Abstract
Few studies have examined the occurrence of minimal change nephrotic syndrome (MCNS) in patients with non-Hodgkin lymphoma (NHL). We report here a series of 18 patients with MCNS occurring among 13,992 new cases of NHL. We analyzed the clinical and pathologic characteristics of this association, along with the response of patients to treatment, to determine if this association relies on a particular disorder. The most frequent NHLs associated with MCNS were Waldenström macroglobulinemia (33.3%), marginal zone B-cell lymphoma (27.8%), and chronic lymphocytic leukemia (22.2%). Other lymphoproliferative disorders included multiple myeloma, mantle cell lymphoma, and peripheral T-cell lymphoma. In 4 patients MCNS occurred before NHL (mean delay, 15 mo), in 10 patients the disorders occurred simultaneously, and in 4 patients MCNS was diagnosed after NHL (mean delay, 25 mo). Circulating monoclonal immunoglobulins were present in 11 patients. A nontumoral interstitial infiltrate was present in renal biopsy specimens from 3 patients without significant renal impairment. Acute kidney injury resulting from tubular lesions or renal hypoperfusion was present in 6 patients. MCNS relapse occurred more frequently in patients treated exclusively by steroid therapy (77.8%) than in those receiving steroids associated with chemotherapy (25%). In conclusion, MCNS occurs preferentially in NHL originating from B cells and requires an aggressive therapeutic approach to reduce the risk of MCNS relapse.
INTRODUCTION
Minimal change nephrotic syndrome (MCNS) is an acquired glomerular disease characterized by massive selective proteinuria and hypoalbuminemia occurring in the absence of glomerular cell infiltrate or immunoglobulin deposits.24 The pathogenesis of this glomerular disease remains poorly understood, but experimental studies and clinical observations point to an origin in the immune system.24,33,36 The current major hypothesis is that MCNS results from immune cell disorders, leading to the release of a putative circulating factor that induces podocyte dysfunction and alters glomerular permeability, resulting in nephrotic proteinuria. Nonetheless, the identity of this factor remains elusive. Several potential candidates, including hemopexin, cardiotrophin-like cytokine 1, interleukin-13, tumor necrosis factor-α, soluble urokinase plasminogen activating receptor (suPAR), and angiopoietin-like 4 have been reported in primary focal segmental glomerulosclerosis (FSGS) and MCNS.9,35,36 The recent identification of new molecules that may also be involved, including CD80 and c-mip, has helped to clarify our understanding of the molecular basis of podocyte dysfunction in MCNS patients.18,37
A large spectrum of glomerular diseases that is considered as paraneoplastic glomerulonephritis (that is, not directly related to monoclonal para-protein deposits in glomeruli) has been described within the context of lymphoid proliferation disorders. However, the underlying molecular mechanisms linking these conditions remain mostly unknown.8,19,22,28 MCNS is the most frequent glomerular disease associated with chronic lymphoid neoplasms and occurs preferentially in patients with classical Hodgkin lymphoma (cHL).8,19,22,28 We previously evaluated the clinical and histologic characteristics of this association in 21 patients, as well as the response of these patients to treatment.4 Moreover, we demonstrated that c-mip overexpression, resulting from a dysregulation of proximal signaling in both podocytes and tumoral cells, may be a molecular signature of this association.5
In contrast to the extensively described association of MCNS with cHL, only a few studies have examined the association of MCNS with non-Hodgkin lymphoma (NHL). NHL is a heterogeneous group of malignancies that originate from either B, T, or NK cells. There are many subtypes of NHL, each of which has distinct clinical, morphologic, and immunophenotypic features.29 A small number of case reports have suggested that MCNS may be associated with several subtypes of NHL; however, this association has not been studied in depth.7,10,13,17,20,21,31 These case reports highlight a close relationship between the progression of NHL and MCNS, suggesting that MCNS may be considered as a paraneoplastic glomerulonephritis in the context of NHL.8,19,22,28
We report here a retrospective French study including 18 patients with MCNS occurring in the context of NHL. We aimed to clarify the pathologic and clinical characteristics of this association and to identify some of its distinctive features, which may provide new insights into the pathophysiology of both diseases.
METHODS
Patients
Eighteen adult patients with biopsy-proven MCNS occurring among 13,992 cases of NHL were retrospectively identified. These patients had been followed between 1997 and 2011 in 10 French departments of nephrology and hematology: Henri Mondor Hospital, La Pitié Salpetrière Hospital, European Georges Pompidou Hospital, Tenon Hospital, Bicètre Hospital, Poissy Saint Germain en Laye Hospital, Charles Nicolle Hospital, Pasteur Hospital, Cambrai Hospital, and Bretonneau Hospital. In each hospital, patients were identified from renal pathology and clinical diagnosis databases and from computerized databases of the LYSA (Lymphoma Study Association). Patients with hemophagocytic syndrome and with MCNS occurring within the context of cHL were excluded from the study. Demographic, clinical, laboratory, and histologic data were assessed for each patient at the time of MCNS diagnosis. This study was approved by our ethical committee of clinical research (Assistance Publique des Hôpitaux de Paris) in accordance with international ethics codes and guidelines.
Three groups of patients were identified according to the timing of the occurrence of the 2 diseases: MCNS preceded NHL (group 1, 4 patients), both diseases occurred simultaneously (group 2, 10 patients), or MCNS followed NHL (group 3, 4 patients). MCNS and NHL were considered as occurring simultaneously when the interval between the 2 diseases was less than 3 months. Follow-up data were obtained for all patients except 1 (Patient [Pt] 11). Adverse events related to the particular strategy of therapeutic management were recorded for all patients who were followed up. The occurrence of death was also recorded.
