Abstract
Giant cell arteritis (GCA) is a relapsing disease. However, the nature, chronology, therapeutic impact, and clinical consequences of relapses have been scarcely addressed. We conducted the present study to investigate the prevalence, timing, and characteristics of relapses in patients with GCA and to analyze whether a relapsing course is associated with disease-related complications, increased glucocorticoid (GC) doses, and GC-related adverse effects. The study cohort included 106 patients, longitudinally followed by the authors for 7.8 ± 3.3 years. Relapses were defined as reappearance of disease-related symptoms requiring treatment adjustment. Relapses were classified into 4 categories: polymyalgia rheumatica (PMR), cranial symptoms (including ischemic complications), systemic disease, or symptomatic large vessel involvement. Cumulated GC dose during the first year of treatment, time required to achieve a maintenance prednisone dose <10 mg/d (T10), <5 mg/d (T5), or complete prednisone discontinuation (T0), and GC-related side effects were recorded. Sixty-eight patients (64%) experienced at least 1 relapse, and 38 (36%) experienced 2 or more. First relapse consisted of PMR in 51%, cranial symptoms in 31%, and systemic complaints in 18%. Relapses appeared predominantly, but not exclusively, within the first 2 years of treatment, and only 1 patient developed visual loss. T10, T5, and T0 were significantly longer in patients with relapses than in patients without relapse (median, 40 vs 27 wk, p < 0.0001; 163 vs 89.5 wk, p = 0.004; and 340 vs 190 wk, p = 0.001, respectively). Cumulated prednisone dose during the first year was significantly higher in relapsing patients (6.2 ± 1.7 g vs 5.4 ± 0.78 g, p = 0.015). Osteoporosis was more common in patients with relapses compared to those without (65% vs 32%, p = 0.001). In conclusion, the results of the present study provide evidence that a relapsing course is associated with higher and prolonged GC requirements and a higher frequency of osteoporosis in GCA.
INTRODUCTION
Giant cell arteritis (GCA) is a granulomatous arteritis predominantly affecting large and medium-sized vessels.18,27 Treatment with high-dose glucocorticoids (GC) results in prompt and remarkable improvement of symptoms and reduces the risk of ischemic complications.2 However, reduced GC doses do not completely abolish essential pathways involved in disease persistence, and consequently, the course of GCA may be troubled by relapses.5,8,27 Recrudescence of GCA activity is common, occurring in at least 43% of patients in population-based studies3,26 and up to 80% in clinical trials with adjuvant therapies.15,16,19,20,22 The remarkable variability in the reported prevalence of relapses may be related to heterogeneity in the definition of relapses and to variability in the GC-tapering schedules. Definition of relapse, flare, or recurrence considerably varies across different studies.16,21–23,26 While in some publications definition of relapse has been based on clinical grounds,15,16,19 in others, isolated increases in acute-phase reactants have been considered disease flares.24
In addition, although this has not been formally evaluated, initial doses and tapering schedules seem to influence relapse rate in GCA.17,20 In this regard, it is noteworthy that the higher relapse rates have been observed in the context of clinical trials with adjuvant therapies where GC tapering is more aggressive than in standard of care settings, and when alternate-day GC tapering is applied.15,16,19,20,22 Consistently, a detailed review of treatments received by patients with isolated polymyalgia rheumatica (PMR) suggests that starting with lower GC doses is associated with higher relapse rates.13
Relapse rate is a commonly used primary endpoint in clinical trials with patients with GCA. However, although frequency of relapses has been reported in various studies,15,16,19,20,22,26 limited information exists regarding the clinical characteristics and predictors of relapses, and accompanying blood test abnormalities, which have been only specifically addressed in a previous study.23 Moreover, it has not been clearly demonstrated whether a relapsing course results in increased disease or treatment-related morbidity in these patients. Therefore, we conducted the present study to investigate the prevalence, timing, predictors, and main features of relapses in a longitudinally followed cohort of patients with GCA with long-term follow-up. In addition, we analyzed whether a relapsing course was associated with disease-related ischemic complications, higher cumulated GC doses, more prolonged treatment periods, and/or higher frequency of GC-related adverse effects.
PATIENTS AND METHODS
Between 1995 and 2007, 187 individuals were diagnosed with biopsy-proven GCA at our institution (Hospital Clínic, Barcelona, Spain). Among them, patients treated by the authors who underwent a regular follow-up for at least 4 years were selected. From the initial 187 patients diagnosed, 81 were excluded for the following reasons: 31 were subsequently treated at other departments or institutions, 19 died early during follow-up, 14 were transferred to nursing homes for advanced dementia, and 17 moved to other regions or had deficient compliance with the scheduled follow-up visits.
