Abstract
Delta-like ligand 4 (DLL4), 1 of the 5 known Notch ligands, is involved in a variety of tumor initiation and progression, particularly in the process of tumor angiogenesis. However, the clinical and prognostic significance of DLL4 in glioblastoma have not been fully elucidated.
Tumor tissues from 69 glioblastoma patients were analyzed using immunohistochemistry for DLL4 expression. Peritumoral brain edema (PTBE) on preoperative magnetic resonance imaging of these patients and the relationship with DLL4 expression were evaluated. The effect on prognosis was assessed by using the Kaplan–Meier survival and the Cox proportional hazard model.
The results showed that elevated DLL4 expression was primarily distributed in the cytoplasm of tumor vascular endothelial cells and rarely detected in tumor cells. Univariate analysis indicated significant correlation of high DLL4 expression with shorter time to progression (TTP) (P < 0.001) and overall survival (OS) (P < 0.001) in glioblastoma. Multivariate analysis confirmed high DLL4 expression as an unfavorable prognostic indicator for TTP (P < 0.001) and OS (P < 0.001), independent of age, gender, symptom duration, resection degree, and PTBE. Importantly, the study also found that DLL4 expression was positively related with PTBE (Spearman’s test: r = 0.845, P < 0.001). A multiple linear regression model was constructed to confirm that the positive index of DLL4 was associated with an increase in maximum extent of PTBE (P < 0.001).
It is thus concluded that DLL4 is correlated with PTBE and may be useful for predicting prognosis in glioblastoma.
INTRODUCTION
Glioblastoma, the most common and lethal primary malignant brain tumor that is characterized by high neovascularization and severe peritumoral brain edema (PTBE) in adults, has a poor prognosis with a median overall survival (OS) of 1–2 years and <3% 5-year survival rate using current standard of care.1,2 PTBE is a frequently encountered phenomenon in patients with glioblastoma, and may be fatal because of its strong contribution to neurological signs and symptoms. Noteworthily, PTBE has been proven as an independent prognostic factor in glioblastoma.3 However, the exact mechanisms responsible for PTBE in glioblastoma are not fully elucidated and are likely to be multifactorial.4,5
Tumor angiogenesis is a prerequisite for tumor growth to provide adequate acquisition of blood supply and is implicated in the development of perilesional edema by increased levels of angiogenic factors and tumor vessels with high permeability,4,6,7 one signaling pathway contributing to this process is Notch pathway.7 Delta-like ligand 4 (DLL4) is 1 of the 5 known transmembranous Notch ligands and is expressed at the site of vascular development and angiogenesis.8,9 Physiologically, upregulated DLL4 activation by vascular endothelial growth factor (VEGF) and hypoxia triggers a negative feedback that inhibits the superfluous VEGF effect of vascular sprouting.10–12 But in tumor angiogenesis, DLL4 induces larger tumor vessel to enhance the vascular structure and function.13,14 Noticeably, DLL4 expression has been proven to be correlated with poor prognosis in breast cancer, nasopharyngeal carcinoma, and colon cancer.15–17 However, whether DLL4 expression can serve as a prognosis predictor in glioblastoma still remains unclear. In addition, based on the correlation of DLL4 with tumor angiogenesis and the critical role of tumor angiogenesis in the progress of PTBE, we speculate that DLL4 is linked to PTBE with possibility. In this study, we have investigated the expression of DLL4 protein, and carried out an initial evaluation for the effect of DLL4 on prognosis and PTBE in patients with primary glioblastoma.