MCNS Characterization
All patients included in the study underwent a renal biopsy for the assessment of nephrotic syndrome (NS) (defined as a urinary protein excretion rate >3000 mg/d and serum albumin concentrations <3 g/dL). MCNS diagnosis was confirmed by a pathologic study showing the presence of minimal change glomerular lesions without segmental sclerosis upon examination by light microscopy. Immunofluorescence did not reveal the presence of immunoglobulin deposits. Pathologic findings included tubular and/or interstitial damage. Extensive immunohistochemistry was systematically performed if interstitial inflammation was observed in renal biopsy specimens. Acute tubular lesions were classified into 4 grades according to the severity of tubular injury30: Grade 0: absent; Grade 1: occasional necrotic tubules dispersed throughout the renal cortex; Grade 2: small groups of necrotic tubules discontinuously distributed throughout the renal cortex; Grade 3: coalescent groups of necrotic tubules easily visualized in the renal cortex; Grade 4: extensive areas of tubular necrosis scattered throughout the renal cortex. Acute kidney injury (AKI) was defined according to AKIN (acute kidney injury network) criteria25: Stage 1 is defined as an increase in serum creatinine of ≥0.3 mg/dL or increase to ≥150%–200% from baseline; Stage 2 as an increase in serum creatinine to >200%–300% from baseline; and Stage 3 as an increase in serum creatinine to >300% from baseline or serum creatinine of ≥4 mg/dL with an acute increase of at least 0.5 mg/dL). For all patients, serum creatinine concentrations were measured at the time of hospital admission for suspected kidney disease, and these concentrations were compared with the baseline value (the lowest serum creatinine concentration measured during the 6 mo preceding hospitalization). Hypertension was defined as blood pressure above 140/90 mm Hg. Treatment of MCNS differed according to patient group but included steroid therapy in all patients and an additional chemotherapy regimen for some of them. Complete remission of NS consisted of a return of urine protein excretion to the normal range (<300 mg/d) combined with normal serum albumin concentrations (>3 g/dL). “Steroid dependence diagnosis" was defined as relapse in proteinuria following the decrease of steroid therapy. “Steroid resistance diagnosis" was defined as the persistence of NS after an average treatment of 10 weeks (range, 8–12 wk) of steroid therapy. Relapse of MCNS was defined as the reappearance of proteinuria (increased urine protein excretion >3000 mg/d). 34
NHL Characterization
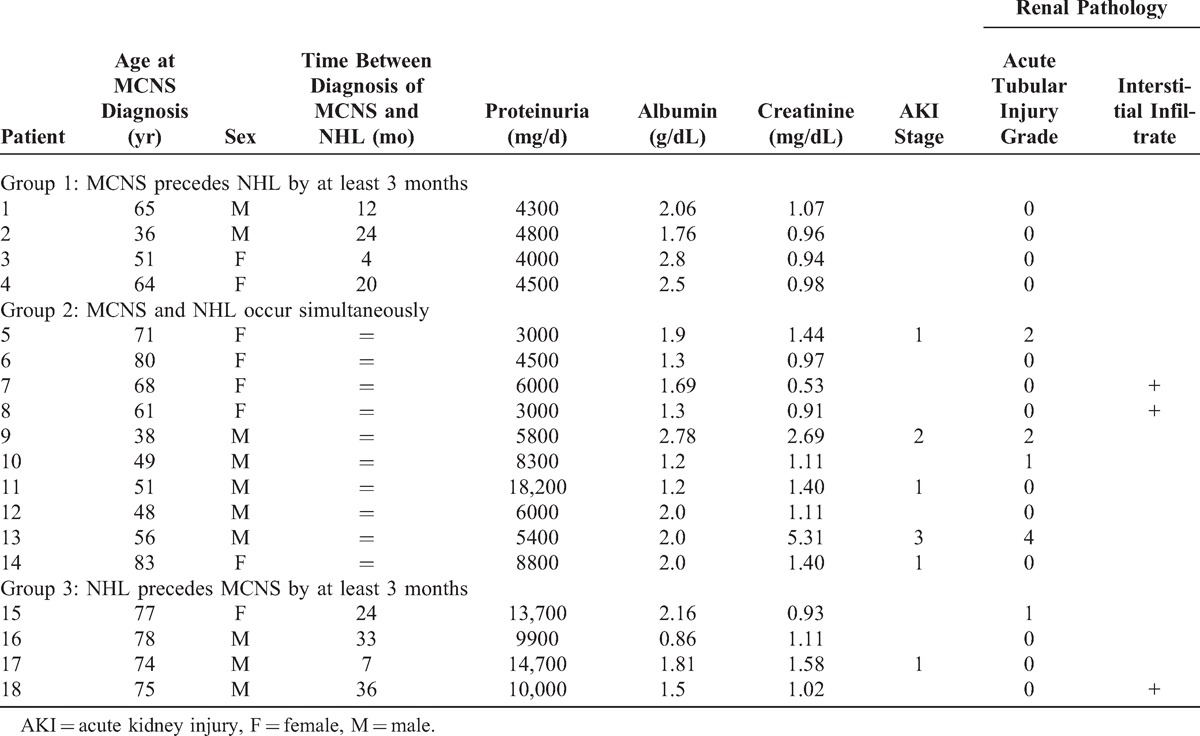
The diagnosis of chronic lymphocytic leukemia (CLL) was made according to standard criteria of cell cytology and immunophenotyping.15 The diagnosis of Waldenström macroglobulinemia (WM) required the presence of serum monoclonal IgM associated with intratrabecular polymorphic lymphoplasmacytic infiltrate in bone marrow.14 Diagnosis of multiple myeloma (MM) was based on the definition by the International Myeloma Working Group.2 Diagnosis of other lymphoproliferative disorders was based on the World Health Organization (WHO) classification and required pathologic confirmation.29 At the time of hematologic diagnosis, Ann Arbor staging was assessed, if appropriate. Systemic symptoms at presentation were recorded. These consisted of fever for >8 days, night sweats, and weight loss of 10% in the last 6 months (see Table 1: A = absence of systemic signs; B = presence). Biological variables indicative of inflammatory syndrome including C-reactive protein (CRP) and fibrinogen concentrations were also evaluated. Patients with concentrations of CRP and/or fibrinogen higher than the normal range were considered to have inflammatory syndrome (see Table 1: a = absence of inflammatory syndrome, b = presence). The first-line chemotherapy regimen administered to our patients differed according to the type of NHL and the date of diagnosis.
TABLE 1.