The remaining 106 patients were uniformly evaluated, treated, and longitudinally followed by the authors for an average of 7.8 ± 3.3 years (range, 4–15 yr). Clinical and laboratory findings at disease diagnosis were recorded. A combination of clinical and blood test abnormalities was used to evaluate the intensity of the systemic inflammatory response (SIR) as previously reported.6,7,14 These included fever >38 °C, weight loss ≥4 kg, hemoglobin (Hb) <11 g/L, and erythrocyte sedimentation rate (ESR) ≥85 mm/h. Patients with 3 or 4 of these items were considered to have a strong SIR, whereas patients with ≤2 were considered to have a weak SIR. Patients underwent clinical assessments in our outpatient facility every 3 months for the first 2 years after diagnosis and approximately every 4–6 months thereafter. ESR, C-reactive protein (CRP), blood cell counts, and Hb concentration were determined at each visit. The treatment protocol consisted of an initial prednisone (PDN) dose of 1 mg/kg per day (up to 60 mg/d) for 1 month. Intravenous methylprednisolone pulse therapy (1 g daily for 3 d) was initially administered to patients with recent (<48 h) visual loss. PDN was subsequently tapered at 10 mg/wk. When reaching 20 mg/d, this dose was maintained for 1–2 weeks and then reduced to 15 mg/d, which was maintained for 1 month. A further reduction to a maintenance dose of 10 mg/d was attempted. If tolerated, a reduction to 7.5 mg/d was tried after 3–6 months. Subsequent tapering was more variable. In general, a reduction to 5 mg/d was attempted approximately 3–6 months later and maintained for 1 year, after which a reduction of 1.25 mg/d was attempted every 6 months. Methotrexate at 15 mg/wk was added when patients experienced ≥2 relapses or had developed GC side effects. Reduction in PDN dose was performed 1 month before the scheduled follow-up visit to evaluate tolerance to the adjustment and to avoid severe relapses. If disease-related symptoms (cranial manifestations or PMR), fever, weight loss, or anemia not attributable to other reasons after the necessary work-up occurred, PDN dose was increased by 10–15 mg/d above the previous effective dose. If asymptomatic increases in acute-phase reactants were detected, PDN dose was held until the next visit. When a relapse could be defined, patients were managed as discussed above. If not, a reduction was attempted regardless of the ESR or CRP levels.
We used a consensus definition of relapse established in the context of international multicenter clinical trials.15,16,19 Relapse or recurrence were indistinctly defined as reappearance of disease-related symptoms, usually accompanied by elevation of acute-phase reactants that required treatment adjustment. Relapses were categorized according to the clinical manifestation into 4 categories: 1) PMR, 2) cranial symptoms (headache, scalp tenderness, jaw claudication, cranial ischemic complications), 3) systemic disease (anemia, fever, and/or weight loss), or 4) symptomatic large vessel involvement (extremity claudication). Cranial ischemic manifestations included stroke, transient ischemic attacks, amaurosis fugax, GCA-related visual loss, or diplopia. Number of relapses, time (in weeks) from the initiation of treatment to first relapse, time required to reach a PDN maintenance dose <10 mg/d (T10), <5 mg/d (T5), and time required to complete GC withdrawal (T0), not followed by a relapse for at least 3 months, were recorded. Cumulated PDN doses received after the first year of treatment were calculated. For each episode of relapsing activity, the ESR, serum CRP, and Hb concentrations were determined, as well as the PDN dose received at that time. In addition, GC-related adverse effects including new or worsening hypertension, diabetes mellitus, hypercholesterolemia, osteoporosis, cataracts, and Cushing appearance were recorded. Measurement of bone mineral density with dual energy X-ray absorptiometry was performed at disease diagnosis and thereafter approximately every 2 years. Osteoporosis was diagnosed using the World Health Organization criteria–-that is, bone mineral density T-score of 2.5 standard deviations (SDs) or more below the young adult mean.4 For screening of diabetes and hyperlipidemia, patients had blood tests prior to each visit, and blood pressure was periodically assessed both at their scheduled visits and by their primary care physicians. We recorded events as adverse events when they appeared or worsened after GC treatment and required new treatment or intensification of previous therapy. The study was approved by the Ethics Committee of Hospital Clínic (Barcelona, Spain).
Statistical Analysis
Continuous variables are presented as mean±SD and/or median and interquartile range (IQR) and categorical data as percentages. Association between relapses and selected covariates was analyzed using the T-test (paired and unpaired) for quantitative variables and the chi-square test for categorical data. Time required to achieve maintenance PDN dose <10 mg/d, <5 mg/d, and time to treatment discontinuation were compared between patients with and without relapses by the Kaplan-Meier survival analysis method. Statistical significance was defined as p < 0.05. Calculations were performed with the statistical package PAWS statistics v 18 (SPSS Inc, Chicago, IL) and GraphPad Prism v 5.04 for Windows (GraphPad Software, La Jolla, CA).
