the English-language literature for all cases of PTM, from 1993 to 2013 to describe their predisposing conditions, route of infection, modalities of treatment, and outcome.
PATIENTS AND METHODS
The French nationwide retrospective RetroZygo study17 aimed to collect all proven or probable mucormycosis cases diagnosed in France between 1 January 2005 and 31 December 2007. Cases were identified through 2 independent sources of recording: the French hospital information system and the National Reference Center for Mycoses and Antifungals (NCRMA). They were defined according to the 2008 European Organisation for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) criteria, only modified by the inclusion of diabetes mellitus and trauma in case of temporal relationship with mucormycosis.17 From these cases, we selected those related to a traumatic injury and compared them with other forms of mucormycosis. A case of PTM was defined as a compatible clinical presentation and histologically and/or culture documented infection occurring after a skin or mucosal injury. Clinical features, diagnostic methods, treatment and outcome of posttraumatic cases were described and compared to other mucormycosis cases of the RetroZygo cohort. The identification of strains was assessed by morphology and confirmed by molecular analysis of the ITS1-5.8S-ITS2 region.10,11 The study was approved by the Institut Pasteur Internal Review Board (2009-34/IRB) and by the Commission Nationale de l’Informatique et des Libertés according to French law.
Literature Review
Search Methods
We retrospectively reviewed the English- and French-language literature from May 1993 to May 2013 to identify all reported cases of PTM. A computer-based search of PubMed (National Library of Medicine, Bethesda, MD) was conducted, using the following keywords: “zygomycosis,” “mucormycosis,” “Rhizopus,” “Mucor,” “Rhizomucor,” “Cunninghamella,” “Mycocladus,” and “Absidia” (now classified as “Lichtheimia”), “Apophysomyces,” “Saksenaea,” “wound,” “trauma,” “injury,” “cornea,” “cutaneous,” and “immunocompetent.” The individual references listed in each publication were also reviewed to identify additional case reports.
Selection Criteria
We restricted our analysis to cases diagnosed either by histology and/or tissue cultures and for which the trauma was identified as the only source of infection. Underlying condition, mechanism of injury, clinical features, and details of antifungal and surgical treatments were required for each reported case. Mortality was assessed as all-cause mortality at 3 months from diagnosis. Because healthcare-related mucormycosis have already been reported,24 we excluded the latter infections. We also excluded burn-related mucormycosis without associated trauma because distinction between colonization and infection requires specific criteria that were not detailed enough in most reports.
Statistical Analysis
Results are expressed as median and interquartile range for continuous variables and as percentage with 95% confidence interval for categorical variables. Comparisons of 2 medians were performed using Wilcoxon rank test. Associations between categorical data were analyzed using the chi square or Fisher exact test, when appropriate. Survival analyses were realized using Kaplan-Meier method for overall survival estimation and the log rank test for survival rates comparison. All analyses were performed using SAS v. 9.1. All tests were bilateral with p < 5%.
RESULTS
Results From the RetroZygo Study
General Results
The RetroZygo study17 included 101 cases of proven (n = 60) and probable (n = 41) mucormycosis. The mean age of the patients was 50.7 years (range, 9–87 yr), with men representing the majority (58%). The 2 most common underlying diseases were hematologic malignancies (50%) and diabetes mellitus (23%). Sites of infection were lungs (28%; 79% in hematology patients), rhinocerebral (25%; 64% in diabetic patients), skin (20%), and disseminated (18%). Ninety-day survival was 56%; it was reduced in cases of dissemination compared with rhinocerebral (hazard ratio [HR], 5.38 [2.0–14.1], p < 0.001), pulmonary (HR, 2.2 [1.0–4.7], p = .04), or skin localization (HR, 5.73 [1.9–17.5], p = .002). Mucormycosis localization remained the only independent factor associated with survival.
Recent trauma were present in 18 (18%) patients. Two cases were subsequently excluded because of absence of identified skin damage in 1 case and because infection occurred at distance from the initial traumatic injury in the other case. Therefore, we considered 16 cases of PTM (15.8%) that actually occurred at the site of injury (Tables 1 and 2). One of these cases is here described in detail.
TABLE 1.
Comparison of Characteristcs of Patients With Posttraumatic Mucormycosis (PTM) and Other Forms of Mucormycosis (Other Patients) Recorded in the RetroZygo Study
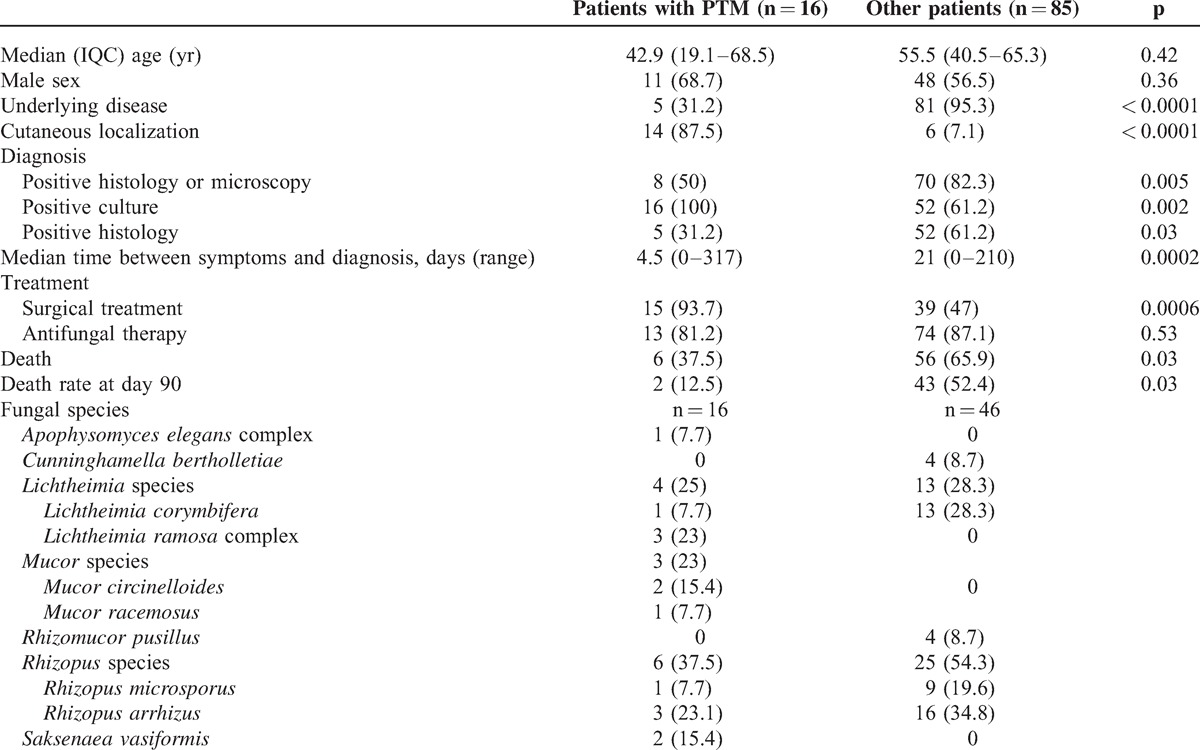
TABLE 2.