METHODS AND MATERIALS
Patients and Tumor Tissues
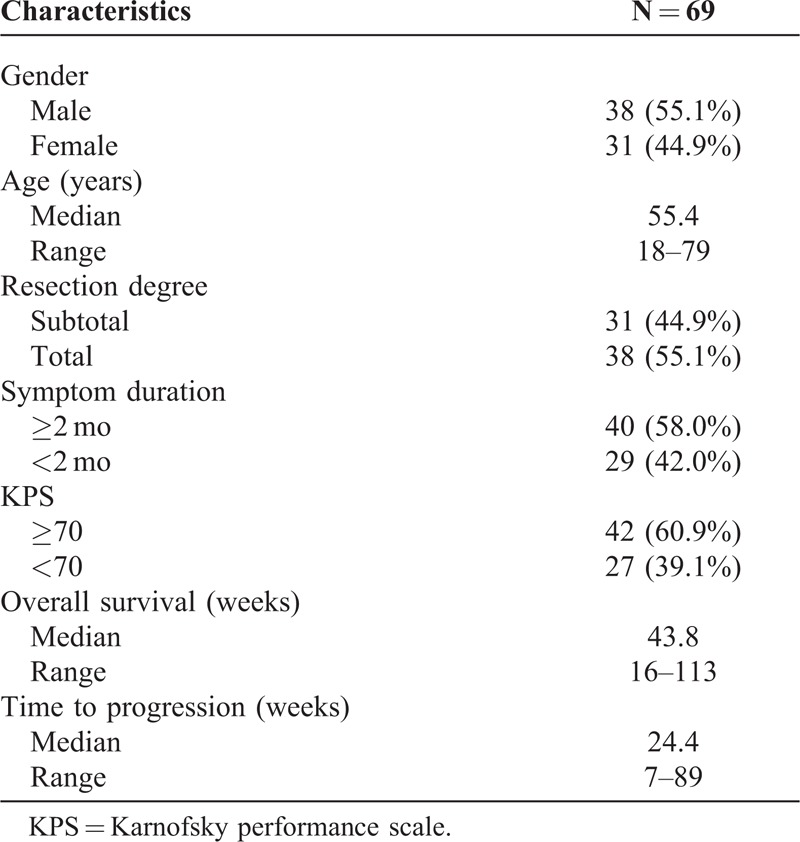
The retrospective study cohort consisted of 69 patients with primary glioblastoma, who underwent surgical resection at the First Affiliated Hospital, Fujian Medical University, Fujian, China, between 2006 and 2012. Informed consent was obtained from each patient according to the research proposals approved by the local ethics committee of the Fujian Medical University. Eligibility criteria included written informed consent and availability of tumor tissue, preoperative magnetic resonance imaging (MRI), and follow-up data. All specimens were obtained from surgical resection and classified according to the World Health Organization classification of brain tumors.18 Clinical information was obtained by reviewing the medical records on radiographic images, by telephone or written correspondence, and by reviewing the death certificate. Patient characteristics are shown in Table 1. Follow-up information was updated every 2 months by telephone interview or questionnaire letters and was last done in March 2014.
TABLE 1.
Clinical Characteristics of All 69 Glioblastoma Patients
Treatment and Clinical Outcome Assessment
Surgical resection was done at diagnosis and adjuvant therapy (radiotherapy and chemotherapy) was administered in every patient enrolled. Before the initial diagnosis of edema, patients did not receive treatment with steroids. For the extent of surgical resection, 31 out of 69 patients received subtotal resection, and other 38 patients received total resection. Postoperatively, all patients received radiotherapy to limited fields (2 Gy per fraction, once a day, 5 days a week, 60 Gy total dose) and adjuvant temozolomide (TMZ) (at 150–200 mg/m2 of body surface area on days 1–5) given at 4-week intervals. In each patient, TMZ was administered for ≥4 cycles in the absence of death or irreversible blood toxicity. No patient received any experimental antitumor vaccination. All patients in this study were followed up in accordance with a strict protocol. After the date of resection, patients were observed at 3-month intervals during the first year and at 6-month intervals thereafter. Tumor progression was determined by the method of Wen et al,19 and “pseudo-progressive” lesions were not included in the analysis.20 The time to progression (TTP) was measured from the date of surgery until the date of tumor progression. The endpoint of the study was OS, which was measured from the day of surgery (equivalent to the day of diagnosis) until death of the patient. For patients who had not experienced tumor progression or death at the time of last follow-up, TTP and OS were censored at the date of last follow-up.
Immunohistochemistry
Tumor specimens were fixed in 10% of formaldehyde and embedded in paraffin for histological sections. Paraffin-embedded sections were deparaffinized in xylene, dehydrated in graded alcohol. Immunohistochemistry for DLL4 protein was performed using a rabbit monoclonal antibody (Abcam, ab7280, Cambridge, UK). Antigen retrieval was performed in citrate buffer pH 6.0, then the sections were incubated overnight at 4°C with the primary antibody at 1:200. Next, they were rinsed with phosphate buffer solution (PBS) and incubated with the horseradish peroxidase-conjugated secondary antibody, followed by a rinse in PBS, incubation with diaminobenzidine staining, and counterstaining with hematoxylin blue. The negative control sections were incubated with PBS in equal concentrations to the primary antibody, and known positive human kidney tissue was performed as positive control.