Demographic, Clinical, and Laboratory Data at the Time of Non-Hodgkin Lymphoma (NHL) Diagnosis
RESULTS
Between 1997 and 2011, 201 patients (from 10 participating centers) diagnosed with NHL (from a total of 13,992 NHL cases diagnosed at those centers during the same time period) underwent a renal biopsy due to significant proteinuria. Among them, 18 patients (8.95%) (10 men and 8 women) exhibited MCNS and were included in our study. The mean age at the onset of MCNS was 62.5 years (range, 36–83 yr), and the mean age at NHL diagnosis was 62.3 years (range, 38–83 yr).
Clinical, Biological, and Pathologic Characteristics of Patients at the Time of MCNS Diagnosis
Laboratory and clinical data of patients at the time of MCNS diagnosis are summarized in Table 2. All patients exhibited typical features of MCNS including nephrotic range proteinuria (mean, 7500 mg/d; range, 3000–18,200 mg/d) and low serum concentrations of albumin (mean, 1.81 g/dL; range, 0.86–2.80 g/dL). Microscopic hematuria was found in 4 patients (Pt 5, Pt 8, Pt 10, Pt 13). Five patients had hypertension (Pt 10, Pt 11, Pt 12, Pt 13, Pt 18) at the time of renal biopsy. AKI was present in 6 patients (Pt 5, Pt 9, Pt 11, Pt 13, Pt 14, Pt 17). Acute tubular injury lesions were observed in 5 patients (Pt 5, Pt 9, Pt 10, Pt 13, Pt 15) and were associated with AKI in 3 patients (Pt 5, Pt 9, Pt 13). Pt 5 and Pt 9 had grade 2 tubular lesions and Pt 13 had grade 4 tubular lesions. Pt 10 and Pt 15 displayed grade 1 lesions without AKI. Renal hypoperfusion was considered as the main cause of AKI in 3 patients without tubular lesions (Pt 11, Pt 14, Pt 17). Glomeruli exhibited normal morphology and no intraglomerular lymphomatous infiltration was found. Patients with a serum monoclonal spike did not have glomerular lesions suggesting monoclonal immunoglobulin-related glomerulonephritis, and Ig deposits were not observed by immunofluorescence study in any patient. In 3 patients without AKI (Pt 7, Pt 8, Pt 18) kidney histologic lesions included a lymphoid interstitial infiltrate of nonlymphomatous origin, as assessed by extensive immunohistochemistry analysis (Figure 1A–C). One patient with AKI (Pt 9) had an interstitial edema infiltrate. None of the patients with MCNS associated with interstitial infiltrate as assessed by renal biopsy was known to have received nonsteroidal antiinflammatory drugs prior to the occurrence of glomerular disease.
TABLE 2.
Demographic, Clinical, and Laboratory Data at the Time of Minimal Change Nephrotic Syndrome (MCNS) Diagnosis
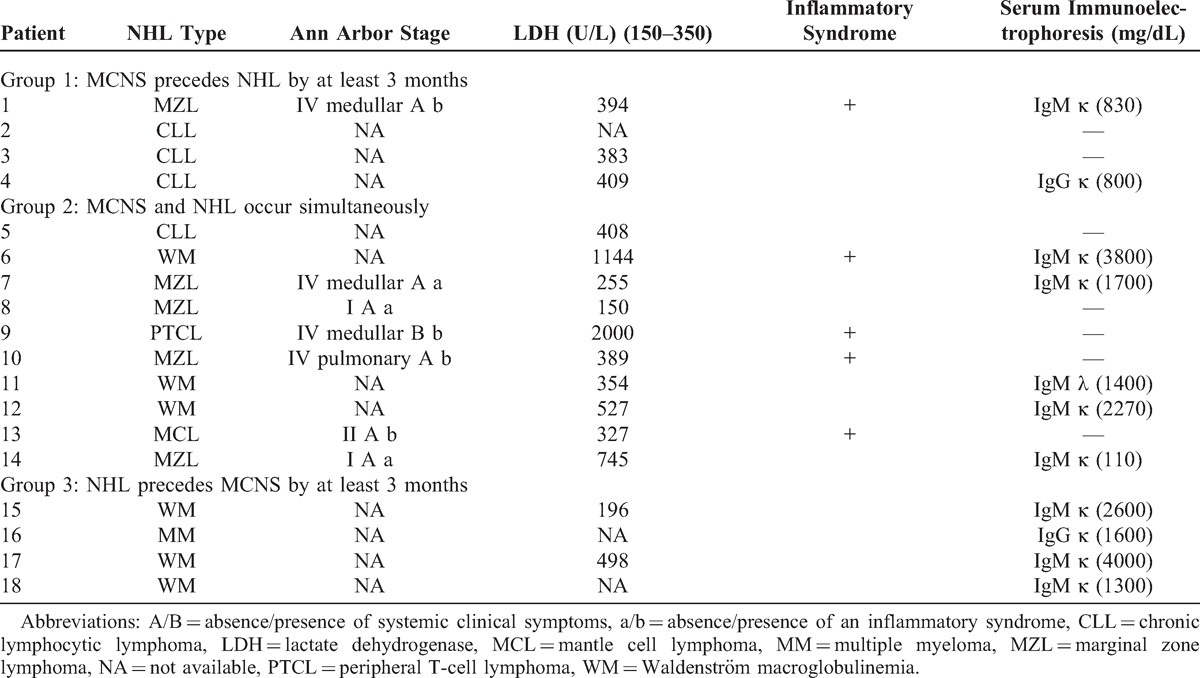
FIGURE 1.