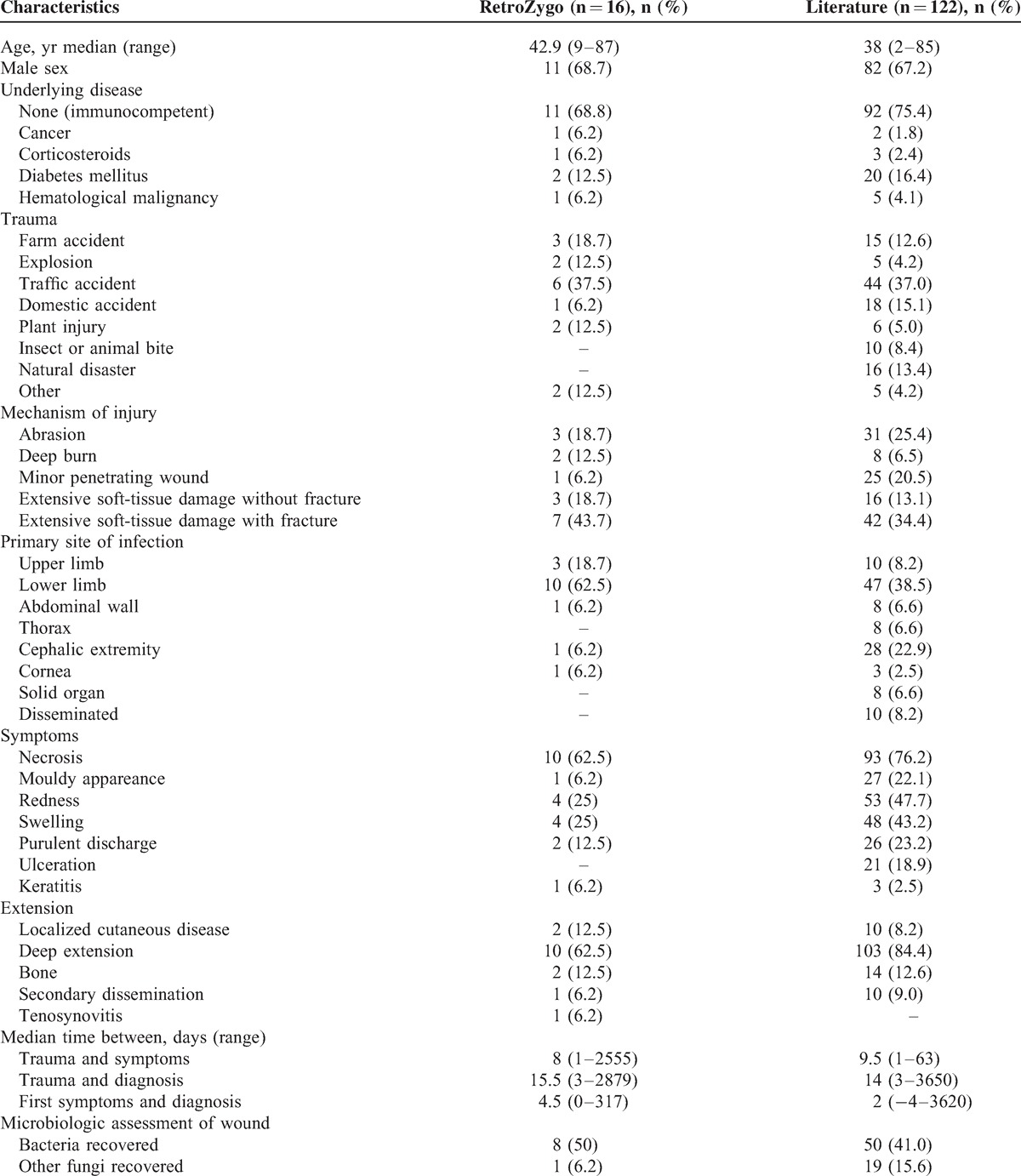
Characteristics of Posttraumatic Mucormycosis in the RetroZygo Study and in the Literature
Illustrative Case From the RetroZygo Study
A 14-year-old girl with no significant medical history was run over by a car while she was walking in a rural setting. She sustained multiple severe traumatic injuries including right lower extremity crush injury with skin degloving from the top of the thigh to the ankle, open fracture of the tibia with loss of bone fragment, deep lacerations of the anterior and posterior tibial muscles with massive contamination of the wounds by soil and plant debris. At the referring hospital, initial surgery consisted of reimplantation of the tibial fragment that was found at the scene of the accident, osteosynthesis with external fixation, and debridement of nonviable tissues. The patient initially received intravenous amoxicillin-clavulanate and massive blood transfusion. Seven days after admission, wounds of the right lower limb developed a necrotic appearance requiring an extensive debridement of all skin, subcutaneous tissue, and muscles of the posterior thigh and the anterior part of the leg (Figure 1). Direct mycologic examination and histology of the debrided tissues showed fungal hyphae within the dermis and deep subcutaneous tissue, confirming a clinically significant mould infection. Several cultures grew with a Mucorale. Rhizopus oryzae was identified on the basis of its phenotype and this identification was confirmed by internal transcribed spacer (ITS) sequencing. Because of positive culture from bone specimen, 12 days later tibial fragment was removed and a cement spacer was placed. At this time, no necrosis was seen and cultures from surgical specimens remained negative. Liposomal amphotericin B (350 mg/d) was started on the day after the first surgical debridement and continued for 21 days. Given the exposition of the tibial diaphysis, a negative pressure dressing (V.A.C therapy) was used for 10 days and reconstruction of the leg was then carried out by a latissimus dorsi flap plus several skin grafts. The patient was discharged after extensive physical therapy at a rehabilitation facility. She remained disease-free at 2 years and was able to ambulate successfully despite an arthrodesis of the right ankle with a 50° equinus deformity.
