Abstract
Cardiac transplantation (CT) has been one of the great medical advances of the last nearly 50 years. We studied the explanted hearts of 314 patients having CT at Baylor University Medical Center Dallas from 1993 to 2012, and compared the morphologic diagnoses to the clinical diagnoses before CT. Among the 314 patients the morphologic and clinical diagnoses were congruent in 272 (87%) and incongruent in 42 (13%). Most of the incongruity occurred among the 166 patients with non-ischemic cardiomyopathy (non-IC) (36/166 [22%]), and of that group the major incongruity occurred among the patients with hypertrophic cardiomyopathy (7/17 [41%]), non-compaction left ventricular cardiomyopathy (NCLVC) (3/3 [100%]), mononuclear myocarditis (3/3 [100%]), arrhythmogenic right ventricular cardiomyopathy (ARVC) (4/4 [100%]), and cardiac sarcoidosis (8/8 [100%]). The phrase “non-IC” is a general term that includes several subsets of cardiac diseases and simply means “insignificant narrowing of 1 or more of the epicardial coronary arteries,” but it does not specify the specific cause of the heart failure leading to CT. A number of cardiac illustrations are provided to demonstrate the morphologic variability occurring among the patients with IC and non-IC.
INTRODUCTION
The first cardiac transplantation (CT) procedure was done in Capetown, South Africa, by Christiaan Barnard in 1967.3 From that time through 2012, more than 90,000 CT procedures were performed worldwide, approximately 3500 a year with about 2200 of that number being performed in the United States.30 The immediate pre-op care, the operation, and the first 6-month post-operative care cost about $1,000,000. Although most have been performed in patients with idiopathic dilated cardiomyopathy (IDC) or ischemic cardiomyopathy (IC), CT is also performed, albeit less frequently, for a variety of other cardiac conditions. In 1998 Waller and colleagues reported clinical and morphologic findings in 92 patients who had had CT for IDC, IC, or hypertrophic cardiomyopathy (HC).71 Of them, 57 had CT at Baylor University Medical Center (BUMC) Dallas from March 1993 through June 1997. (The remaining 35 patients had CT at other institutions and are not included in the present analysis.) Surprisingly, that clinico-pathologic study appears to have been the first to report morphologic findings in a systematic fashion in the recipient hearts in those 3 conditions. The present report was prompted by finding incongruity between the clinical and morphologic diagnoses in some patients having CT, and that incongruity led to a thorough analysis of that frequency in 314 patients who had had CT at BUMC in the past 20 years.
METHODS
The recipient hearts in each of the 314 patients were submitted to the surgical pathology division of the department of pathology of BUMC Dallas. The patients included had undergone CT at BUMC from March 1993 through June 2012. All hearts during this period were described and classified by 1 of the authors (WCR). Figure 1 displays the number of CTs done at BUMC during each of those 20 years and the 3 major reasons for CT among the 314 patients. Each heart after formalin fixation and “cleaning” was weighed carefully by WCR, opened by him, and photographed (most by JMK). Although some were opened by parasagittal cuts, especially in those patients with HC, the cardiac ventricles in most were incised by transverse cuts parallel to the posterior atrioventricular sulcus. The resulting “bread-loaf” slices were approximately 1 cm in thickness except for the most apical portion, which was about 3 cm thick. The heart was weighed after these cuts and after all extraneous tissues were removed. The left ventricular cavity was measured both from anterior to posterior and from right to left dimensions, and the largest of these cavity numbers were used as the maximal cavity dimension. The right ventricular cavity dimension was the length from the right ventricular aspect of the ventricular septum to the endocardial lining of the right ventricular free wall along the posterior wall of this chamber. Figures 2–30
FIGURE 1.