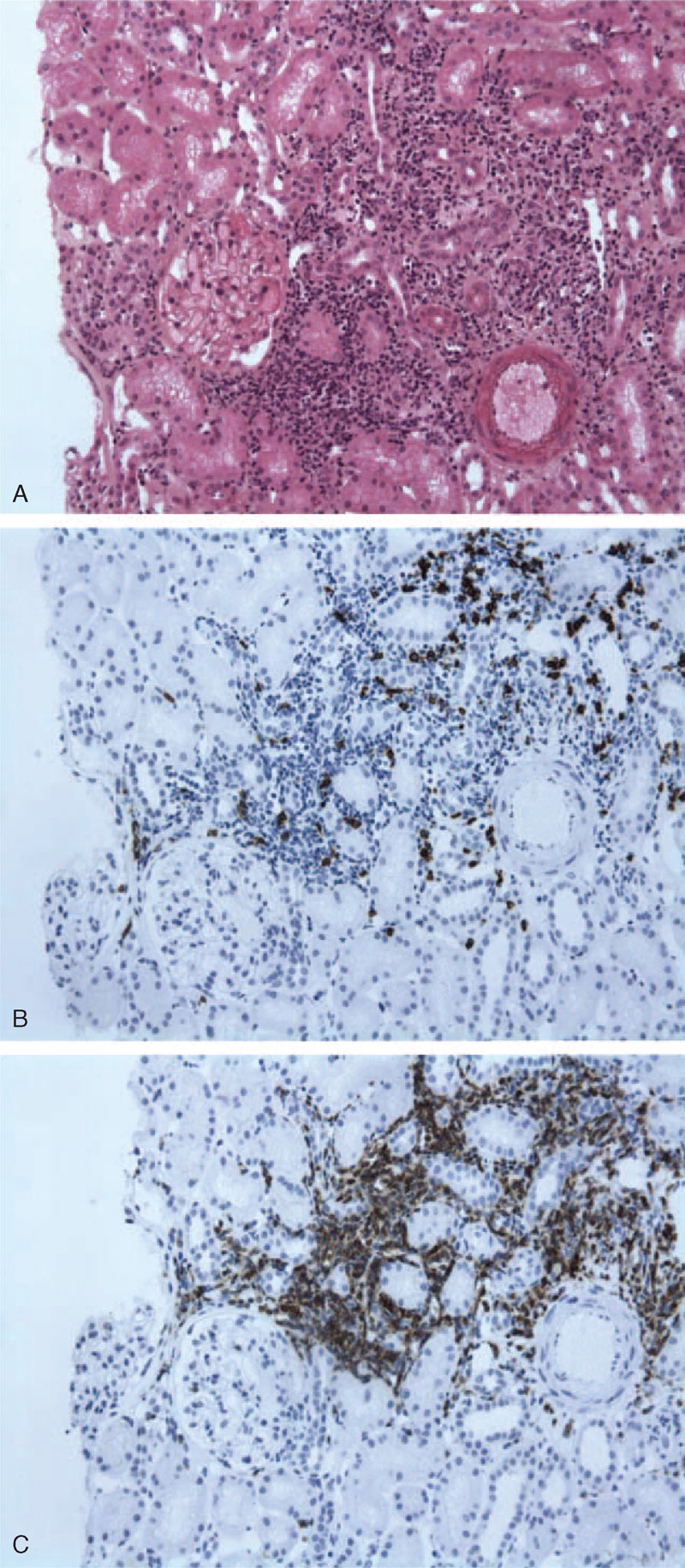
Kidney biopsy showing nontumoral interstitial infiltration from a patient with MCNS and marginal zone lymphoma (Pt 8). A, Interstitial infiltrate of the interstitium showing normal glomeruli (light microscopy Masson trichrome stain). B, Immunohistochemical staining of cellular infiltrates showing a few CD3+ lymphoid cells in the interstitial infiltrate. C, Cellular infiltrate stained brightly for CD20+ cells. Interstitial infiltrate was negative for heavy and light immunoglobulin chains (data not shown).
Clinical, Biological, and Pathologic Characteristics of NHL
MCNS was associated with a broad spectrum of lymphoproliferative disorders (Table 1). All patients in our series except for 1 (Pt 9) had NHL of B-cell origin. The most frequent lymphoid neoplasms included WM (n = 6, 33.3%), marginal zone B-cell lymphoma (MZL) (n = 5, 27.8%), and CLL (n = 4, 22.2%). The remaining 3 patients displayed MM (Pt 16), mantle cell lymphoma (Pt 13), or peripheral T-cell lymphoma (PTCL) (Pt 9). The patient with MM presented with asymptomatic myeloma according to the International Myeloma Working Group definition: bone marrow aspirate contained more than 10% plasma cells and there was no organ damage except for glomerular disease that was not related to monoclonal immunoglobulin deposits. Eleven patients (61.1%) had monoclonal circulating immunoglobulins (IgM in 9 cases and IgG in 2 cases) with a mean concentration of 1860 mg/dL at the time of NHL diagnosis. Human immunodeficiency virus serology was negative for all patients. The mean serum lactate dehydrogenase (LDH) activity (assessed from 15 patients with available data) was 545 U/L (range, 150–2000 U/L). Ann Arbor staging was detailed for 7 patients without WM, MM, or CLL (see Table 1). Systemic symptoms were present in 1 patient with PTCL (Pt 9), whereas 5 patients (Pt 1, Pt 6, Pt 9, Pt 10, Pt 13) exhibited an inflammatory syndrome at the time of hematologic management.
Description of MCNS Associated With NHL According to the Interval Between the Onset of the 2 Diseases
Group 1: MCNS Precedes NHL (at least 3 months between the occurrence of the 2 diseases)
Four patients (Pt 1 to Pt 4) with a mean age of 54 years (range, 36–65 yr) had MCNS prior to NHL. The average interval between the 2 diseases was 15 months (range, 4–24 mo). The underlying hematologic disorder was CLL in 3 patients (Pt 2, Pt 3, Pt 4) and stage IV MZL related to bone marrow infiltration in 1 patient (Pt 1). Proteinuria level was between 4000 and 4800 mg/d (mean, 4400 mg/d), and all patients exhibited significant hypoalbuminemia (2.28 g/dL, range 1.76–2.80 g/dL). Renal function was not altered at the time of renal biopsy, whereas LDH level was high in all patients tested. Inflammatory biological syndrome was present in 1 case (Pt 1). Renal biopsy confirmed the diagnosis of MCNS and the study of interstitial and tubule area showed no significant findings.