RESULTS
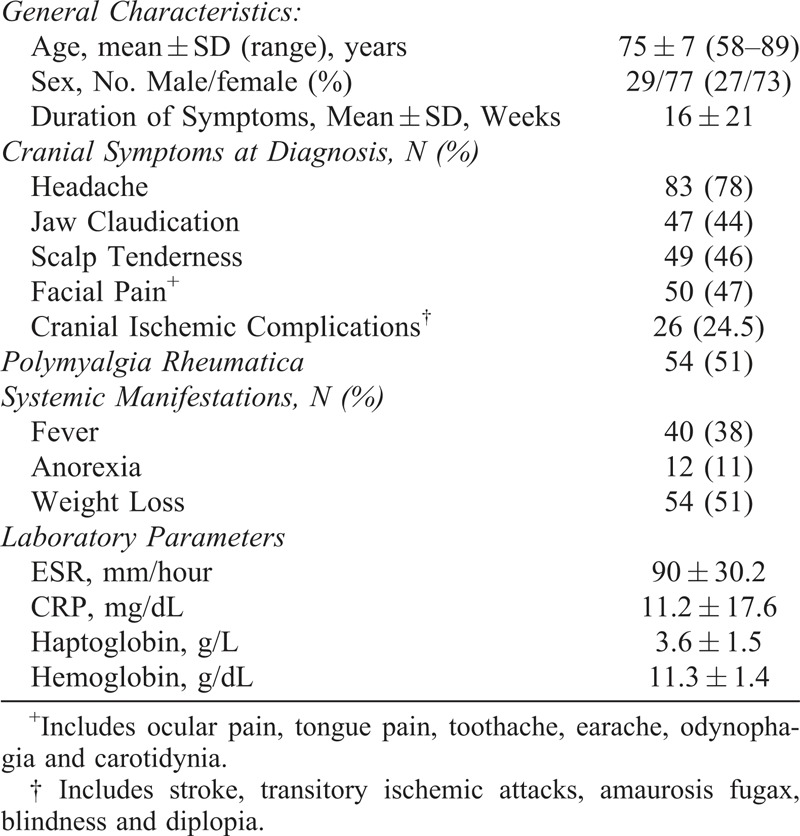
We analyzed 106 patients. Mean age at diagnosis was 75 ± 7 years (range, 58–89 yr) with a male to female ratio of 1:2.6. Demographic data and main clinical features at disease onset are depicted in Table 1.
TABLE 1.
Baseline Characteristics at Diagnosis (n=106)
Chronology and Characteristics of Relapses
Sixty-eight patients (64%) relapsed during follow-up (mean, 7.8 ± 3.3 yr; range, 4–15 yr) (Figure 1). Mean time to first relapse was 79 ± 75 weeks (range, 11–339 wk) with a median of 51 (IQR, 89) weeks. Thirty-four of the 68 patients (50%) relapsed during the first year after diagnosis (Figure 2A).
FIGURE 1.
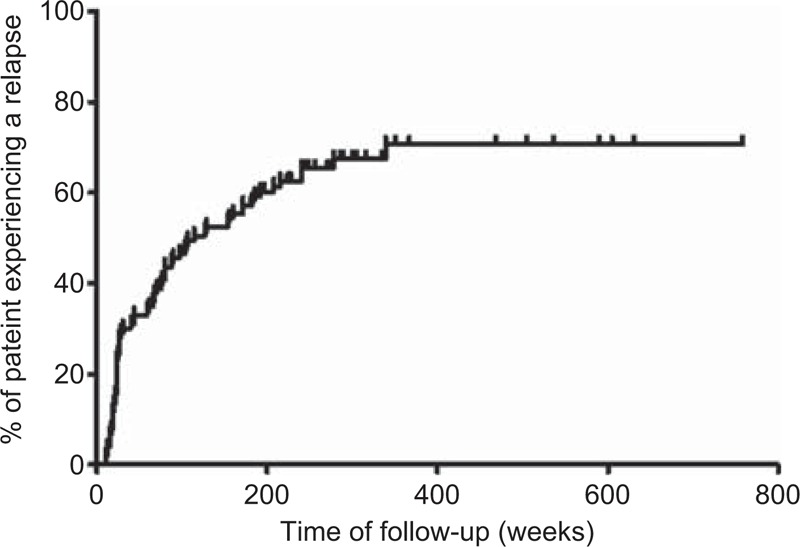
Kaplan-Meier plot of the entire series showing the probability of relapse over time.
FIGURE 2.