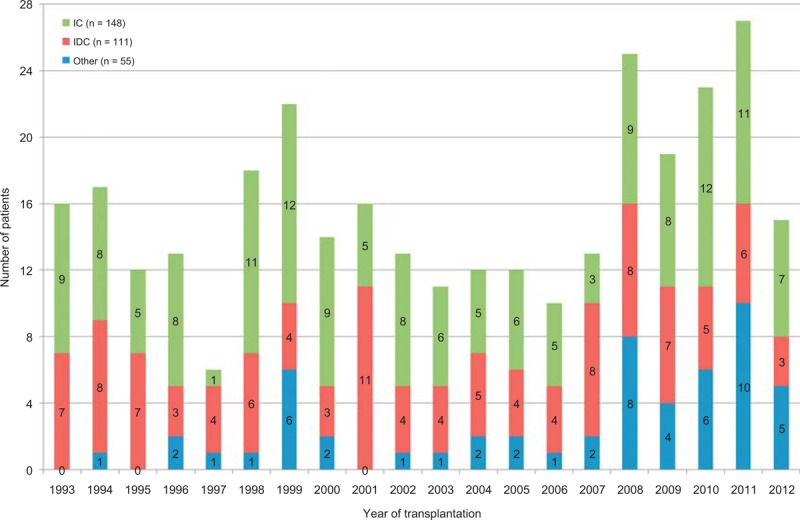
Bar graph showing the number of cardiac transplantation procedures done each year at Baylor University Medical Center 1993–2012 (through June 2012), and the 3 major reasons for the transplant (IC = ischemic cardiomyopathy [green]; IDC = idiopathic dilated cardiomyopathy [red], and other [blue]).
FIGURE 2.
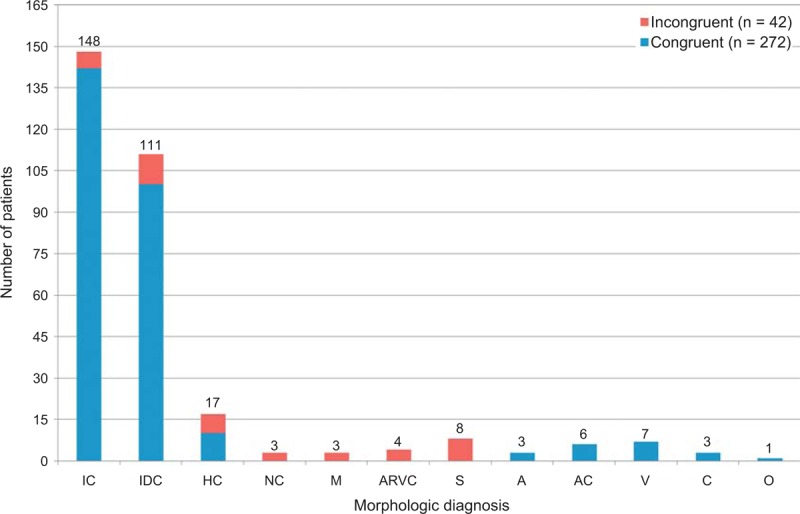
Bar graph showing frequency of congruence and incongruence among the various conditions cardiac transplantation among the 314 patients. IC = ischemic cardiomyopathy; IDC = idiopathic dilated cardiomyopathy; HC = hypertrophic cardiomyopathy; NC = non-compaction left ventricular cardiomyopathy; M = mononuclear myocarditis; ARVC = arrhythmogenic right ventricular cardiomyopathy; S = sarcoidosis; A = amyloidosis; V = valvular heart disease; C = congenital heart disease; O = other.
FIGURE 30.