FIGURE 1.

Right leg wound 7 days after a traffic accident in a young girl. The presence of subcutaneous necrosis suggested a Mucorale infection, thereafter confirmed by mycologic culture.
Circumstances of Trauma and Mechanism of Injury
Trauma occurred outside Europe in 3 cases: 2 terrorist blasts in Lebanon and Egypt, and 1 traffic accident in Tunisia. In France, traumas were mostly due to traffic accident (n = 5) or farm working accident (n = 3). With the exception of 1 case, all infections consecutive to a minor injury (n = 4) occurred in context of soil-contaminated wounds: 2 patients had plant injury while gardening and 1 patient had minor abrasion of the foot after a stone fall (see Table 2). The remaining case was considered healthcare-associated mucormycosis and occurred 2 weeks after setting up a plaster in a young girl suffering from osteosarcoma.27 Finally, 1 patient had deep burn related community-acquired mucormycosis secondary to house fire. Compared with patients who had other forms of mucormycosis, the presence of an underlying disease was less frequently reported in patients who developed PTM (31.2% vs 81%, p < 0.0001).
Clinical Presentation
First symptoms occurred a median of 8 days (1 d to 7 yr) after the trauma. For 13 of the 16 patients, the lesion developed on the limbs (see Table 2). Except 2 cases (1 tenosynovitis and 1 keratitis), all infections involved cutaneous or subcutaneous tissues (87.5%). Only 1 patient had a secondary pulmonary dissemination of the infection. Necrosis was observed in 10 patients (62.5%), while a moldy appearance wound was noted in only 1 case. In PTM, cutaneous location was more frequent than for nontrauma-related mucormycosis (87% vs 7%, p < 0.0001).
Diagnosis
Diagnostic findings are represented in Figure 2. All cases had a mucorale growing in culture. Mucormycosis was proven for 8 patients; diagnosis was assessed by positive histology: muscle (n = 3), skin (n = 1), and bone biopsy (n = 1) or by the presence of Mucorales species in culture from a sterile site: bone biopsy (n = 1) and muscle biopsy (n = 2). Mucormycosis was probable for the remaining 8 patients. Diagnosis was based on a positive culture from a nonsterile site either alone-cutaneous biopsy (n = 3), cornea (n = 1), wound swab (n = 1)-or associated with positive direct examination (n = 3). Of note, bacteria grew from the wound in half of the patients (at the same time of PTM diagnosis in 5 cases and before diagnosis in 2 cases). Species involved were Acinetobacter baumannii (n = 1), Aeromonas species (n = 1), Bacillus cereus (n = 1), Enterobacter cloacae (n = 2), Enterococcus species (n = 2), Escherichia coli (n = 2), Morganella morganii (n = 1), Pseudomonas aeruginosa (n = 5), and Staphylococcus species (n = 2). Aspergillus niger was also recovered from the wound in 1 patient after a bomb explosion in Egypt. The median time between first symptoms and diagnosis was significantly shorter in patients with PTM than in those with other forms (4.5 vs 21 d, p = 0.0002).
FIGURE 2.
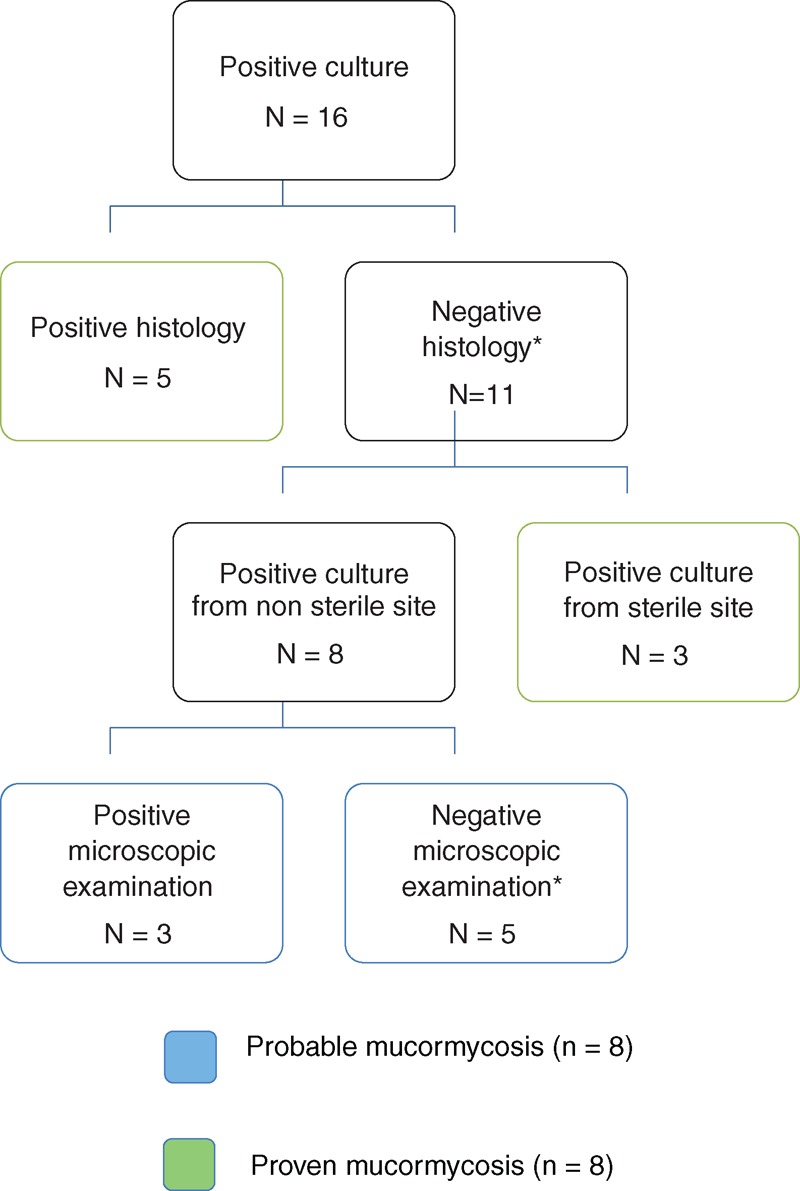
Histologic and mycologic results obtained in 16 proven or probable cases of posttraumatic mucormycosis from the RetroZygo study. ∗Negative or not done.