Evaluation of DLL4 Immunohistochemical Staining
Two pathologists, who were blinded to the pathological diagnosis and clinical data, observed the immunohistochemical staining results. The number of positive endothelial or tumor cells was counted using a light microscope at a magnification of ×200. In each tumor specimen, 5 fields were examined. The expression was classified as low, if <10% of the cells were positive staining, and high, otherwise.21 The positive index (PI) was defined as the percentage of immunopositive cells (ratio of positively stained cells to the total number of cells per slide, multiplied by 100). The total concordance of scoring between the 2 observers was 92.8% (64/69) (Cohen’s kappa coefficient of 0.901, P < 0.001), indicating substantial agreement.
Measurement of Preoperative PTBE on MRI and Evaluation of the Postoperative KPS
All preoperative MRI at initial diagnosis were performed at the First Affiliated Hospital, Fujian Medical University (1.5 T scanner, slice thickness 5 mm). Spin echo sequences of MRI scans included axial and sagittal T1WI (with and without contrast enhancement) and axial T2WI. Peritumoral edema was defined as a region of increased T2 signal intensity on the tumor margin. Accordingly, the preoperative PTBE in glioblastoma was measured on first diagnostic MRI with an easy applicable and simple technique, edema extending <1 cm from the tumor margin was defined as minor and edema extending more than 1 cm from the tumor margin was defined as major.3
The Karnofsky performance scale (KPS) has been established as one of the major prognostic indicators for glioblastoma survival. A KPS, 70 has been shown to be significantly associated with a poor survival rate. All patients enrolled in the present study were evaluated with respect to a postoperative KPS above or below 70 who were assessed 10–14 days after surgery according to the protocol described previously.22
Statistical Analysis
All data were analyzed using SPSS 19.0 software. Associations between DLL4 expression and categorical variables were analyzed by Chi-square test or Spearman’s rank correlation (r) analysis, as appropriate. The correlation of PTBE with DLL4 and other potential clinical variables (age, KPS, symptom duration) were assessed using multiple linear regression model. Survival curves were constructed using the Kaplan–Meier method, and survival differences were evaluated by the log-rank test. A multivariate analysis was done by using Cox proportional hazards model to determine the prognostic effect of DLL4 expression, PTBE degree, and potential clinical variables (age, gender, KPS, symptom duration, and extent of resection) on TTP and OS. Backward selection applying a stopping rule based on the Akaike information criterion was used to exclude redundant or unnecessary variables. The proportional hazards assumptions were confirmed graphically. Hazard ratios and their corresponding 95% confidence intervals were computed to provide quantitative information about the relevance of results of the statistical analysis. Differences with a P value of 0.05 or less were considered to be statistically significant.
RESULTS
DLL4 Expression Levels and Patterns in Glioblastoma Tissues
Immunohistochemical staining showed that in primary glioblastoma, 46 out of 69 cases (66.7%) exhibited high-level expression of DLL4 compared with adjacent nonneoplastic brain tissues with rare positive immunoreactivity, the median PI of DLL4 expression was 18.3 (range 3.1–43.9). DLL4 expression was primarily observed in the cytoplasm of endothelial cells of glomeruloid and nonglomeruloid blood cells within glioblastoma (Figure 1A–C). In all endothelial subpopulations, DLL4 expression was distributed similarly in glomeruloid vascular proliferation (seen in N = 51 cases), and nonglomeruloid endothelium both with single-distributed (seen in N = 46 cases) and in cluster (seen in N = 47 cases). Of interest, positive cytoplasmic staining of DLL4 was rarely detected in tumor cells (seen in N = 6 cases) (Figure 1D–F). Several patterns of DLL4 expression by tumor cells were seen, including perivascular (N = 6 cases), adjacent necrosis (N = 4 cases), and occasion diffused (N = 6 cases).
FIGURE 1.