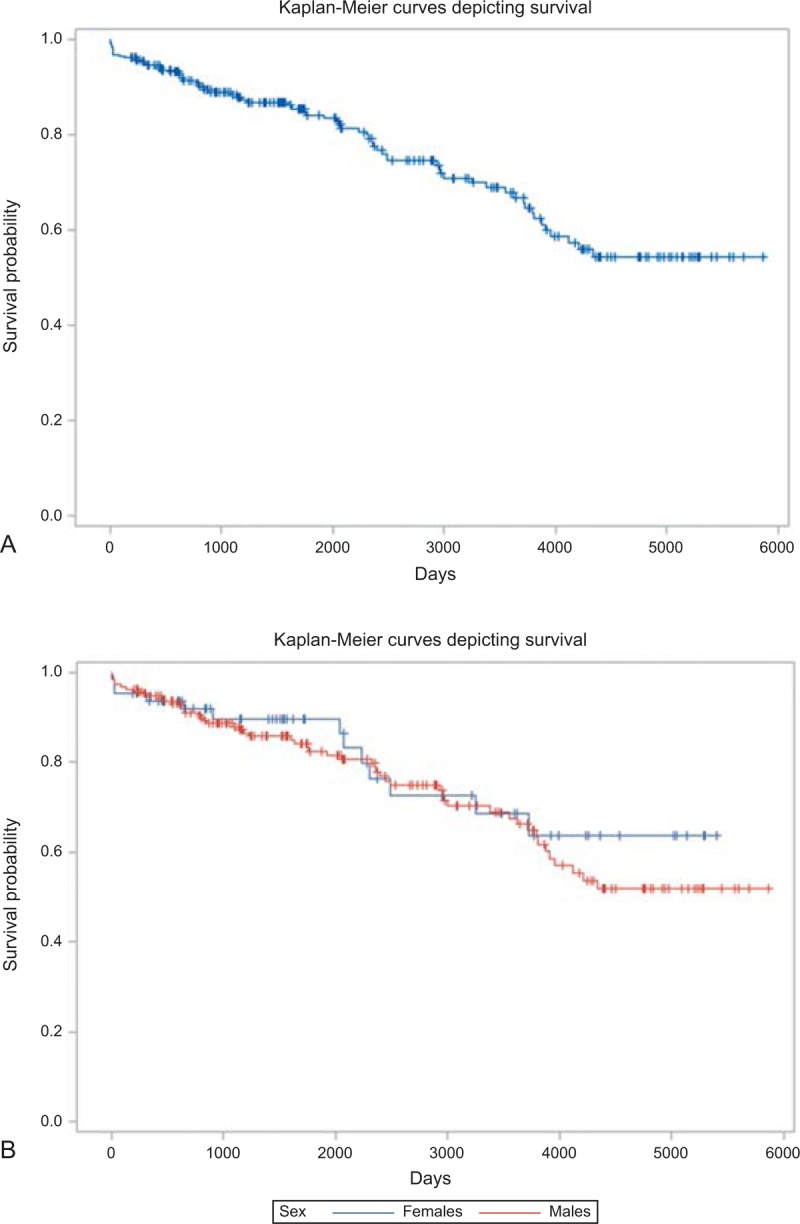
Kaplan-Meier survival curves for 257 patients whose survival data were available. (a) The unadjusted survival curve. (b) The unadjusted survival curves by sex.
FIGURE 4.

Ischemic cardiomyopathy. Photographs of sections of the cardiac ventricles caudal to the mitral valve in a 54-year-old woman who had an acute myocardial infarction when she was 33 years of age and underwent percutaneous coronary intervention at that time. At age 39 years, she had another acute myocardial infarct and underwent coronary artery bypass grafting. Cardiac catheterization 2 years later disclosed that all bypass conduits were closed. At age 42 years, she had another percutaneous coronary intervention. The left ventricular ejection fraction shortly before cardiac transplantation was 10%. These views show a healed myocardial infarct in the anterior wall of left ventricle and another in the posterior wall opposite one another. The quantity of subepicardial adipose tissue is clearly increased. The left ventricular cavity is quite dilated.
FIGURE 5.