Mucorales Species Involved
Molecular identification to the species level was obtained from culture for 13 (81%) patients (see Table 1). Rhizopus species were predominant (n = 6) followed by Lichtheimia (n = 4), Mucor (n = 3), Saksenaea vasiformis (n = 2), and Apophysomyces elegans complex (n = 1). Remarkably, no Mucor, Saksenaea, or Apophysomyces species were found in patients with other forms of mucormycosis. However, Rhizopus and Lichtheimia species were found in the same proportion in PTM and non-PTM patients.
Treatment
In most cases, initial management consisted of a combination of medical and surgical treatments (n = 12) (Table 3). No systemic antifungal treatment was recorded for 3 patients due to ocular location, immediate premortem diagnosis, or favorable response following surgery alone. Multiple debridements were performed in 6 cases (37.5%) and the number of surgical procedures ranged from 1 to 16 (median, n = 1). Two patients needed an amputation. Only 1 patient, with favorable outcome, did not undergo surgical intervention, but liposomal amphotericin B monotherapy. Antifungal therapy was prescribed for a median duration of 23 days (range, 5–216 d). The most commonly used drug was liposomal amphotericin B (L-AmB), administered in 11 patients at a median daily dose of 5 mg/kg (range, 3–10 mg/kg). When prescribed, local antifungal therapy was always associated with systemic therapy excepted for keratitis. It consisted of antifungal drops for ocular localized infection, local application of amphotericin and amphotericin-impregnated beads in 1 case each. Most of patients (n = 9, 60%) were admitted to intensive care unit and all except the 1 with keratitis received concomitant antibacterial therapy. Compared to patients with other forms of mucormycosis, surgery was more frequently performed in patients with PTM (93.7% vs 47%, p = 0.0006).
TABLE 3.
Treatment and Outcome of Patients With Posttraumatic Mucormycosis in the RetroZygo Study and in the Literature

Outcome
The global survival rate was 62.5% (median duration of follow-up, 36 months; range 0–67), which was significantly higher than patients with other form of infection (34.1%, p = 0.03). Similarly, survival rate at day 90 was significantly higher than patients with other forms of infection (87.5% vs 47.6%, p = 0.03) (Figure 3).
FIGURE 3.

Survival of posttraumatic (dotted line) versus other forms of mucormycosis (blue line) in the RetroZygo study.
Review of 122 Other Cases of PTM From the Literature
A total of 122 individual cases of PTM were identified from our literature review between 1993 and 2013, of which 85 (69.7%) were recorded after 2002 (Figure 4). Of these cases, 99 were defined as proven and 23 as probable mucormycosis. The majority of cases were from Europe (n = 34) or North America (n = 40), but a significant number occurred in Middle East or in India (n = 23) (Figure 5). There was a larger proportion of male (67.2%) with a median age of 38 years (see Table 2). An underlying disease was reported for 30 patients (24.6%). Most common traumatisms were traffic accident (37%), domestic accidents (15.1%), natural disaster (14%), farm working accident (12.6%), and animal or insect bite (8.4%) (including 2 scorpion stings, 1 spider bite, 1 magpie peck, and 1 dog scratch).2,3,15,18,23,29,34,35,37,39 Natural disasters were Joplin tornado (13 cases),22 collapse of building (1 case),2 and Asian tsunami (2 cases)1,33 (Figure 6). Half of wounds were described as extensive soft-tissue damage, 8 (6.5%) deep burns that occurred in a soil contamination context, 25 (20.5%) as penetrating wound and 31 (25.4%) as superficial lesions. The latter 2 included a great proportion of insect or animal bites,2,3,15,18,23,29,34,35,37,39 penetrating injury with plant material (for example, a thorn), but also surprising traumas as crab trap16 or wooden matchstick injuries.13 More than one-third of the wounds were observed on the lower limbs (n = 47), followed by the cephalic extremity (n = 28) (see Table 2). Three patients had keratitis and solid organ was the primary site of infection in 8 cases: kidney (n = 1), liver (n = 1), stomach (n = 1), lungs (n = 2) and peritoneum (n = 2). Of the 98 patients with available data, patients developed symptoms of mucormycosis after a median of 9.5 days (range, 1–63 d) after trauma. Necrosis was reported in 93 (76.2%) patients while a mould appearance was noted in 27 (22.1%) patients. Subsequent dissemination occurred in 10 cases, including 2 cases in a context of immunosuppression. Organs involved were lungs (n = 4), kidney (n = 1), abdominal cavity (n = 2), stomach and brain (n = 1); the remaining 2 patients had multivisceral dissemination, which was diagnosed at autopsy.