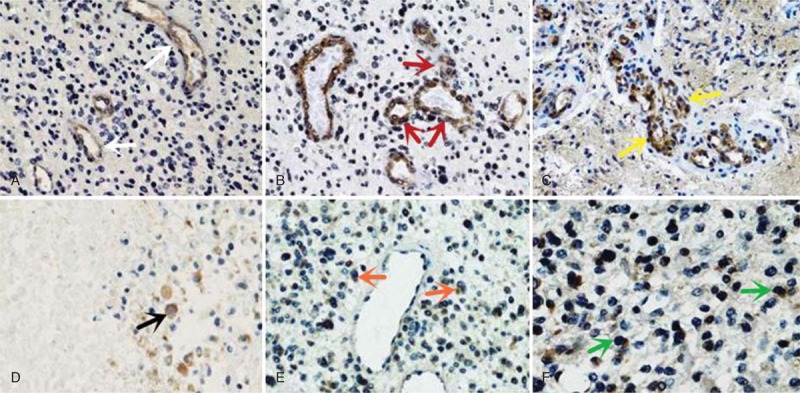
Cellular expression of DLL4 protein in glioblastoma specimens (immunohistochemistry). (A–C) Positive staining of DLL4 is primarily distributed in the cytoplasm of tumor endothelial cells: A, single nonglomeruloid vessel (white arrow); B, nonglomeruloid vessel in cluster (red arrow); C, glomeruloid vessel (yellow arrow). (D–F) Positive cytoplasmic staining of DLL4 is seldom detected in some tumor cells: D, adjacent necrosis (black arrow); E: perivascular (orange arrow); F: occasion-diffused (green arrow).
Relationship Between DLL4 Expression and Clinical Features
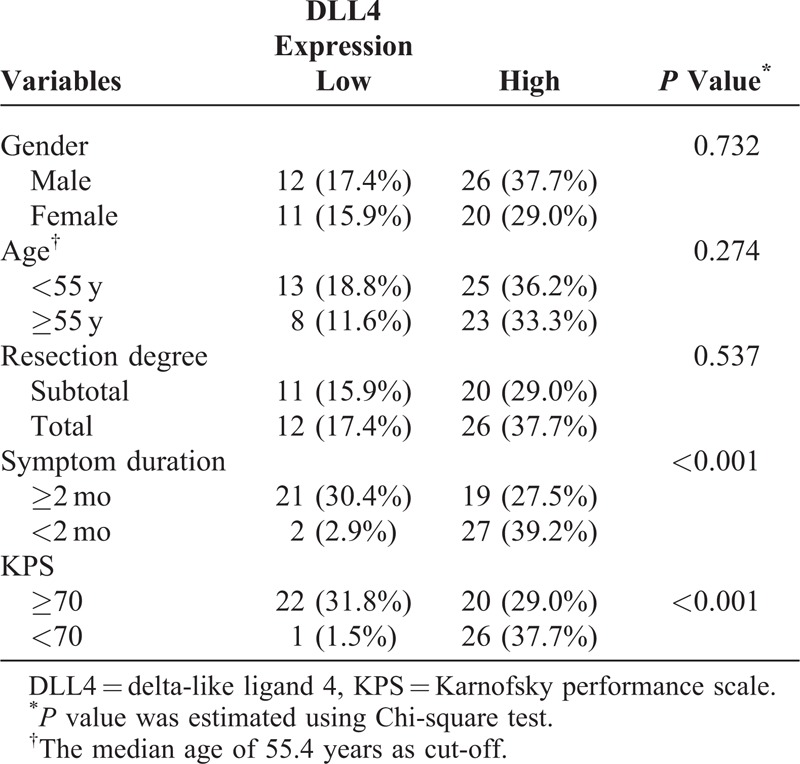
The relationships between DLL4 expression and clinical features in glioblastoma are listed in Table 2. The level of DLL4 expression was statistically associated with KPS, DLL4 expression was higher in patients with KPS <70 than those with KPS ≥70 (P < 0.001). In addition, a relationship between DLL4 expression and symptom duration was detected; for patients with DLL4-high expression, tumor-related symptom seemed to process more rapidly (P < 0.001). However, we failed to detect relationships between DLL4 and other patient characteristics, including age, gender, and resection degree.
TABLE 2.
Correlations of DLL4 and Clinical Characteristics
Correlation of DLL4 Expression With PTBE
PTBE has been proven as an independent prognosis factor in glioblastoma for its strong contribution to neurological signs and symptoms.3 Then based on the evidence of DLL4 expression related to symptom duration and performance status as indicated above and the important role of DLL4 in tumor angiogenesis, we further evaluated the relationship between DLL4 expression and PTBE in glioblastoma. According to the standard of Schoenegger et al,3 the degree and maximum extent of PTBE were assessed in all 69 patients’ preoperative MRIs. The degree of PTBE was positively associated with the expression of DLL4 (r = 0.845, P < 0.001), maximum extent of PTBE increased with elevated DLL4 expression, the PI of DLL4 expression in minor PTBE was lower than that in major PTBE (Figure 2).
FIGURE 2.