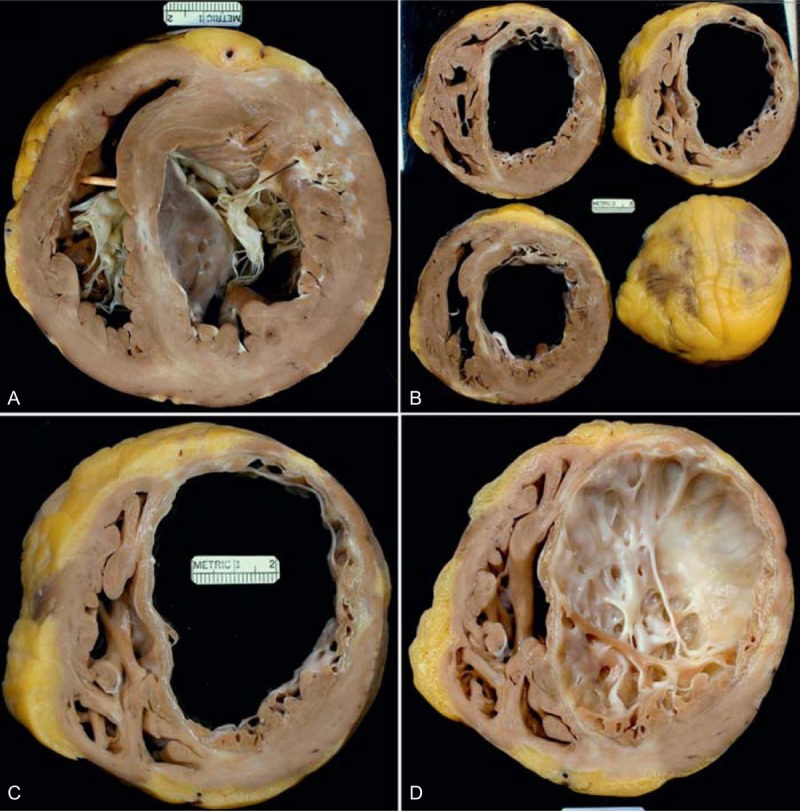
Ischemic cardiomyopathy. Heart in a 56-year-old man who had a large anterior wall acute myocardial infarct at age 46 years. Subsequently, a stent was inserted into the left anterior descending and left circumflex coronary arteries. His left ventricular ejection fraction shortly before cardiac transplantation was 10%. (a) View of the ventricular cavities at the base showing both tricuspid and mitral valves. A scar is present in the central portion of the ventricular septum and in the anterior left ventricular free wall. (b) Views of the cardiac ventricles caudal to the base showing a huge amount of scaring in the anterior wall of left ventricle with scaring of the entire ventricular septum. Less than half of the left ventricular free wall is devoid of scars. (c) A close-up view of a portion of ventricular walls showing full thickness scarring of the ventricular septum and anterior lateral left ventricular free wall. (d) View of the interior of the left ventricular apex showing the extensive septal and free wall left ventricular scaring.
FIGURE 6.
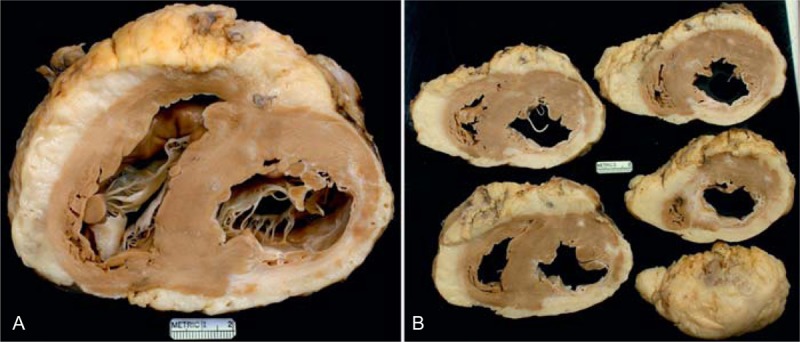
Ischemic cardiomyopathy. Heart in a 56-year-old man who at age 49 years had an acute myocardial infarction, and percutaneous coronary intervention with multiple stents was performed. Six months later he underwent coronary artery bypass grafting. Heart failure gradually progressed thereafter such that the left ventricular ejection fraction fell to 25%. (a) View of the basal portion of the heart exposing both tricuspid and mitral valves. The posterior wall of left ventricular is transmurally scarred resulting in the ventricular septum’s being thicker than the left ventricular free wall. The quantity of subepicardial adipose tissue is enormous, such that the heart floated in a container of formaldehyde. (b) Views of the ventricles more caudally showing that the posterior wall infarct extends from apex to base.