Group 2: MCNS Occurs Simultaneously With NHL (within an interval of 3 months)
This group included 10 patients (55.6%) (Pt 5 to Pt 14). The mean age was 61.8 years (range, 38–83 yr). These patients exhibited a heterogeneous group of lymphoid proliferation disorders including MZL in 4 patients (Pt 7, Pt 8, Pt 10, Pt 14), and WM in 3 patients that involved IgMλ in 1 case (Pt 11), and IgMκ in 2 cases (Pt 6, Pt 12). The remaining 3 patients exhibited CLL (Pt 5), PTCL (Pt 9), or mantle cell lymphoma (Pt 13). Mean proteinuria level at the onset of renal involvement was 6900 mg/d (range, 3000–18,200 mg/d) and mean serum albumin concentration was 1.74 g/L (range, 1.20–2.78 g/L). Among the 10 patients with MCNS occurring simultaneously with NHL, 5 of them had AKI: 3 patients had stage 1 AKI (Pt 5, Pt 11, Pt 14), 1 patient had stage 2 AKI (Pt 9), and 1 patient had stage 3 AKI (Pt 13) requiring hemodialysis therapy. Mean serum concentration of creatinine was 1.69 mg/dL (range, 0.53–5.31 mg/dL). LDH activity was abnormally high in 7 patients (Pt 5, Pt 6, Pt 9, Pt 10, Pt 11, Pt 12, Pt 14) and 4 patients had a biological inflammatory syndrome (Pt 6, Pt 9, Pt 10, Pt 13). Renal biopsy specimen revealed tubular lesions in 4 patients (Pt 5, Pt 9, Pt 10, Pt 13) leading to AKI in all cases except Pt 10. Two patients (Pt 7, Pt 8) exhibited a nontumoral renal infiltrate mainly composed of CD3+ and CD20+ lymphocytes, without acute renal impairment (see Figure 1A–C).
Group 3: MCNS Follows NHL (at least 3 months between the occurrence of the 2 diseases)
This group included 4 patients (22.2%) (Pt 15 to Pt 18) who developed MCNS with an average delay of 25 months (range, 7–36 mo) after NHL. Their mean age at MCNS diagnosis was 76 years (range, 74–78 yr). Lymphoproliferative neoplasms included WM disease in 3 patients (Pt 15, Pt 17, Pt 18), with a mean IgMκ peak of 2630 mg/dL, and MM disease in 1 patient (Pt 16), with an IgGκ monoclonal component (peak concentration, 1600 mg/dL). None of these patients displayed clinical manifestations consistent with lymphoproliferative disorders at the time of NS diagnosis. Laboratory parameters were not suggestive of biological inflammatory syndrome. At the time of hematologic diagnosis, lymphoproliferative disorders observed in these patients were considered as indolent and did not require specific therapy. At the time of MCNS diagnosis, the mean daily proteinuria of these patients was 12,100 mg (range, 9900–14,700 mg/d) and their mean serum albumin concentration was 1.58 g/dL (range, 0.86–2.16 g/dL). Deep vein thrombosis occurred in 1 patient (Pt 15). The mean serum concentration of creatinine was 1.16 mg/dL. One patient (Pt 17) displayed AKI (stage 1 according to AKIN criteria) simultaneous with NS, without acute tubular lesion or acute interstitial nephritis as assessed by renal biopsy. Renal biopsy demonstrated the presence of a nonlymphomatous interstitial lymphoid infiltrate in 1 patient (Pt 18) and acute tubular lesions in 1 case (Pt 15), without significant impairment of renal function in both cases.
Treatment and Outcome of MCNS and NHL
Follow-up data about the course of NS in response to particular therapies were available for 17 patients (Pt 11 was lost to follow-up). All patients received steroid therapy, either alone in 9 patients (Pt 1, Pt 2, Pt 3, Pt 4, Pt 7, Pt 8, Pt 14, Pt 16, Pt 18) or associated with chemotherapy in 9 patients (Pt 5, Pt 6, Pt 9, Pt 10, Pt 11, Pt 12, Pt 13, Pt 15, Pt 17). The different chemotherapy regimens are listed in Table 3. The mean duration of follow-up from the time of MCNS diagnosis was 44.8 months (range, 2.8–153.4 mo). All patients were specifically treated for MCNS and went into complete remission. Fourteen patients were treated by standard steroid protocol (usually administered in the case of “idiopathic” MCNS) either with or without chemotherapy (Pt 1, Pt 2, Pt 3, Pt 4, Pt 5, Pt 7, Pt 8, Pt 11, Pt 12, Pt 14, Pt 15, Pt 16, Pt 17, Pt 18). The mean duration of steroid therapy among these patients was estimated at 6.2 months for group 1, 6.9 months for group 2, and 13 months for group 3. Relapse of NS occurred in 9 patients (all patients included in group 1 and group 3 and 1 patient [Pt 14] in group 2), with an average delay of 15.9 months (range, 4–24 mo). MCNS relapse occurred in 7 of 9 patients (77.8%) who were treated exclusively by steroid therapy (see Table 3). By contrast only 2 of 8 patients (25%) who were treated by chemotherapy displayed relapse of NS. Moreover, 5 patients (Pt 1, Pt 6, Pt 9, Pt 10, Pt 13) had inflammatory syndrome at the time of NHL diagnosis. MCNS relapse occurred in only 1 of these patients (20%). By contrast, among the 13 patients without inflammatory syndrome at the time of baseline hematologic evaluation, relapse of NS occurred in 8 cases (62%). The mean proteinuria level at the end of the follow-up was 1400 mg/d (range, 0–4800 mg/d): mean values were 900 mg/d in group 1, 800 mg/d in group 2, and 2800 mg/d in group 3.
TABLE 3.