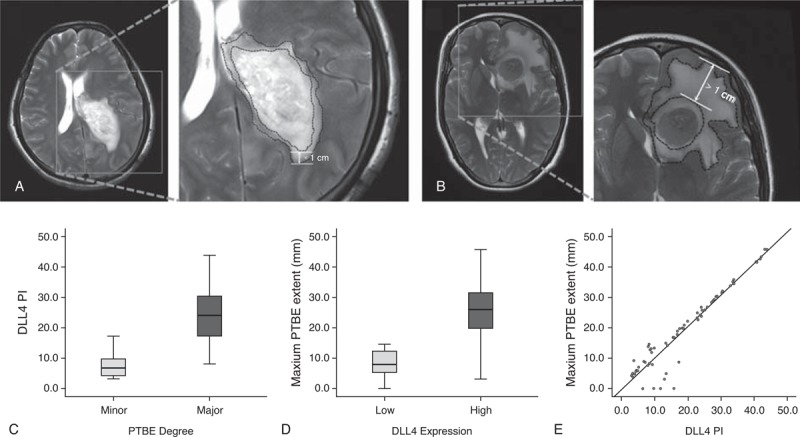
Correlation of DLL4 with PTBE in all 69 glioblastomas. (A,B) T2-weighted images showing peritumoral edema extending <1 cm from the tumor margin, defined as (A) minor edema and extending >1 cm from the tumor margin, defined as (B) major edema. (C) PI of DLL4 expression in minor PTBE is lower than that in major PTBE. (D,E) maximum extent of PTBE increases with elevated DLL4 expression (r = 0.845, P < 0.001).
Furthermore, a multivariable assessment of the association between DLL4 expression and PTBE that took into account other potential factors (age, KPS, symptom duration) influencing this relationship was performed by building a multiple linear regression model, which was evaluated using a stepwise variable selection method. The results showed that the coefficient of determination (R2) was 0.712, only the PI of DLL4 expression was associated with an increase in maximum extent of PTBE (P < 0.001).
Effect of DLL4 and PTBE on Patient Prognosis
Follow-up was available for all patients and the median follow-up period was 53.2 weeks (range: 20–124); during the follow-up period, 61 patients (88.4%) had died with glioblastoma. Kaplan–Meier curves were used to show that high DLL4 expression was statistically associated with both reduced TTP (P < 0.001) and OS (P < 0.001) in glioblastoma (Figure 3A). Similar results were also observed between survival estimates of patients with minor PTBE and major PTBE (Figure 3B).
FIGURE 3.
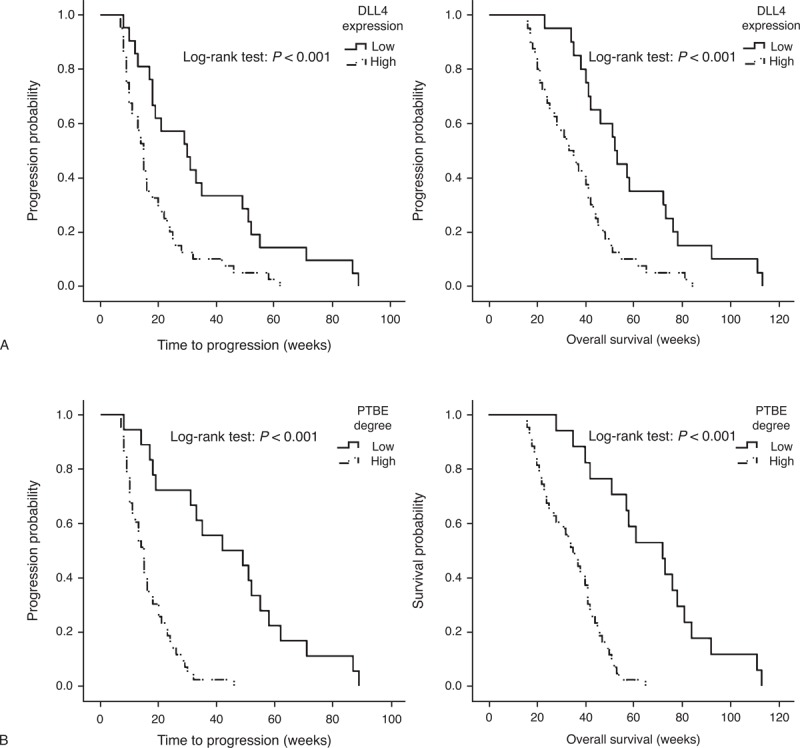
Effect of DLL4 and PTBE on TTP and OS in glioblastoma. (A) High DLL4 expression and (B) major PTBE are associated with both reduced TTP and OS.