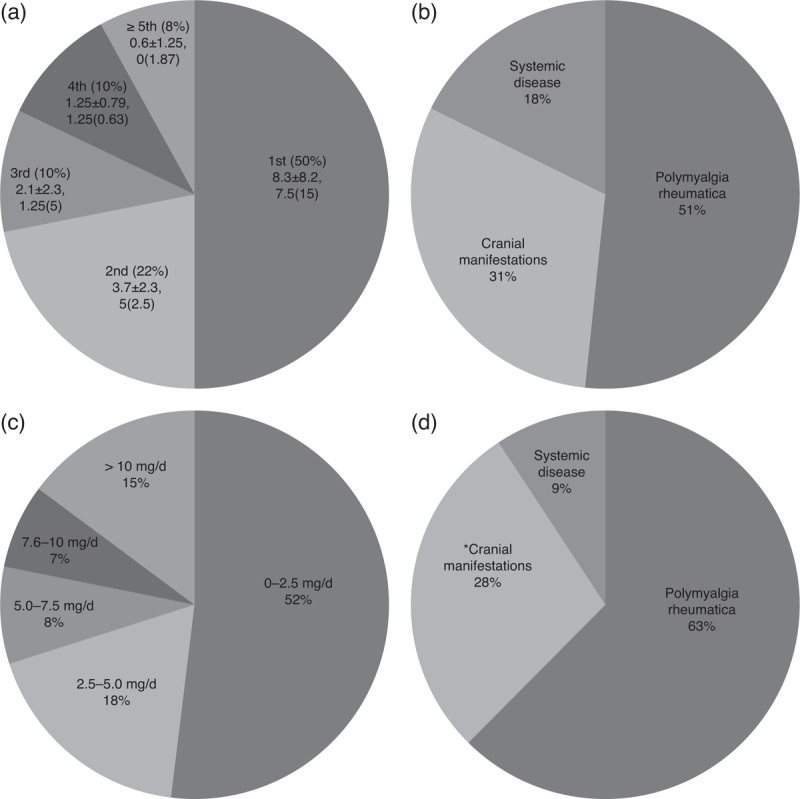
Characteristics and timing of flares in relapsing patients (n = 68). A) Percentage of patients relapsing per year of follow-up and mean±SD and median (IQR) prednisone dose (mg/d) received at the time of relapse. B) Percentage of relapsing patients with a given clinical type of relapse. C) Percentage of patients receiving the indicated dose of prednisone (mg/d) at the time of the first relapse. D) Clinical type of relapse at the second recurrence. *One patient developed a severe ischemic complication (anterior ischemic optic neuritis).
PMR was the most frequent clinical manifestation observed during the first flare (Figure 2B). Of note, severe ischemic complications were not developed by any patient as part of the first relapsing episode. Most patients relapsed with the same features originally present at GCA diagnosis (n = 52, 78%). For those who developed a different clinical manifestation, PMR was the most frequent new feature (n = 9, 75%). No patients in this series relapsed with symptomatic large vessel involvement.
Figure 2A shows the mean±SD and median (IQR) of PDN used by patients at the time of relapse during the first 5 years of follow-up. Mean PDN dose received by the 68 patients at the first relapse was of 5.3 ± 6.5 mg/d with a median of 2.5 (IQR, 7.5) mg/d. Fifty-two percent were receiving doses ≤2.5 mg/d (Figure 2C). PDN doses at the time of relapse tended to decrease over time (Figure 2A). Patients who relapsed during the first year received 8.3 ± 8.2 mg/d, median 7.5 mg (IQR, 15), whereas patients who relapsed during the second year were receiving 3.7 ± 2.3 mg, median 5 mg (IQR, 2.5).
Mean ESR, CRP, and Hb levels at the time of the first relapse were 61 ± 29 mm/h, 4.0 ± 3.8 mg/dL, and 12 ± 1.4 g/L, respectively. The inflammatory response at that time was comparatively lower than that observed at GCA onset (ESR 88 ± 33 vs 61 ± 29 mm/h, p < 0.0001; CRP 11 ± 19 vs 4.0 ± 3.8 mg/dL, p = 0.001; and Hb 11.3 ± 1.6 vs 12 ± 1.4 g/L, p < 0.0001). We observed an increase of 33 ± 14 mm/hr in ESR level, an increase of 2.9 ± 2.2 mg/dL in CRP concentration, and a decrease of 0.7 ± 0.1 g/L for Hb values between the previous laboratory tests while in remission and the ones performed at disease relapse.
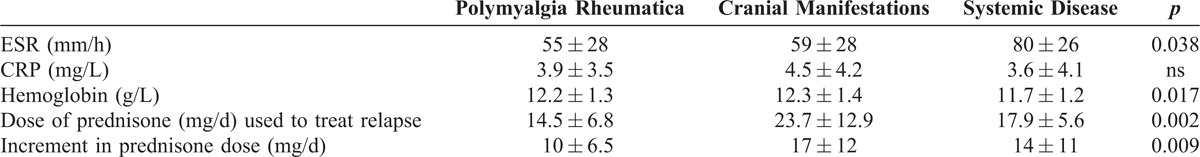
There were significant differences in the PDN doses used to treat relapses according to the type of recurrence (Table 2). The lowest doses were used to treat PMR symptoms (14.5 ± 6.8 mg/d) whereas higher doses were employed to treat cranial manifestations (23.7 ± 12.9 mg/d). ESR levels and Hb concentrations were significantly more deviated from normal values in patients who relapsed with systemic manifestations (see Table 2).
TABLE 2.