MCNS Treatment and Outcome
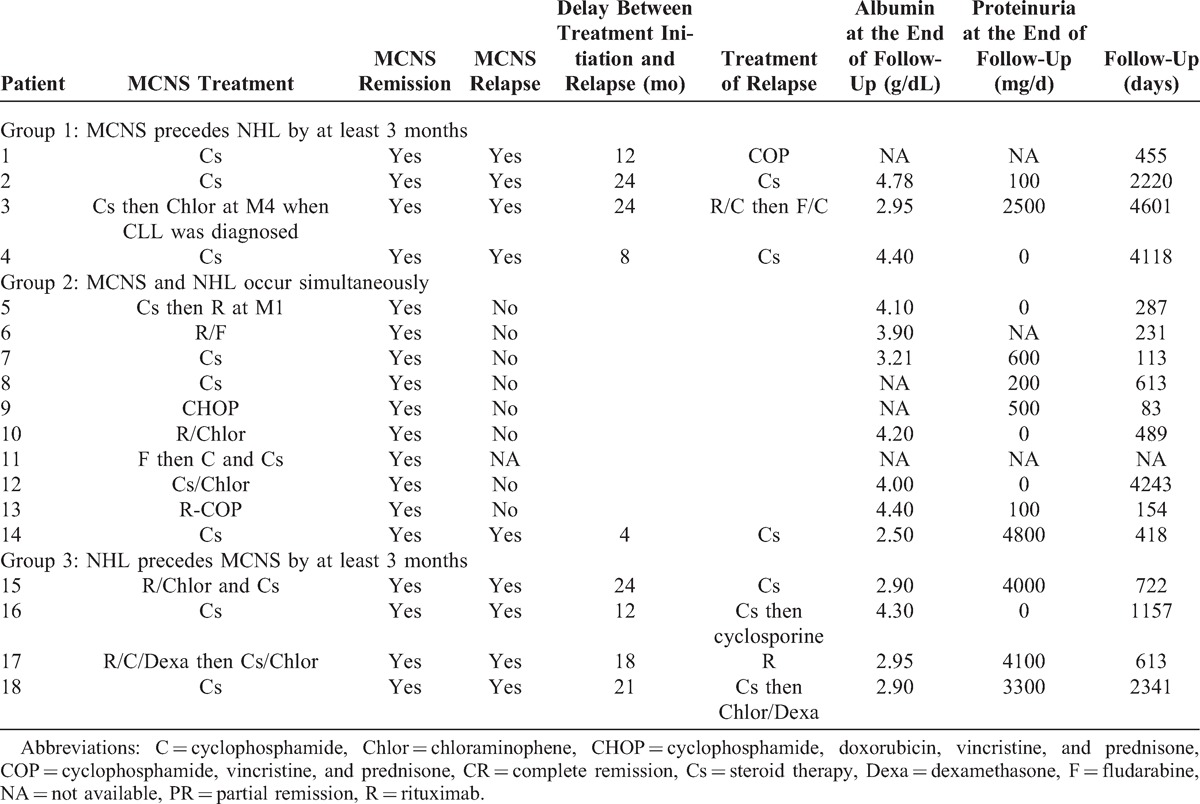
Response to treatment was evaluated according to the temporal relationship between the occurrence of MCNS and NHL.
Group 1: MCNS Precedes NHL (at least 3 months between the occurrence of the 2 diseases)
Steroid therapy was given to all patients, leading to complete remission of NS in 3 cases. One patient (Pt 3) was considered in partial remission from NS when CLL was diagnosed 4 months later and required additional treatment with chloraminophene, which was associated with complete remission from NS. MCNS relapse occurred in all patients with a mean delay of 17 months (range, 8–24 mo). In 2 cases (Pt 1, Pt 2) relapse occurred at the time of the diagnosis of lymphoproliferative disease. In 1 patient, (Pt 3), relapse was associated with an uncontrolled hematologic disorder. One patient of this group had septic complications consisting of repeated bouts of pneumonia (Pt 4).
Group 2: MCNS Occurs Simultaneously With NHL (within an interval of 3 months)
Steroid therapy alone was used to treat 3 patients successfully (Pt 7, Pt 8, Pt 14). Additional drugs were not given to these patients because the lymphoid disorder was not considered to be aggressive. Seven other patients received either steroid therapy included in chemotherapy regimen (Pt 6, Pt 9, Pt 10, Pt 13) or were started on steroid therapy in parallel with chemotherapy (Pt 5, Pt 11, Pt 12). All patients who were treated simultaneously for both diseases went into complete remission from NS. MCNS reoccurred 4 months after the end of steroid treatment in only 1 patient (Pt 14), who was treated exclusively by steroid therapy for MZL. Death resulting from septic shock (Pt 9) or hemorrhagic shock (Pt 14) occurred in 2 patients. At the end of follow-up, relapse of NHL had occurred in 1 (Pt 12) of 7 patients who were treated exclusively by chemotherapy. In this patient, the recurrence of WM without MCNS relapse was successfully treated by rituximab.
Group 3: MCNS Follows NHL (at least 3 months between the occurrence of the 2 diseases)
No specific treatment was given for NHL before the occurrence of MCNS because NHL was considered to be indolent in these patients at the time of hematologic diagnosis. Steroid therapy alone (Pt 16, Pt 18) or associated with chemotherapy (Pt 15, Pt 17) was started following the diagnosis of MCNS, leading to complete remission of NS in all cases. MCNS relapse occurred in all patients with a mean delay of 18.7 months (range, 12–24 mo), simultaneously with the relapse of lymphoid disorder in Pt 15 and Pt 17, and simultaneously with uncontrolled hematologic disease in Pt 16 and Pt 18 (who did not receive any specific treatment for NHL). Three patients of this group developed septic complications consisting of pneumonia (Pt 18), herpes zoster thoracic infection (Pt 17), and acute prostatitis (Pt 16).
DISCUSSION
MCNS is the most common glomerular disease associated with cHL.1,4,22 However, few studies have investigated the occurrence of MCNS within the context of NHL. In a large series of 700 patients with NHL that included patients with CLL, glomerular disease was diagnosed in 5 cases with 1 case each of membranous nephropathy, FSGS, diffuse proliferative glomerulonephritis, mesangio-capillary glomerulonephritis, and membranoproliferative glomerulonephritis.11 The authors of this report found that glomerular diseases had been previously reported only in 37 patients with NHL and in 42 patients with CLL. Of these cases, MCNS was found in only 4 patients with NHL (10.8%) and in 4 patients with CLL (9.5%), demonstrating that MCNS is a rare finding in the context of NHL.