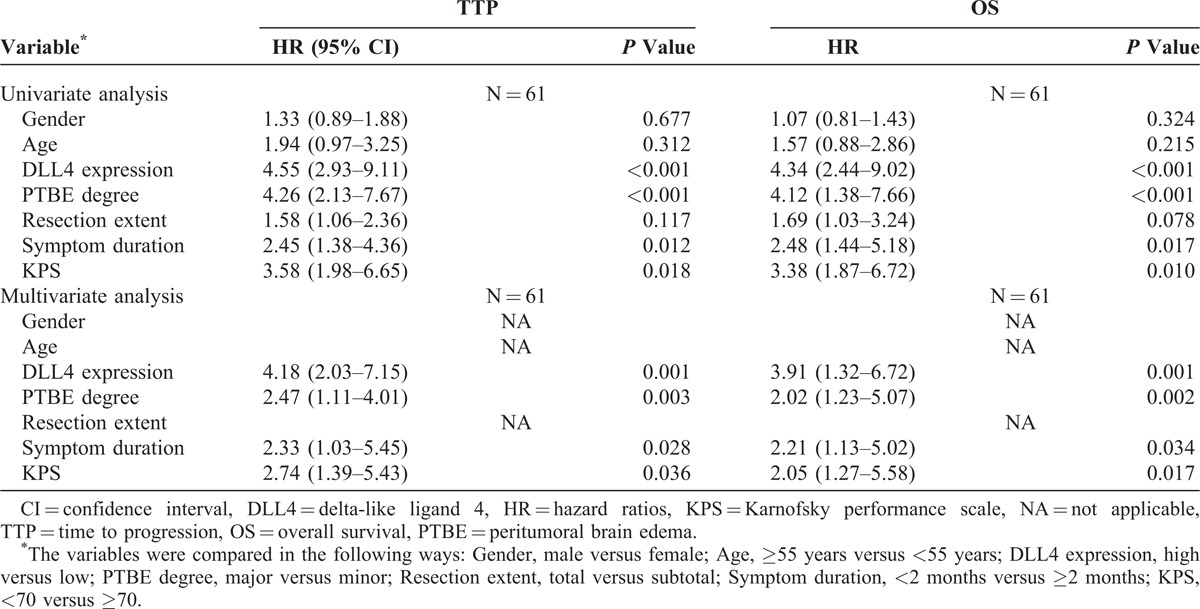
Next, a Cox multivariable proportional hazard model was constructed to examine independent prognostic significance of DLL4 expression with clinical characteristics including age, gender, resection degree, symptom duration, KPS, and PTBE. Considering a strong correlation between DLL4 expression and PTBE as indicated, we tested for multicollinearlity that included the above variables to eliminate the influence on proportional hazard analysis (TTP: maximum variance inflation factor [VIFmax] = 2.382, minimum tolerance [TOLmin] = 0.420; OS: VIFmax = 2.430, TOLmin = 0.411). Assumptions of proportional hazards were checked and found to be satisfactory with a log–log plot. Then the results of Cox multivariable proportional hazard model confirmed that DLL4-high expression was a significant predictor for shorter TTP and OS in glioblastoma, independent of age, gender, symptom duration, KPS, resection degree, and PTBE (Table 3).
TABLE 3.
Cox Proportional Hazards Regressions for TTP and OS in Glioblastoma
DISCUSSIONS
Glioblastoma, which is characterized by high neovascularization and severe PTBE, is the most common primary malignancy in the central nervous system. In glioblastoma, PTBE may cause severe neurological signs and symptoms, thereby seriously influencing survival. The exact mechanisms of PTBE are likely to be multifactorial. VEGF is a pivotal molecular mediator of PTBE in glioma,23,24 and anti-angiogenic therapy targeted on VEGF pathway dramatically reduces PTBE.25 VEGF interacts with many pathways as a complex network to modulate angiogenesis and glioma-related edema, including Notch, hypoxia inducible factor, aquaporin-4.26–28 But to date, the most recently identified member of Notch ligand family DLL4 and VEGF are the only 2 genes involved in vascular defects.29 In addition, a positive relationship between DLL4 and VEGF has been observed in human glioma.21 Thus, a hypothesis has been given that glioblastoma may have DLL4-dependent pathway leading to PTBE.