FIGURE 7.
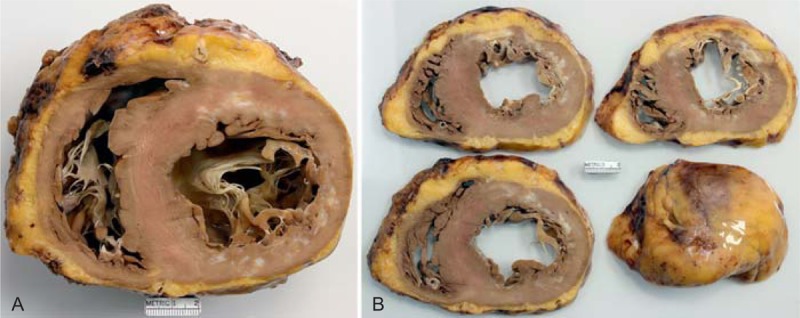
Ischemic cardiomyopathy. Views of the heart showing a transmural left ventricular scar from apex to base and a lateral and posterior walls scar which is probably not transmural in a 57-year-old man who had an acute myocardial infarct at age 48 years followed later by a coronary bypass grafting. Heart failure gradually progressed through the years, and his lowest left ventricular ejection fraction was 30%. (a) View of the base of the heart exposing both tricuspid and mitral valves. There is scarring in the anterior wall and in the posterior lateral wall. The lateral wall is much thinner than the ventricular septum. (b) Views of the ventricles more caudally showing extensive transmural anterior wall scarring. The quantity of subepicardial adipose tissue is increased.
FIGURE 8.
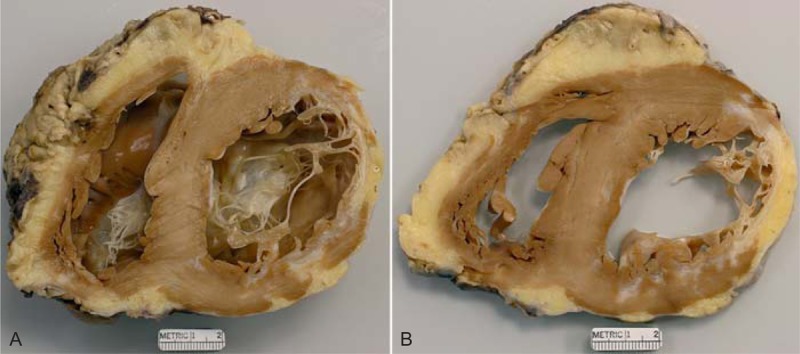
Ischemic cardiomyopathy. Heart in a 58-year-old man who had an acute myocardial infarct at age 29 years. A sibling and a parent had had myocardial infarcts at age 42 years. He clearly had heterozygous familial hypercholesterolemia. At age 30 years, he had coronary bypass grafting. His lowest left ventricular ejection fraction was 20%. (a) View of the basal portion of the heart exposing both tricuspid and mitral valves. The anterolateral wall is transmurally scarred such that the ventricular septum is thicker than the left ventricular free wall. (b) A more caudal view of the left ventricular wall showing the severe degree of scarring in the lateral wall with lesser degrees of scarring in the anterior and posterior walls. The quantity of subepicardial adipose tissue is increased.
FIGURE 9.