FIGURE 4.

Distribution of 122 published cases of posttraumatic mucormycosis, 1993–2013. Each bar represents a 5-year period except for the last, which represents 6 years.
FIGURE 5.
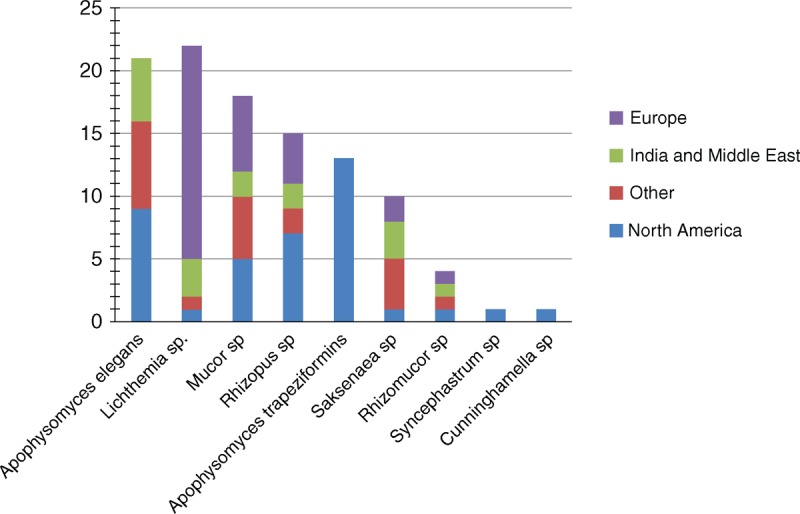
Distribution of Mucorales species according to the geographical origin in the literature review.
FIGURE 6.
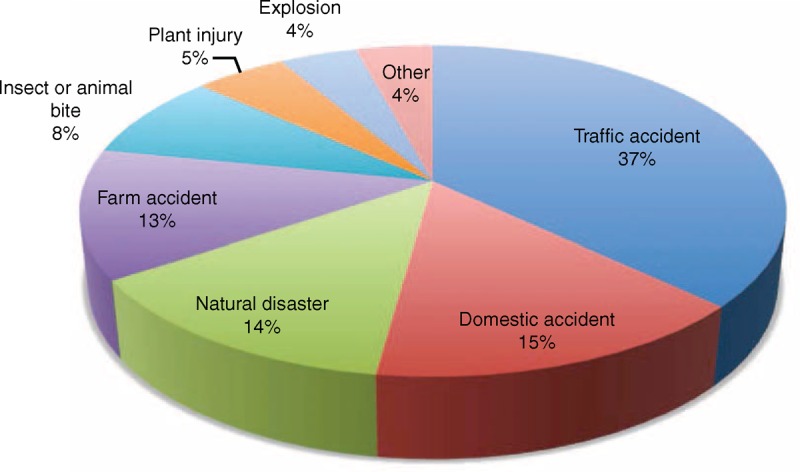
Traumas responsible for 122 mucormycosis in the literature.
Culture was positive in 111 cases leading to the identification of Absidia and Apophysomyces species as the most frequent pathogens (21.1% and 31.7%, respectively), followed by Rhizopus (14.4%), Mucor species (15.4%), and Saksenaea species (9%) (see Figure 5). The median interval between symptoms onset and diagnosis was 2 days (range, −4–3620 d]. Bacteria and other fungi were recovered from incident wounds in 50 (41%) and 19 (15.6%) patients, respectively. The majority of patients underwent surgery (91.6%). As the infection progressed, additional debridements were performed for 85 (69.7%) patients with a median of 2 surgical procedures per patient (range, 0 to 19). Amputation and enucleation were performed in 18 (16.5%) and 9 (8.1%) patients respectively. Antifungal treatment consisted of monotherapy of amphotericin B deoxycholate for 54 patients (44.3%) or L-AmB for 45 patients (36.9%). Finally, 90 (74.4%) patients had a favorable outcome.
DISCUSSION
To our knowledge, this is the largest series to date of trauma-related mucormycosis on the basis of a nationwide retrospective study. Based on 2 independent data sources, these cases are estimated to be representative of 77% of all PTM cases occurring in the country over a 3-year period.5 Although hematologic malignancy is the predominant risk factor of mucormycosis, PTM are a major concern as they represent the third cause of mucormycosis in the RetroZygo study (15.8%)17 and the second cause (17%, equal with diabetes mellitus) in a recent European study.32 Moreover, concordant with the increased description of mucormycosis in Europe, United States, and India,8,21,32 the number of published cases increased significantly over time.
It is noteworthy that epidemiologic, clinical, mycologic and outcome characteristics of PTM differ from those observed during other forms of mucormycosis. Indeed, most patients with PTM both in the RetroZygo cohort and in the present literature survey did not present any underlying condition, in accordance with results of a European study, that reported 35/39 PTM patients to be immunocompetent.32 In contrast with other forms of mucormycosis, PTM occurs as the result of direct inoculation of spores into damaged skin with contaminated material during trauma. In addition to huge number of spores present in soil contaminated wounds, acidosis due to large soft tissue damage and lack of tissue viability associated with local immunodepression could explain the pathogenicity of Mucorales after trauma. Indeed, a major trauma by itself has been shown to cause a systemic immunocompromised state.38
In both RetroZygo and literature studies, PTM occurred mainly after extensive tissue damage as degloving, amputation and tear, where a massive soil contamination is observed. However, the initial trauma can also be minimal since 18% to 25% cases in our study were related to superficial abrasion. Cutaneous inoculation of Mucorales may have underestimated consequences since infection can rapidly invade deeper tissue, and eventually disseminate, that occurred in 84.4% and 9% cases in our review, respectively. A necrotic lesion in an injured patient should raise suspicion of mucormycosis. However, this sign may be absent in the first stages of the disease, and symptoms may be non-specific as those commonly observed during bacterial infections.