In this study, we analyzed the correlation of DLL4 expression with peritumoral edema; the results indicated that edema degree was positively associated with the DLL4 expression, and the mean edema maximum extent in the DLL4-high group was significantly higher than those in the DLL4-low group. The association of enhanced degree of edema with higher rate of DLL4 positive expression may be attributable to the role of DLL4 in tumor angiogenesis.30 Tumor angiogenesis is a process of acquiring an adequate blood supply for tumor growth and progression, and has been implicated in the development of perilesional edema by increased levels of angiogenic factors and tumor vessels with high permeability. In tumor angiogenesis, upregulated DLL4 activation enhances the vasculature structure and function within a tumor. The vessels within tumor have an increased permeability associated with blood–brain barrier disruption, which results in vasogenic cerebral edema.31 This explanation is in line with anti-angiogenic therapy reducing peritumoral edema.25,32
Furthermore, we evaluated the effect of DLL4 expression on patient outcome in glioblastoma. Noticeably, our study provided the direct evidence that high DLL4 expression was related with poor outcome in glioblastoma. First, by using immunohistochemical technique, we assessed the pattern and level of DLL4 protein expression in glioblastoma. Herein, we found that positive staining of DLL4 was primarily distributed in the cytoplasm of tumor vascular endothelial cells and rarely detected in some tumor cells of glioblastoma. Next, we observed close associations of DLL4 expression with KPS and symptom duration, 2 adverse factors influencing patient survival.22,33 Then Kaplan–Meier curves were used to indicate that both the median TTP and OS in patients with DLL4-high expression were significantly shorter than those in patients with DLL-low expression. Moreover, a Cox multivariable proportional hazard model revealed that DLL4 was an independent unfavorable predictor of TTP and OS in glioblastoma. The mechanism for DLL4 affecting patient outcome may be multiple. Part of the deleterious effect of DLL4 on survival may be because of the correlation of DLL4 with edema and a portion of the effect; other properties of DLL4, the role in tumorigenesis, may also contribute to shortened survival. DLL4-Notch signaling plays a critical role in the maintenance of glioma stem cell (GSC); knockdown of DLL4 decreases GSC population in glioma neurosphere in vitro and inhibits co-cultured glioma neurosphere propagation in vivo.34 Moreover, Notch pathway also interacts with several signaling molecules and pathways, including p53, rat sarcoma protein, nuclear factor-κB, transforming growth factor-β, phosphatidyl inositol 3-kinase, and epidermal growth factor receptor (EGFR), all of which are important in tumor carcinogenesis, invasion, and metastasis.35,36
In conclusion, our study indicated that DLL4 expression was positively correlated with peritumoral edema degree and was an independent predictor of patient prognosis in glioblastoma. However, our research refers to preliminary clinical observation and is limited by a number of factors. First and foremost, the findings is limited by relatively less generalizability because of few samples and the fact that this study was retrospective in nature and, therefore, not of the highest quality. Furthermore, the results of the association between DLL4 and PTBE does not mean that DLL4 is the cause of PTBE; indeed, whether abnormal DLL4 expression plays a role in the progression of PTBE or occurs in response to expression changes of other molecules that are related with PTBE is unclear. Thus, more molecular investigations into the interplay of DLL4 and other signaling events implicated in edema are needed to develop a potential strategy for controlling PTBE and improving patient outcome in glioblastoma.
Footnotes
Abbreviations: DAB = diaminobenzidine, DLL4 = delta-like ligand-4, GSC = glioma stem cell, KPS = Karnofsky performance scale, MRI = magnetic resonance imaging, OS = overall survival, PBS = phosphate buffer solution, PI = positive index, PTBE = peritumoral brain edema, TMZ = temozolomide, TOL = tolerance, TTP = time to progression, VEGF = vascular endothelial growth factor, VIF = variance inflation factor.
The authors would like to thank the Department of Pathology, First Affiliated Hospital, for their help in immunohistochemistry design, and the Public Health School, Fujian Medical University, Fujian, China, for assistance in data processing and statistical analysis.
This study was supported in part by the grants from the Key Clinical Special Discipline Construction Program of Fujian, P. R. China, in part by the National Natural Science Foundation of China, under Grant 30973083, in part by the Science and Technology Commission, Fujian, China, under Grant 2007I0014, and in part by the Science Research Foundation, Ministry of Health and United Fujian Provincial Health and Education Project for Tackling the Key Research, under Grant WKJ2008-2-45.