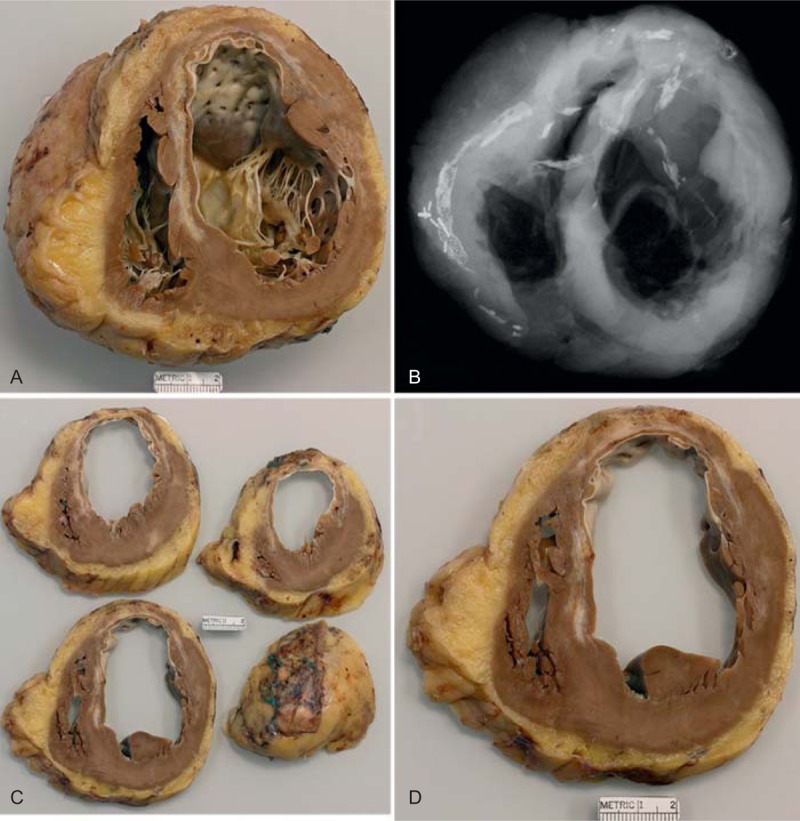
Ischemic cardiomyopathy. Heart in a 63-year-old man who had an acute myocardial infarct when he was 51 years of age. Subsequently, he underwent coronary artery bypass grafting. Percutaneous coronary intervention followed the bypass operation, and at age 58 years an implantable cardioverter defibrillator was inserted. Not long before cardiac transplantation his ejection fraction was 15%. At age 61 years, he had a stroke, and also during that year he underwent bilateral iliac stent implantation. (a) View of the basal portion of the heart exposing both tricuspid and mitral valves. Nearly the entire septum is scarred as is the anterior left ventricular free wall. The left ventricular cavity is greatly dilated. The quantity of subepicardial adipose tissue is considerably increased. (b) Radiograph of the basal portion of the heart showing a metallic stent in the right coronary artery with considerable scarring of the right and left anterior descending coronary arteries. (c) These more basal portions of the ventricular wall show that the large infarct extends to the cardiac apex. (d) View of 1 of the slices of left ventricular free wall showing marked thinning due to scar of the anterior wall.
FIGURE 10.
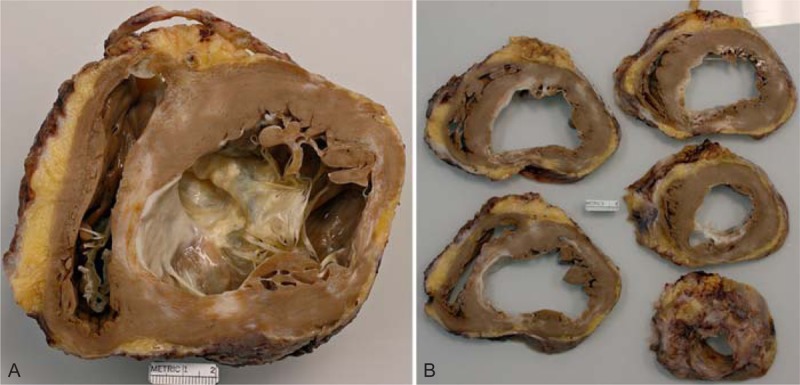
Ischemic cardiomyopathy. Heart in a 61-year-old man who had a acute myocardial infarct at age 53 years followed by a coronary artery bypass grafting. By age 59 years, his left ventricular ejection fraction was as low as 15%. An intracardiac defibrillator had been inserted also at age 54 years. (a) View of the basal portion of the heart showing a portion of tricuspid valve and a good bit of the anterior mitral leaflet. A large transmural scar is present in the lateral wall between the 2 left ventricular papillary muscles and in the ventricular septum in apposition to the lateral wall infarct. The left ventricular cavity is greatly dilated. (b) View of the more caudal portions of the ventricular wall again showing the 2 infarcts extending almost to the apex of the heart.