Laboratory Characteristics and PDN Dose at Each Relapse Type
Thirty-eight patients (36%) had 2 or more relapses. Distribution of relapse types was similar to that observed at the first episode (Figure 2D). One patient developed a severe ischemic complication (anterior ischemic optic neuritis) as part of her second recurrence. This patient was treated for 3.5 years and regularly followed for 5 years. She subsequently abandoned regular visits and presented with a relapse including malaise, ischemic optic neuritis, and elevation of ESR, 4 years later. The mean PDN dose at the second flare was 4.3 ± 2.7 mg/d with a median of 5 (IQR 2.5) mg. ESR (mm/h), CRP (mg/dL), and Hb (g/L) levels were 55 ± 30, 3.9 ± 6.1, and 12.1 ± 1.4 respectively.
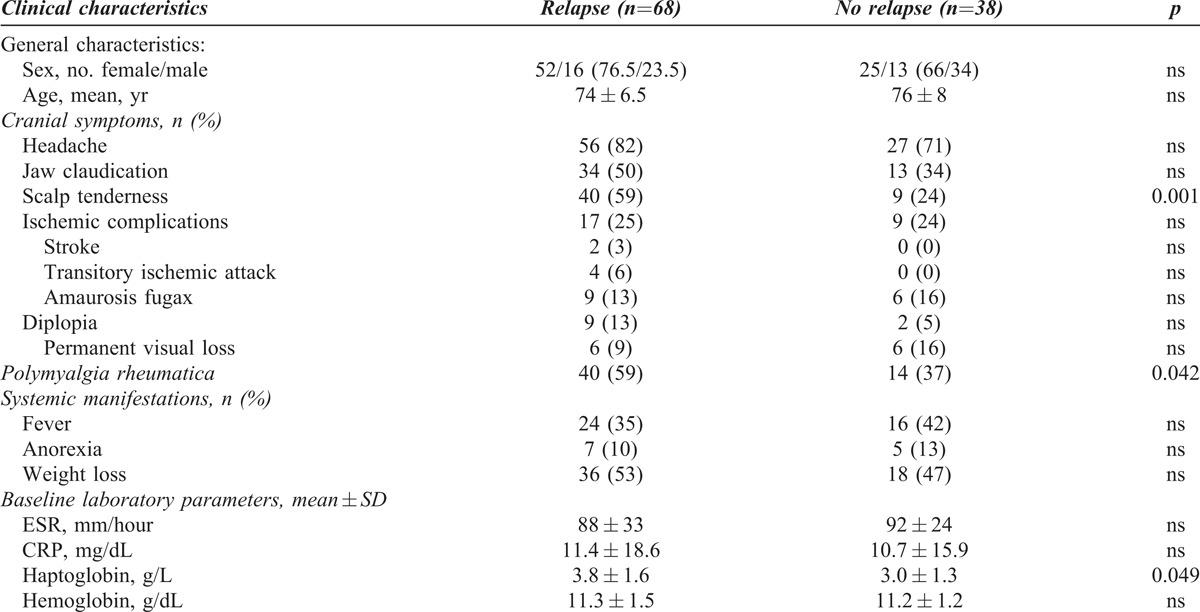
Predictors of Relapse
To search for predictors of relapses, we compared initial clinical and laboratory findings between patients with or without recurrent disease. At disease onset, PMR and scalp tenderness were more frequently observed in relapsing patients (Table 3). Acute-phase reactants tended to be higher in patients with recurrences, but only haptoglobin reached statistical significance (3.8 ± 1.6 g/L vs 3.0 ± 1.3 g/L, p = 0.042). When the intensity of the SIR was evaluated combining clinical and laboratory findings, patients with multiple relapses were significantly more frequent among those with a strong SIR: 23 of 27 (85%) patients with a strong SIR relapsed 2 or more times compared with 15 of 41 (37%) of those with a weak SIR. No other predictors of recurrences could be identified.
TABLE 3.
Clinical Manifestations at Diagnosis in Patients With and without Relapses
Glucocorticoid Requirements and Side Effects According to Disease Recurrences
Treatment requirements were different in both groups of patients (Table 4). Patients with relapses required significantly longer periods of time to reach a maintenance dose of PDN <10 mg/d, <5 mg/d, and to completely discontinue GC therapy (Figure 3). Cumulated PDN dose during the first year was significantly higher in relapsing patients (6.2 ± 1.7 g vs 5.4 ± 0.78 g, p = 0.015). Relapsing patients had an increased prevalence of osteoporosis (65% for relapsing patients vs 32% for nonrelapsing, p = 0.001). Other adverse effects also tended to be more frequent in patients with relapses, but differences did not reach statistical significance. As expected, methotrexate was administered more frequently in patients with relapses than in patients in sustained remission (22% vs 3%, p = 0.009).
TABLE 4.
Treatment Requirements and Side Effects During Follow-up

FIGURE 3.