We report here for the first time, to our knowledge, a large series of 18 adult patients who presented with both MCNS and NHL. The current study shows that MCNS may precede, occur simultaneously with, or follow a broad spectrum of non-Hodgkin lymphoproliferative disorders. MCNS was preferentially associated with neoplasm originating from B cells (94.4% of cases). Consistent with this observation, there is compelling evidence that Hodgkin Reed-Sternberg (HRS) cells, the histologic hallmark of the cHL, are also derived from mature B cells.12 In the current study, the most frequent lymphoproliferative disorders associated with MCNS were WM (33.3% of cases), MZL (27.7% of cases), and CLL (22.2% of cases). These lymphoid disorders are currently considered to be indolent lymphomas according to WHO classification.16 NHLs that are more aggressive than WM, MZL, or CLL are often associated with interstitial, intraglomerular, or intravascular lymphomatous infiltration, which may be revealed by nephrotic range proteinuria.32 The spectrum of renal lesions associated with WM usually includes kidney injury directly resulting from IgM monoclonal deposits,3 and CLL is most commonly associated with membranoproliferative glomerulonephritis.26 Our results suggest that the diagnosis of MCNS should be considered in cases of NS occurring within the context of lymphoid proliferation, regardless of the subtype of lymphoid proliferation disorder. Electron microscopy analysis was not performed for our series of patients because we did not observe immunoglobulin deposits by immunofluorescence.
MCNS occurred simultaneously with NHL in 10 patients (55.5% of the current series), suggesting a possible molecular link between these conditions. Moreover, 3 patients (in group 1) experienced MCNS relapse at the time of NHL diagnosis or in the context of an uncontrolled hematologic disease. In the situation where MCNS precedes NHL, it is possible that some patients showed signs of early hematologic disease that was not clinically diagnosed at the time of MCNS diagnosis. In group 1 and group 3, the occurrence of NHL and MCNS were non-overlapping (by a period of at least 3 mo). In these patients, the time intervals separating the 2 diseases were shorter than those observed for the association MCNS with cHL.4
MCNS was associated with AKI in 6 patients and was preferentially observed in patients exhibiting the simultaneous occurrence of MCNS and NHL (5 of 6 patients). AKI involved acute tubular lesions in 3 of these patients (Pt 5 grade 2, Pt 9 grade 2, and Pt 13 grade 4) and was related to renal hypoperfusion in 3 patients without tubular lesions. It is noteworthy that 50% of patients from group 2 presented with AKI at the time of glomerular disease. This result strongly suggests that clinicians should be encouraged to consider, in some particular cases, the possibility of underlying NHL in patients presenting with AKI and MCNS. Renal histology showed that tubular injury was not related to “cast nephropathy” as frequently observed in patients with monoclonal gammopathy.6 In the context of MCNS associated with NHL, the presence of AKI has been attributed to renal infiltration by malignant cells.31 In contrast, we failed to detect cells of tumoral origin in these infiltrates by extensive immunohistochemistry in our patients with AKI. Similar findings were reported for patients with MCNS associated with cHL, in whom interstitial infiltrates were not considered to be caused by malignant infiltration.4 Altogether these data suggest that nontumoral interstitial infiltrate associated with minimal change glomerular lesions may occur in a context of underlying lymphoproliferative neoplasm without significant renal failure.
The current study demonstrated that MCNS associated with NHL is frequently sensitive to steroids, as is the case for MCNS patients without lymphoproliferative disorders. However, relapse was more frequently observed for patients treated exclusively by steroid therapy (77.8%) than for patients receiving both steroids and a regimen of chemotherapy (25%). Similar findings have been reported for patients with MCNS associated with cHL. Indeed, MCNS preceding cHL was frequently found to be either steroid dependent or steroid resistant: MCNS became sensitive to treatment upon the cure of hematologic disease in these patients.4 In contrast, in patients with MCNS that followed NHL, MCNS was sensitive to steroids; however, MCNS relapsed if NHL reoccurred. Collectively, these observations suggest that aggressive chemotherapy may be an effective treatment to induce complete and prolonged remission of MCNS associated with NHL. In agreement with this hypothesis, it was recently reported that serum permeability activity significantly decreased in response to chemotherapy treatment in 1 patient with steroid-resistant NS associated with cHL.1
The close temporal relationship between the occurrence of MCNS and NHL, as well as the high sensitivity of MCNS to therapy during the concomitant treatment of NHL, suggests a pathophysiologic link between these disorders. Although the mechanisms underlying these observations are unknown, it has been suggested that in cases of MCNS associated with cHL, the excessive production of inflammatory cytokines could alter the glomerular filtration barrier.27 However, only 5 patients in the current study had significant inflammatory syndrome, which was much lower than the proportion of patients who have inflammatory syndrome in cases of MCNS associated with cHL.4 Unexpectedly, patients were seemingly less likely to relapse if inflammatory syndrome was present at the time of non-Hodgkin lymphoid disorder diagnosis than if it was not (20% vs 62%, respectively). Strikingly, 61% of our patients had NHL associated with a serum monoclonal component (IgM in 9 cases and IgG in 2 cases). However, these patients did not show immunoglobulin deposits in the renal parenchyma. Several decades ago, the clinical course of 12 patients with isolated circulating monoclonal immunoglobulin and MCNS was reported.23 Nonetheless, the potential role of immunoglobulin in the pathogenesis of MCNS has not been explored. Indeed, the particular role of immunoglobulin monoclonal components in patients with MCNS associated with NHL requires investigation.
In conclusion, the findings of the current study suggest that a wide spectrum of NHL (preferentially originating from B cells) may promote the occurrence of MCNS. The close temporal relationship between the occurrence of lymphoma and glomerular disease strongly suggests that these diseases share a causative mechanism, which remains to be determined. Chemotherapy appears to significantly and positively influence the course of glomerular disease.
ACKNOWLEDGMENTS
We acknowledge Pr. Bruneval (Service de Pathologie Hôpital Européen Georges Pompidou, Paris), Dr. Charlotte (Service de Pathologie Hôpital de la Pitié Salpétrière, Paris), and all of the clinicians and pathologists from LYSA (Lymphoma Study Association) for their help in the care and follow-up of the patients.
Footnotes
Abbreviations: AKI = acute kidney injury, AKIN = acute kidney injury network, cHL = classic Hodgkin lymphoma, CLL = chronic lymphocytic leukemia, CRP = C-reactive protein, FSGS = focal segmental glomerulosclerosis, LDH = lactate dehydrogenase, MCNS = minimal change nephrotic syndrome, MM = multiple myeloma, MZL = marginal zone B-cell lymphoma, NHL = non-Hodgkin lymphoma, NS = nephrotic syndrome, Pt = patient, PTCL = peripheral T-cell lymphoma, WHO = World Health Organization.