Most patients developed symptoms in the first 10 days after injury, but the disease can progress slowly, especially in cases of superficial abrasions. Recently, Lu et al reported 5 cases of PTM from China caused by Mucor irregularis (formerly Rhizomucor variabilis) that all shared unusual clinical features.19,20 Following minor trauma, infection was confined to superficial tissues and progressed very slowly, in the range of 7 months to 18 years. As a consequence, Mucorales should always be suspected as potential agents of soft-tissue infections in injured patients even in case of minor injury, indolent development or aspecific symptoms. This is of major concern because early diagnosis improves outcome.7 To prove fungal tissue infection, biopsy sample from infected lesions should be performed systematically for histologic and mycologic investigations. We evidence that bacterial coinfection can frequently be recovered from wounds either before or together with Mucorales, which may finally lead to misdiagnose isolated bacterial infection. Recovering bacteria should rather make clinicians even more suspicious since it reflects generally a massive soiled contamination. Furthermore, it was recently reported that bacterial infections of wounds increased the risk of subsequent mucormycosis after tornado injury.22
Of major importance, distribution of Mucorales species differed between PTM patients compared to others, especially for 2 species. Indeed, S. vasiformis and A. elegans complex were respectively responsible for 2 and 1 cases in RetroZygo study (including 2 cases for whom trauma occurred in Liban [Lebanon] and in Egypt) and for 9% and 18.9% cases of PTM in the literature, respectively. In contrast, these species were not recovered in other patients from RetroZygo study and recovered in only 5% and 6% cases in the review of 929 cases of mucormycosis by Roden et al.26S. vasiformis and A. elegans complex, are different from the typical opportunistic Mucorales as they are mainly involved in cutaneous infections from immunocompetent patients, especially from southern countries.12 Warkentien et al have recently reported a series of 37 invasive mold infections following combat-related injuries in United States military personnel.36 Of the 16 Mucorales infections reported, 6 were related to A. elegans and 2 to S. vasiformis. Most cases occurred in Afghanistan. To our knowledge, A. elegans was first isolated in 1979 from soil samples in a mango orchard in Northern India. Since that time, this species has been reported with increasing frequency as a cause of cutaneous PTM, especially on the Indian subcontinent, where it is the second most common isolate (19%) following Rhizopus arrhizus (oryzae).6 Other forms of mucormycosis due to A. elegans are rare and seem to occur mostly in apparently immunocompetent patients.12 Two other species of the genus Apophysomyces were recently described: Apophysomyces variabilis was identified as the infecting pathogen in 5 Indian patients with primary cutaneous mucormycosis14 and Apophysomyces trapeziformis was responsible for 13 cases of PTM following the tornado that occurred in Missouri in 2011.22 Similarly, S. vasiformis has increasingly been reported as a cause of cutaneous infections in immunocompetent patients, but only rarely as a cause of rhino-orbito-cerebral, disseminated, and pulmonary infections.25
Most patients with PTM underwent surgical treatment including multiple debridements or amputation. Surgical resection is of primary importance in PTM as infection spreads rapidly, leading to a large area of necrosis with poor penetration of antifungal agents. Surgery was demonstrated as predictive of better outcome in previous studies.26,30,32 Both recent third European Conference of Infections in Leukemia (ECIL-3) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommendations emphasize a timely debridement of all devitalized tissues to treat PTM. The wound must be closely monitored and surgery must be repeated when new clinical features strongly suggest disease progression.9,31
Prognosis of PTM is better than that observed during other forms of mucormycosis (90-day mortality of 12.5% vs 52.4%). This is in accordance with a previous major report in which patients with PTM had an 86% smaller probability of death compared with patients with hematological malignancies.32 Such differences may have several explanations. First, fewer patients had an underlying disease in the PTM group; secondly, median time between first symptoms and diagnosis was significantly shorter in patients with PTM. Finally, we can raise the question of the role of the greater proportion of patients with PTM who underwent surgery may contribute to a better outcome.
In conclusion, this study provides new insights into PTM by comparison with other forms of mucormycosis. Fungal species involved vary with geographical location and are frequently associated with bacterial coinfection. Increased attention to environmental fungi as a possible cause of soft-tissue infection in trauma patients is thus warranted and early surgical treatment and adapted antifungal therapy are required to avoid local extension and fungal dissemination, even in apparently nonimmunocompromised patients.
ACKNOWLEDGMENTS
The authors thank Pr. Françoise Dromer, Dr. Dounia Bitar, and Gloria Morizot for their major contributions to the RetroZygo study.