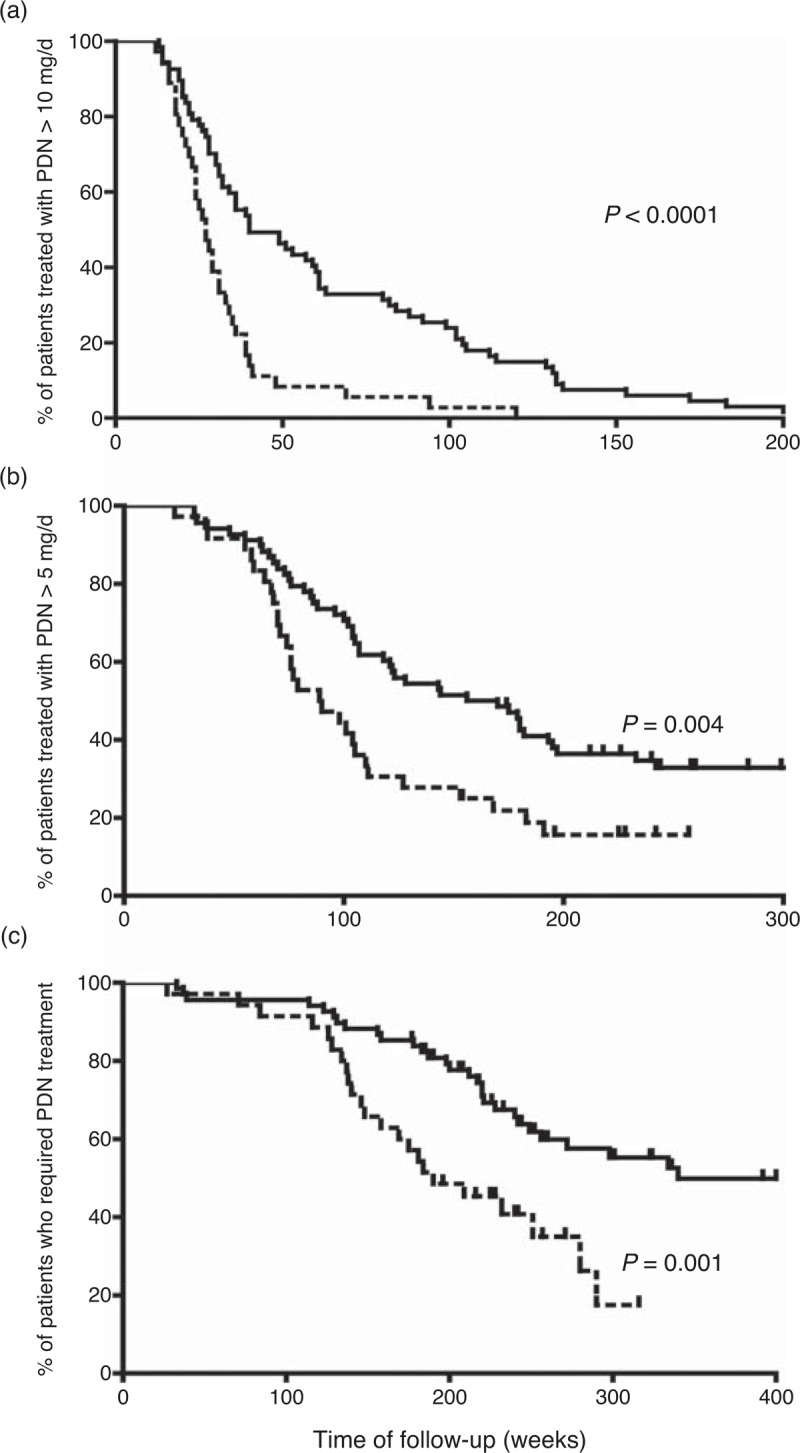
Survival curves showing the time required to reach a stable dose of prednisone <10 mg/d (A), <5 mg/d (B), and 0 mg/d (C) in patients with relapses (solid line) and with sustained remission (broken line). [Note the scale for time of follow-up is different among the 3 figure parts].
DISCUSSION
Limited information about the characteristics of recurrences occurring in patients with GCA is available.23 Here, we present detailed data about clinical and laboratory characteristics of relapses from a cohort of uniformly treated patients with GCA with long-term follow-up. The definition of relapse used in the present study was similar to that used in randomized, controlled clinical trials evaluating adjunctive therapies for GCA.15,16,19
In spite of the satisfactory initial response to GC treatment, 64% of patients relapsed in the present series. This percentage is somewhat higher than that reported in population-based studies,3,26 possibly due to the more extended follow-up of our patient cohort, but some selection bias cannot be excluded. Although most relapses occurred within the first 2 years of treatment, recurrences also developed subsequently. PMR was the most frequent symptom (51%) at the time of relapse, followed by cranial manifestations (31%). In previous studies16,22,23 headache was the leading feature (44%-60%), followed by PMR (19%-30%),16,23 and constitutional syndrome (28%).23 Therefore, the distribution found in the current cohort is close to that found in other studies. It is noteworthy that disease-related ischemic complications seem to be extremely infrequent in the context of controlled relapses. In previous studies the occurrence of ischemic manifestations has been also found to be infrequent during follow-up (0%-6%).1,16,23 Only 1 patient in the current series suffered anterior ischemic optic neuropathy in the context of a delayed relapse, but this patient had interrupted regular control visits at the time of disease recurrence. No patient in our series relapsed with symptomatic involvement of large vessels.