FIGURE 11.
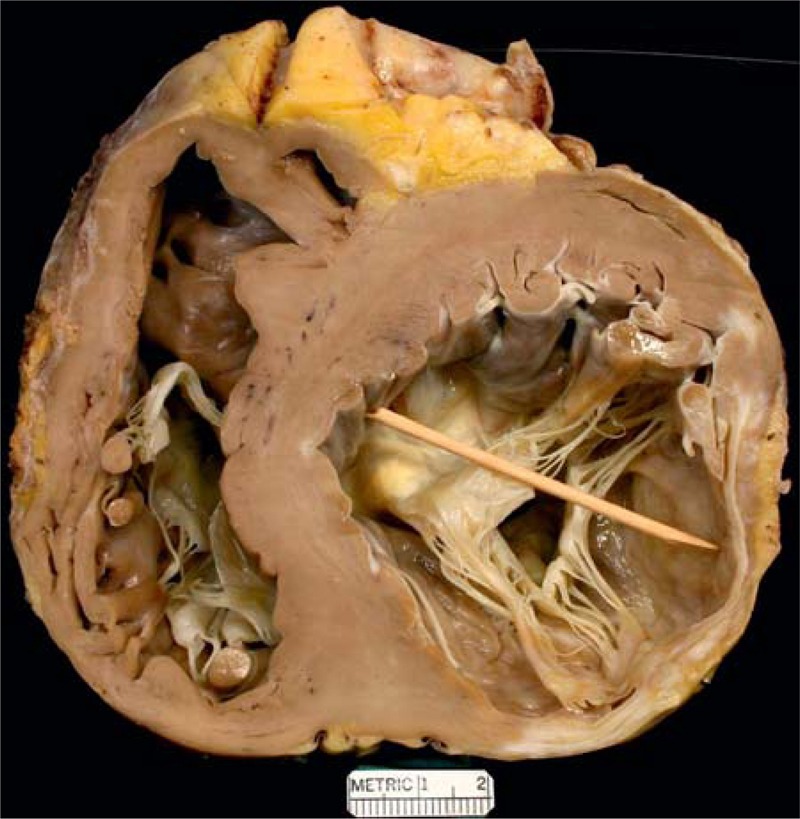
Ischemic cardiomyopathy. Heart in a 67-year-old man who had a large acute myocardial infarction at age 53 followed by coronary artery bypass grafting. At age 60, he had another bypass grafting operation. Heart failure progressed and was accompanied by several mitral regurgitation. His ejection fraction not long before cardiac transplantation was 10%. The view of the heart shows its basal portion exposing both mitral and tricuspid valves. The posterior and lateral walls of the heart are extremely thin from the extensive scarring. The posteromedial papillary muscle is flattened from the scarring and the anterolateral 1 is atrophied. The ventricular septum as a consequence is much thicker than the left ventricular free wall.
FIGURE 12.