APPENDIX: The French Mycosis Study Group
The authors thank the following principal investigators of the French Mycosis Study Group (France), who contributed to the current database. Those outside Paris are as follows (in alphabetical order by city): Agen (C. Freimann, P. Rispal), Amiens (E. Lewandowski, T. Chouaki, H. Dupont, G. Damaj, P. Mertl), Angers (P. Six, J. P. Bouchara, H. Hitoto, N. Ifrah, T. Urban), Apt (T. Carrelet), Arles (J. P. Pellegrini), Aurillac (A. Mosser), Auxerre (A. Calmelet), Bagnères de Bigorre (F. Braman), Bain de Bretagne (D. Bordes), Belfort (D. Pobel), Besanxcon (A. Dussaucy, J. F. Viel, F. Grenouillet, G. Blasco, F. Legrand, J. C. Navellou, E. Plouvier, L. Tavernier, B. Kantelip), Bordeaux (A. Kostrzewa, B. Couprie, J. P. Bebear, E. Blanchart, L. De Gabory, M. Dupon, C. Deminière), Boulogne Billancourt (L. Comar), Bourg en Bresse (N. Peslin), Brest (J. M. Cauvin), Bry sur Marne (G. B. Ibara), Caen (M. J. D’Alche-Gautier, C. Duhamel, R. Verdon), Cahors (A. Simons), Chalons en Champagne (F. Canas, P. Berger), Charleville (I. Frangi), Chartres (P. Denier), Cherbourg (G. Acher, M. Barre), Clamart (C. Soler, L. Bargues, D. Brousse, J. P. Perez), Clermont Ferrand (J. Afonso, D. Pons, J. O. Bay, C. Jacomet, P. Dechelotte), Créteil (S. Bretagne, K. Belhadj), Dijon (C. Quantin, D. Caillot), Doullens (M. Derancourt), Dreux (J. M. Vogt), Echirolles (J. F. Peresse), Foix (E. Hornus), Frejus (Y. Pinier, J. Gutnecht), Grande Synthe (F. Pierchon), Garches (S. Baron), Grenoble (J. Fauconnier, B. Lebeau), Ispoure (J. Sahuc), Lens (C. Courouble, P. Morel), Libourne (D. Crenn), Lievin (B. Duquesne), Lille (B. Sendid, C. Berthon, V. Coiteux), Limoges (A. Vergnenègre, D. Ajzenberg, I. Pommepuy), Lorient (J. B. Boubier), Lyon (P. Messy, A. L. Bienvenu, Y. Franxcois, M. Michallet, J. L. Peix), Mainvilliers (T. Al Jijakli), Mantes la Jolie (M. Meyer), Marseille (M. Bertrand, V. J. Bardou, M. H. Giovannini, B. Beylot, S. Ranque, A. Stein, S. Masson, P. Metellus, A. M. Stoppa), Meaux (A. Fiacre, G. Kassab), Melun (G. Amghar), Montpellier (C. Michelon-Meslé, P. Rispail, L. Durand), Nancy (K. Alzahouri, M. Machouart, A. Debourgogne, Dr Witz, J. F. Chassagne, A. Gerard, E. Simon), Nanterre (D. Carradot), Nantes (D. Antonioli, F. Gay-Andrieu, N. Corradini), Nice (G. Daideri, M. Gari-Toussaint, H. Hyvernat, M. Poiree, P. M. Roger), Nimes (C. Lechiche, L. Lachaud), Niort (A. Garcia, S. Labbe), Peronne (P. Decroix), Perpignan (M. Saada, C. Billotet), Poitiers (C. Kauffman-Lacroix, C. Godet), Pontoise (D. Decup), Reims (D. Blanc, H. Daragon, D. Toubas, C. Nérot), Rennes (A. Fresnel, X. Carsin, J. P. Gangneux, C. Arvieux, T. Lamy de la Chapelle, N. Rioux), Ruffec (E. P. Satre), Salons de Provence (G. Gineyt), Sens (M. Fourre), Saint-Etienne (J. M. Rodrigues, B. Trombert, P. Vercherin, H. Raberin, J. Cornillon, F. Lucht), Saint Julien en Genevois (D. Pietri), Sainte Catherine (P. H. Miquel), Strasbourg (F. Binder, N. Roeslin, V. Letscher-Bru, J. P. Dupeyron, R. Herbrecht), Suresnes (C. Leclerc), Toulon (C. Michel, H. Redier), Toulouse (L. Molinier, S. Cassaing, M. Alvarez, L. Esposito, B. Marchou, X. Brousset, E. Uro-Coste), Tourcoing (I. Vérin, P. Patoz, S. Alfandari, X. Lemaire, Y. Yazdanpanah, X. Froment), Tours (S. Sunder, J. Chandenier, E. Gyan), Troyes (F. Benaoudia), Valence (D. Le Magny), Valenciennes (J. Kohler), Vannes (H. Journel), Verdun (J. Deleau), Vesoul (P. Selles), Villiers Semeurs (F. Sini).
Principal investigators of the study group in Paris are as follows (in alphabetical order by affiliation): Antoine Beclère (F. Doucet-Populaire, L. Savale), Avicenne (C. Bouges-Michel), Bichat (C. Chochillon, O. Brugière, M. Wolff, V. Joly, H. Adle-Biassette), Cochin (M. T. Baixench), HEGP (G. Chatellier), Hôtel Dieu (M. Cornet), Institut Montsouris (Y. Péan), Lariboisière (T. Meas, R. Kania), Necker (C. Lebihan, M. E. Bougnoux), Pitié Salpétrière (A. Fekkar, A. Datry, L. Bodin, Y. Samson), Robert Debré (B. Brethon), Saint-Antoine (M. Develoux, F. Isnard), Saint-Joseph (H. Pons-Pouchelle), Saint-Louis (C. Lacroix, P. Ribaud, A. Xhaard, A. Bergeron-Lafaurie, O. Marie, J. Pavie, E. Raffoux), Tenon (A. Parrot).
Footnotes
Abbreviations: ITS = internal transcribed spacer, L-AmB = liposomal amphotericin B, PTM = posttraumatic mucormycosis.
Financial support and conflicts of interest: The authors have no financial support to declare for this work. F.L. has received travel grants or speaker's fees from MSD and Gilead Science. O.L. has been a consultant for Gilead Sciences, and has received grants or speaker's fees from Astellas, Pfizer, Merck, and Gilead Sciences. All other authors report no potential conflicts.