Conflicts of interest and funding sources: The authors of this manuscript have no conflicts of interest to disclose and no funding sources to declare.
REFERENCES
- 1.Aggarwal N, Batwara R, McCarthy ET, et al. Serum permeability activity in steroid-resistant minimal change nephrotic syndrome is abolished by treatment of Hodgkin disease. Am J Kidney Dis. 2007;50:826–829. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 3.Audard V, Georges B, Vanhille P, et al. Renal lesions associated with IgM-secreting monoclonal proliferations: revisiting the disease spectrum. Clin. J Am Soc Nephrol. 2008;3:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Audard V, Larousserie F, Grimbert P, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–2260. [DOI] [PubMed] [Google Scholar]
- 5.Audard V, Zhang SY, Copie-Bergman C, et al. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-Reed Sternberg cells and podocytes. Blood. 2010;115:3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basnayake K, Stringer SJ, Hutchison CA, et al. The biology of immunoglobulin free light chains and kidney injury. Kidney Int. 2011;79:1289–1301. [DOI] [PubMed] [Google Scholar]
- 7.Belghiti D, Vernant JP, Hirbec G, et al. Nephrotic syndrome associated with T-cell lymphoma. Cancer. 1981;47:1878–1882. [DOI] [PubMed] [Google Scholar]
- 8.Cambier JF, Ronco Onco-nephrology: P. Glomerular diseases with cancer. Clin J Am Soc Nephrol. 2012;7:1701–1712. [DOI] [PubMed] [Google Scholar]
- 9.Clement LC, Avila-Casado C, Mace C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LJ, Rennke HG, Laubach JP, et al. The spectrum of kidney involvement in lymphoma: a case report and review of the literature. Am J Kidney Dis. 2010;56:1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da’as N, Polliack A, Cohen Y, et al. Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67:158–164. [DOI] [PubMed] [Google Scholar]
- 12.Farrell K, Jarrett RF. The molecular pathogenesis of Hodgkin lymphoma. Histopathology. 2011;58:15–25. [DOI] [PubMed] [Google Scholar]
- 13.Ghiggeri GM, Bleid D, Garaventa A, et al. Recurrent lymphomatoid papulosis associated with nephrotic syndrome. An occurrence of uncertain origin. Pediatr Nephrol. 2009;24:189–192. [DOI] [PubMed] [Google Scholar]
- 14.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–685. [DOI] [PubMed] [Google Scholar]
- 15.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. [DOI] [PubMed] [Google Scholar]
- 17.Hory B, Saunier F, Wolff R, et al. Waldenstrom macroglobulinemia and nephrotic syndrome with minimal change lesion. Nephron. 1987;45:68–70. [DOI] [PubMed] [Google Scholar]
- 18.Ishimoto T, Shimada M, Araya CE, et al. Minimal change disease: a CD80 podocytopathy? Semin Nephrol. 2011;31:320–325. [DOI] [PubMed] [Google Scholar]
- 19.Jhaveri KD, Shah HH, Calderon K, et al. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013;84:34–44. [DOI] [PubMed] [Google Scholar]
- 20.Kasmani R, Marina VP, Abidi S, et al. Minimal change disease associated with MALT lymphoma. Int Urol Nephrol. 2012;44:1911–1913. [DOI] [PubMed] [Google Scholar]
- 21.Kowalewska J, Nicosia RF, Smith KD, et al. Patterns of glomerular injury in kidneys infiltrated by lymphoplasmacytic neoplasms. Hum Pathol. 2011;42:896–903. [DOI] [PubMed] [Google Scholar]
- 22.Lien YH, Lai Pathogenesis LW. Diagnosis and management of paraneoplastic glomerulonephritis. Nat Rev Nephrol. 2011;7:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallick NP, Dosa S, Acheson EJ, et al. Detection, significance and treatment of paraprotein in patients presenting with proteinuria without myeloma. Q J Med. 1978;47:145–175. [PubMed] [Google Scholar]
- 24.Mathieson PW. Minimal change nephropathy and focal segmental glomerulosclerosis. Semin Immunopathol. 2007;29:415–426. [DOI] [PubMed] [Google Scholar]
- 25.Mehta R, Kellum J, Shah S, et al. The Acute Kidney Injury N. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moulin B, Ronco PM, Mougenot B, et al. Glomerulonephritis in chronic lymphocytic leukemia and related B-cell lymphomas. Kidney Int. 1992;42:127–135. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama S, Yokote T, Kobayashi K, et al. Minimal-change nephrotic syndrome preceding Hodgkin lymphoma by 5 years with expression of tumor necrosis factor alpha in Hodgkin-Reed-Sternberg cells. Hum Pathol. 2010;41:1196–1199. [DOI] [PubMed] [Google Scholar]
- 28.Ronco PM. Paraneoplastic glomerulopathies: new insights into an old entity. Kidney Int. 1999;56:355–377. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow SHCE, Harris NL, Jaffe ES, et al. Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed Lyon, France: IARC (International Agency for Research on Cancer) Press; 2008. [Google Scholar]
- 30.Tavares MB, Chagas de Almeida Mda C, Martins RT, et al. Acute tubular necrosis and renal failure in patients with glomerular disease. Ren Fail. 2012;34:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrier B, Buzyn A, Hummel A, et al. Serum monoclonal component and nephrotic syndrome—it is not always amyloidosis. Diagnosis: WM complicated by retroperitoneal and renal infiltration and associated with a minimal change disease. Nephrol Dial Transplant. 2006;21:3327–3329. [DOI] [PubMed] [Google Scholar]
- 32.Tornroth T, Heiro M, Marcussen N, et al. Lymphomas diagnosed by percutaneous kidney biopsy. Am J Kidney Dis. 2003;42:960–971. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berg JW, JJ. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin Sci. 2004;107:125–136. [DOI] [PubMed] [Google Scholar]
- 34.Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2:445–453. [DOI] [PubMed] [Google Scholar]
- 35.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang SY, Audard V, Fan Q, et al. Immunopathogenesis of idiopathic nephrotic syndrome. Contrib Nephrol. 2011;169:94–106. [DOI] [PubMed] [Google Scholar]
- 37.Zhang SY, Kamal M, Dahan K, et al. C-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal. 2010;3:ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]