Relapses were usually accompanied by elevated levels of ESR and CRP that were, nevertheless, lower than those observed at disease onset. In accordance, PDN doses much lower than the starting doses were usually effective for treating controlled relapses. However, it must be stressed that the reported features were obtained from patients who were closely followed with re-assessments performed approximately every 3 months during the first 2 years after diagnosis. We cannot exclude that severe relapses requiring higher GC doses may occur in patients controlled less tightly. These findings indicate that patients with GCA need to be indefinitely observed even after successful GC discontinuation.
As for the time at greatest risk for relapse, in 50% of patients who relapsed, recurrences occurred during the first year. Mean time to first relapse was 19.7 ± 18.7 months, similar to what has been reported by others.21,23,26 However delayed relapses also occurred.
As shown in Figure 2, PDN dose received at the time when relapses occurred decreased over time, suggesting that disease activity progressively decreases and, over the years, lower PDN doses are required to maintain remission. Overall, relapses occurred when patients were receiving a mean PDN dose of 5.3 ± 6.5 mg/d with median 2.5 mg/d (IQR, 7.5). This dose is lower than that reported in other series. This may be due to variability in the rate of initial PDN tapering across different studies or to other reasons. No patient in our cohort relapsed with PDN higher than 25 mg/d.
Patients with relapses required longer periods of treatment and were exposed to higher cumulated PDN doses, similar to what was found in a previous study.23 All patients in our cohort experienced at least 1 GC-related side effect. Other studies have reported GC adverse effects in 90%-95% of GCA patients within the first 3 years of therapy.24,26 These include new or worsening hypertension (22%-84%),19,24,26 infections (22%-56%),19,22,24,26 osteoporosis and bone fractures (8%-38%),19,22–24,26 new or worsening diabetes mellitus (7%-37%),19,22–24,26 and cataracts (4%-41%).19,24,26 The higher frequency of side effects in our patient cohort may be related to the longer follow-up period. We observed that patients with recurrences presented more GC-related toxicity, in particular osteoporosis despite the administration of calcium supplements, vitamin D, and bisphosphonates. These data highlight the need for more efficient and safer therapies.
In the current cohort, relapses could not be attributed to insufficient treatment because relapsing patients received more GC and for more extended periods of time. This observation indicates that some patients have a more resistant disease. From the clinical standpoint, an intense acute-phase response was associated with higher risk of relapse. Other investigators have also observed that abnormalities related to the acute-phase response are predictors of relapse.6,9,12,14,23,25,28 Among other findings, only scalp tenderness and PMR were slightly more frequent in relapsing patients. Although this association may be spurious, a similar trend has been observed in other studies.23 Previous studies have investigated tissue and serum biomarkers associated with persistent disease activity and relapsing course. Elevated serum concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and soluble intercellular adhesion molecule (ICAM)-1 are associated with relapsing disease.10,12,28 Increased expression of TNF-α or chemokine (C-C motif) ligand (CCL)-2 mRNA in involved arteries is associated with recurrent disease and higher GC requirements. However, TNF-α blockade was not sufficient to reduce relapses and spare corticosteroids,16 indicating that association does not imply causality and suggesting that TNF-α effects may be compensated by other cytokines and that upstream mediators may be more relevant to perpetuate disease activity. Elevated mRNA concentrations of Th1 cytokines IL-12/23p40 and interferon (IFN)-γ have been observed after 1 year of treatment in second temporal artery biopsies of relapsing patients, suggesting re-activation of initial events able to drive subsequent inflammatory cascades.28 In contrast, increased IL-17 expression in GCA lesions is a predictor of sustained response to GC.11 Further research is needed to elucidate the mechanisms involved in disease persistence, to enable the design of more specific and efficient targeted therapies.
Footnotes
Abbreviations: CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GC = glucocorticoids, GCA = giant cell arteritis, Hb = hemoglobin, IQR = interquartile range, PDN = prednisone, PMR = polymyalgia rheumatica, SD = standard deviation, SIR = systemic inflammatory response, TNF = tumor necrosis factor.
Results partially presented at the Annual Scientific Meeting of the American College of Rheumatology 2012, Washington, DC (November 10–14, 2012).
Conflicts of Interest and Funding Sources: The authors declare no conflicts of interest. Supported by Ministerio de Economía y Competitividad (SAF 08/04328 y SAF 11/30073). MA Alba was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT), Mexico and by the Agencia de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) (Generalitat de Catalunya). G Espígol-Frigolé was supported by Instituto de Salud Carlos III. M Butjosa and S Prieto-González were supported by Premi Fi de Residència (Hospital Clínic).