The authors have no conflicts of interest to disclose.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2.Oike T, Suzuki Y, Sugawara K, et al. Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma: Japanese mono-institutional results. PloS One. 2013;8:e78943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoenegger K, Oberndorfer S, Wuschitz B, et al. Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol. 2009;16:874–878. [DOI] [PubMed] [Google Scholar]
- 4.Stummer W. Mechanisms of tumor-related brain edema. Neurosurg Focus. 2007;22:E8. [DOI] [PubMed] [Google Scholar]
- 5.Lin ZX. Glioma-related edema: new insight into molecular mechanisms and their clinical implications. Chinese J Cancer. 2013;32:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. [DOI] [PubMed] [Google Scholar]
- 7.Gridley T. Notch signaling during vascular development. Proc Nat Acad Sci USA. 2001;98:5377–5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv W, Chen L, Zhou DH, Wei B. Influence of specific blocking of the delta-like ligand 4/notch signal transduction pathway on the biological behavior of human umbilical vein endothelial cells. Cancer. 2010;25:449–454. [DOI] [PubMed] [Google Scholar]
- 9.Mailhos C, Modlich U, Lewis J, et al. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135–144. [DOI] [PubMed] [Google Scholar]
- 10.Shutter JR, Scully S, Fan W, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Develop. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams CK, Li JL, Murga M, et al. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu ZJ, Shirakawa T, Li Y, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. [DOI] [PubMed] [Google Scholar]
- 14.Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. [DOI] [PubMed] [Google Scholar]
- 15.Jubb AM, Soilleux EJ, Turley H, et al. Expression of vascular notch ligand delta-like 4 and inflammatory markers in breast cancer. Am J Pathol. 2010;176:2019–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JX, Cai MB, Wang XP, et al. Elevated DLL4 expression is correlated with VEGF and predicts poor prognosis of nasopharyngeal carcinoma. Med Oncol. 2013;30:390. [DOI] [PubMed] [Google Scholar]
- 17.Jubb AM, Turley H, Moeller HC, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Brit J Cancer. 2009;101:1749–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica. 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. [DOI] [PubMed] [Google Scholar]
- 20.Sanghera P, Perry J, Sahgal A, et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Canad J Neurol Sci. 2010;37:36–42. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Wang J, Gong L, et al. Correlation of Delta-like ligand 4 (DLL4) with VEGF and HIF-1alpha expression in human glioma. Asian Pacific J Cancer Prevent. 2011;12:215–218. [PubMed] [Google Scholar]
- 22.Stark AM, van de Bergh J, Hedderich J, et al. Clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg. 2012;114:840–845. [DOI] [PubMed] [Google Scholar]
- 23.Carlson MR, Pope WB, Horvath S, et al. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592–2598. [DOI] [PubMed] [Google Scholar]
- 24.Senger DR, Galli SJ, Dvorak AM, et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. [DOI] [PubMed] [Google Scholar]
- 25.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer. 2007;67:2729–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Wang X, Zhen S, et al. Aquaporin-4 upregulated expression in glioma tissue is a reaction to glioma-associated edema induced by vascular endothelial growth factor. Oncol Rep. 2012;28:1633–1638. [DOI] [PubMed] [Google Scholar]
- 27.Li JL, Sainson RC, Oon CE, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer. 2011;71:6073–6083. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Fan F, Wang A, et al. Dll4-Notch signaling in regulation of tumor angiogenesis. J Cancer Res Clin Oncol. 2014;140:525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. [DOI] [PubMed] [Google Scholar]
- 30.El Hindy N, Keyvani K, Pagenstecher A, et al. Implications of Dll4-Notch signaling activation in primary glioblastoma multiforme. Neuro-oncology. 2013;15:1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees JH, Smirniotopoulos JG, Jones RV, et al. Glioblastoma multiforme: radiologic-pathologic correlation. Radiographics. 1996;16:1413–1438. [DOI] [PubMed] [Google Scholar]
- 32.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. [DOI] [PubMed] [Google Scholar]
- 33.Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:8205–8212. [DOI] [PubMed] [Google Scholar]
- 34.Zhu TS, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing NOTCH ligands that nurture self-renewal of cancer stem-like cells. Cancer. 2011;71:6061–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chigurupati S, Venkataraman R, Barrera D, et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer. 2010;70:418–427. [DOI] [PubMed] [Google Scholar]
- 36.Sivasankaran B, Degen M, Ghaffari A, et al. Tenascin-C is a novel RBPJkappa-induced target gene for Notch signaling in gliomas. Cancer. 2009;69:458–465. [DOI] [PubMed] [Google Scholar]