Contributor Information
Collaborators: and the French Mycosis Study Group
REFERENCES
- 1.Andresen D, Donaldson A, Choo L, et al. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005; 365:876–878. [DOI] [PubMed] [Google Scholar]
- 2.Arnaiz-Garcia ME, Alonso-Pena D, Gonzalez-Vela M, et al. Cutaneous mucormycosis: report of five cases and review of the literature. J. Plast. Reconstr. Aesthetic Surg. JPRAS. 2009; 62:434–441. [DOI] [PubMed] [Google Scholar]
- 3.Bearer EA, Nelson PR, Chowers MY, Davis CE. Cutaneous zygomycosis caused by Saksenaea vasiformis in a diabetic patient. J Clin Microbiol. 1994; 32:1823–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitar D, Van Cauteren D, Lanternier F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009; 15:1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitar D, Morizot G, Van Cauteren D, et al. Estimating the burden of mucormycosis infections in France (2005-2007) through a capture-recapture method on laboratory and administrative data. Rev Epidemiologie Sante Publique. 2012; 60:383–387. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Chatterjee SS, Das A, et al. Invasive zygomycosis in India: experience in a tertiary care hospital. Postgrad Med J. 2009; 85:573–581. [DOI] [PubMed] [Google Scholar]
- 7.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008; 47:503–509. [DOI] [PubMed] [Google Scholar]
- 8.Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis. 2006; 25:215–229. [DOI] [PubMed] [Google Scholar]
- 9.Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014; 20 Suppl 3:5–26. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Hermoso D, Dannaoui E, Lortholary O, Dromer F. Agents of Systemic and Subcutaneous Mucormycosis and Entomophthoromycosis. Man Clin Microbiol. 10th Ed Washington, DC: ASM Press; 2011. [Google Scholar]
- 11.Garcia-Hermoso D, Hoinard D, Gantier J-C, et al. Molecular and phenotypic evaluation of Lichtheimia corymbifera (formerly Absidia corymbifera) complex isolates associated with human mucormycosis: rehabilitation of L. ramose. J Clin Microbiol 2009; 47:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes MZR, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011; 24:411–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal A, Tyagi I, Syal R, et al. Apophysomyces elegans causing acute otogenic cervicofacial zygomycosis involving salivary glands. Med Mycol. 2007; 45:457–461. [DOI] [PubMed] [Google Scholar]
- 14.Guarro J, Chander J, Alvarez E, et al. Apophysomyces variabilis infections in humans. Emerg Infect Dis. 2011; 17:134–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hicks WL, Jr, Nowels K, Troxel J. Primary cutaneous mucormycosis. Am J Otolaryngol. 1995; 16:265–268. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M, Smith MB, McGinnis MR. Zygomycosis due to Apophysomyces elegans: report of 2 cases and review of the literature. Arch Pathol Lab Med. 1999; 123:386–390. [DOI] [PubMed] [Google Scholar]
- 17.Lanternier F, Dannaoui E, Morizot G, et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin Infect Dis. 2012; 54 Suppl 1:S35–43. [DOI] [PubMed] [Google Scholar]
- 18.Lechevalier P, Hermoso DG, Carol A, et al. Molecular diagnosis of Saksenaea vasiformis cutaneous infection after scorpion sting in an immunocompetent adolescent. J Clin Microbiol. 2008; 46:3169–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Liu Z, Shen Y, et al. Primary cutaneous zygomycosis caused by rhizomucor variabilis: a new endemic zygomycosis? A case report and review of 6 cases reported from China. Clin Infect Dis. 2009; 49:39–43. [DOI] [PubMed] [Google Scholar]
- 20.Lu X-L, Najafzadeh MJ, Dolatabadi S, et al. Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Persoonia. 2013; 30:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr KA, Carter RA, Crippa F, et al. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002; 34:909–917. [DOI] [PubMed] [Google Scholar]
- 22.Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012; 367:2214–2225. [DOI] [PubMed] [Google Scholar]
- 23.Pourahmad M, Sepidkar A, Farokhnia MH, et al. Mucormycosis after scorpion sting: case report. Mycoses. 2013; 56:589–591. [DOI] [PubMed] [Google Scholar]
- 24.Rammaert B, Lanternier F, Zahar J-R, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012; 54 Suppl 1:S44–54. [DOI] [PubMed] [Google Scholar]
- 25.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000; 13:236–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41:634–653. [DOI] [PubMed] [Google Scholar]
- 27.Roux BG-L, Mechinaud F, Gay-Andrieu F, et al. Successful triple combination therapy of disseminated absidia corymbifera infection in an adolescent with osteosarcoma. J Pediatr Hematol Oncol. 2010; 32:131–133. [DOI] [PubMed] [Google Scholar]
- 28.Saegeman V, Maertens J, Meersseman W, et al. Increasing incidence of mucormycosis in University Hospital, Belgium. Emerg Infect Dis. 2010; 16:1456–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saravia-Flores M, Guaran DM, Argueta V. Invasive cutaneous infection caused by Apophysomyces elegans associated with a spider bite. Mycoses. 2010; 53:259–261. [DOI] [PubMed] [Google Scholar]
- 30.Singh N, Aguado JM, Bonatti H, et al. Zygomycosis in solid organ transplant recipients: a prospective, matched case-control study to assess risks for disease and outcome. J Infect Dis. 2009; 200:1002–1011. [DOI] [PubMed] [Google Scholar]
- 31.Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013; 98:492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011; 17:1859–1867. [DOI] [PubMed] [Google Scholar]
- 33.Snell BJ, Tavakoli K. Necrotizing fasciitis caused by Apophysomyces elegans complicating soft-tissue and pelvic injuries in a tsunami survivor from Thailand. Plast Reconstr Surg. 2007; 119:448–449. [DOI] [PubMed] [Google Scholar]
- 34.Tapish S, Taha M, Naresh G, et al. Primary mucormycosis of abdominal wall: A rare fungal infection in a immunocompetent patient. Indian J Surg. 2010; 72 Suppl 1:306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trotter DJ, Gonis G, Cottrill E, Coombs C. Disseminated Saksenaea vasiformis in an immunocompetent host. Med J Aust. 2008; 189:519–520. [DOI] [PubMed] [Google Scholar]
- 36.Warkentien T, Rodriguez C, Lloyd B, et al. Invasive mold infections following combat-related injuries. Clin Infect Dis. 2012; 55:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberg WG, Wade BH, Cierny G, 3rd, et al. Invasive infection due to Apophysomyces elegans in immunocompetent hosts. Clin Infect Dis. 1993; 17:881–884. [DOI] [PubMed] [Google Scholar]
- 38.Wichmann MW, Ayala A, Chaudry IH. Severe depression of host immune functions following closed-bone fracture, soft-tissue trauma, and hemorrhagic shock. Crit Care Med. 1998; 26:1372–1378. [DOI] [PubMed] [Google Scholar]
- 39.Wilson PA. Zygomycosis due to Saksenaea vasiformis caused by a magpie peck. Med J Aust. 2008; 189:521–522. [DOI] [PubMed] [Google Scholar]