References
- 1.Aiello PD, Trautmann JC, McPhee TJ, et al. Visual prognosis in giant cell arteritis. Ophthalmology. 1993;100:550–555. [DOI] [PubMed] [Google Scholar]
- 2.Alba MA, Espigol-Frigole G, Butjosa M, et al. Treatment of large vessel vasculitis. Curr Immunol Rev. 2011;7:435–442. [Google Scholar]
- 3.Andersson R, Malmvall BE, Bengtsson BA. Long-term corticosteroid treatment in giant cell arteritis. Acta Med Scand. 1986;220:465–469. [DOI] [PubMed] [Google Scholar]
- 4.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. Technical Report Series. Vol. 843 World Health Organization; 1994: 1–129. [PubMed] [Google Scholar]
- 5.Bongartz T, Matteson EL. Large-vessel involvement in giant cell arteritis. Curr Opin Rheumatol. 2006;18:10–17. [DOI] [PubMed] [Google Scholar]
- 6.Ciccia F, Alessandro R, Rizzo A, et al. IL-33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis. 2013;72:258–264. [DOI] [PubMed] [Google Scholar]
- 7.Cid MC, Font C, Oristrell J, et al. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 1998;41:26–32. [DOI] [PubMed] [Google Scholar]
- 8.Cid MC, Garcia-Martinez A, Lozano E, et al. Five clinical conundrums in the management of giant cell arteritis. Rheum Dis Clin North Am. 2007; 33:819–834, vii. [DOI] [PubMed] [Google Scholar]
- 9.Cid MC, Hoffman MP, Hernandez-Rodriguez J, et al. Association between increased CCL2 (MCP-1) expression in lesions and persistence of disease activity in giant-cell arteritis. Rheumatology (Oxford). 2006;45:1356–1363. [DOI] [PubMed] [Google Scholar]
- 10.Coll-Vinent B, Vilardell C, Font C, et al. Circulating soluble adhesion molecules in patients with giant cell arteritis. Correlation between soluble intercellular adhesion molecule-1 (sICAM-1) concentrations and disease activity. Ann Rheum Dis. 1999;58:189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espigol-Frigole G, Corbera-Bellalta M, Planas-Rigol E, et al. Increased IL-17A expression in temporal artery lesions is a predictor of sustained response to glucocorticoid treatment in patients with giant-cell arteritis. Ann Rheum Dis. 2013;72:1481–1487. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Martinez A, Hernandez-Rodriguez J, Espigol-Frigole G, et al. Clinical relevance of persistently elevated circulating cytokines (tumor necrosis factor alpha and interleukin-6) in the long-term followup of patients with giant cell arteritis. Arthritis Care Res (Hoboken). 2010;62:835–841. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Rodriguez J, Cid MC, Lopez-Soto A, et al. Treatment of polymyalgia rheumatica: a systematic review. Arch Intern Med. 2009;169:1839–1850. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Rodriguez J, Garcia-Martinez A, Casademont J, et al. A strong initial systemic inflammatory response is associated with higher corticosteroid requirements and longer duration of therapy in patients with giant-cell arteritis. Arthritis Rheum. 2002;47:29–35. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman GS, Cid MC, Hellmann DB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–1318. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman GS, Cid MC, Rendt-Zagar KE, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med. 2007;146:621–630. [DOI] [PubMed] [Google Scholar]
- 17.Hunder GG, Sheps SG, Allen GL, et al. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med. 1975;82:613–618. [DOI] [PubMed] [Google Scholar]
- 18.Jennette JC, Falk RJ, Bacon PA, et al. Revised international Chapel Hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 19.Jover JA, Hernandez-Garcia C, Morado IC, et al. Combined treatment of giant-cell arteritis with methotrexate and prednisone. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2001;134:106–114. [DOI] [PubMed] [Google Scholar]
- 20.Kyle V, Hazleman BL. Treatment of polymyalgia rheumatica and giant cell arteritis. I. Steroid regimens in the first two months. Ann Rheum Dis. 1989;48:658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyle V, Hazleman BL. The clinical and laboratory course of polymyalgia rheumatica/giant cell arteritis after the first two months of treatment. Ann Rheum Dis. 1993;52:847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahr AD, Jover JA, Spiera RF, et al. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Lado L, Calvino-Diaz C, Pineiro A, et al. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore). 2011;90:186–193. [DOI] [PubMed] [Google Scholar]
- 24.Mazlumzadeh M, Hunder GG, Easley KA, et al. Treatment of giant cell arteritis using induction therapy with high-dose glucocorticoids: a double-blind, placebo-controlled, randomized prospective clinical trial. Arthritis Rheum. 2006;54:3310–3318. [DOI] [PubMed] [Google Scholar]
- 25.Nesher G, Nesher R, Mates M, et al. Giant cell arteritis: intensity of the initial systemic inflammatory response and the course of the disease. Clin Exp Rheumatol. 2008;26:S30–S34. [PubMed] [Google Scholar]
- 26.Proven A, Gabriel SE, Orces C, et al. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum. 2003;49:703–708. [DOI] [PubMed] [Google Scholar]
- 27.Salvarani C, Pipitone N, Versari A, et al. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol. 2012;8:509–521. [DOI] [PubMed] [Google Scholar]
- 28.Visvanathan S, Rahman MU, Hoffman GS, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis—a prospective longitudinal study. Rheumatology (Oxford). 2011; 50:2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]