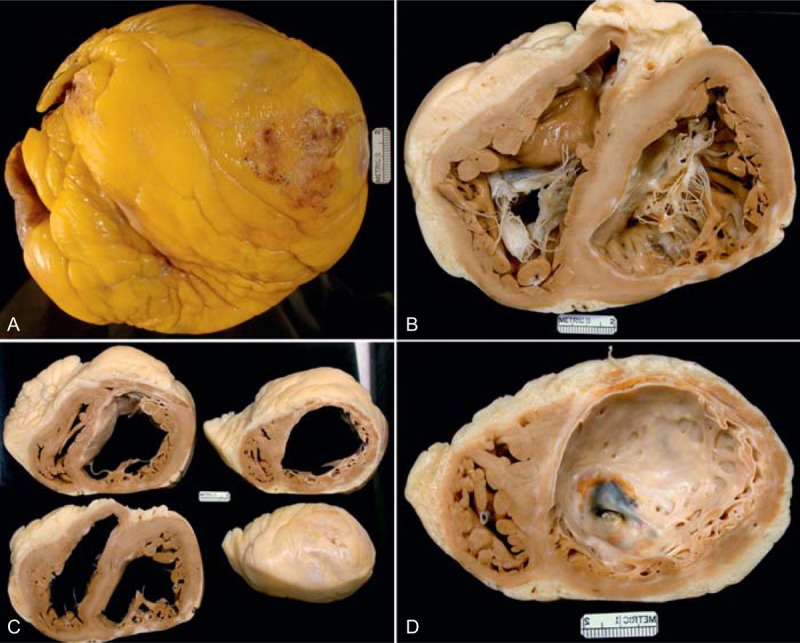
Ischemic cardiomyopathy. Heart in a 67-year-old man who had a large anterior wall infarct and another posterior wall infarct in the distant past. The exact dates of the infarcts are unclear, but radiograph of the excised heart showed calcium in the wall of the apex of the left ventricle indicating that the infarct was probably at least 10 years earlier. Heart failure had been a problem for many years. The left ventricular ejection fraction not long before cardiac transplantation was as low as 5%. (a) View of the exterior of the heart showing that most of the myocardial walls are covered by adipose tissue. (b) View of the base of the heart exposing both tricuspid and mitral valves. The posterior left ventricular free wall is thinned by scar tissue. (c) Views of the ventricles more caudally now showing both anterior and posterior wall infarcts. (d) View of the apex showing scarring in probably 300° of the 360° left ventricular wall. The heart contained so much adipose tissue that it floated in formaldehyde.
FIGURE 15.
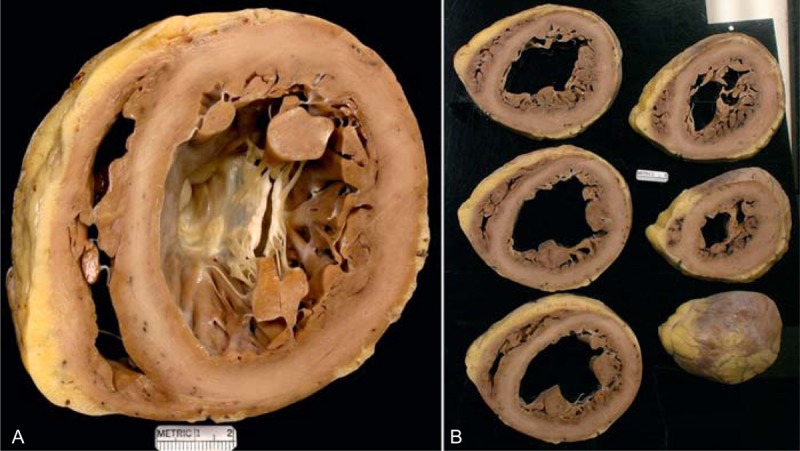
Idiopathic dilated cardiomyopathy. Views of the heart in a 61-year-old woman who had had evidence of chronic heart failure since age 51 years. She did reasonably well on medical therapy until age 59 years, when the heart failure worsened considerably and an implantable cardiac defibrillator was inserted. During the 2 years before transplant, the heart failure progressively worsened, and the left ventricular ejection fraction fell to a low of approximately 10%. She never had chest pain. Earlier in life she had had several children. (a) Basal portion of heart exposing well the mitral valve. The left ventricle is greatly enlarged measuring up to 7.5 cm. No foci of fibrosis or necrosis are seen in the ventricular walls. (b) Views of the ventricles caudal to the mitral valve, again showing considerable left ventricular cavity dilatation.
FIGURE 16.
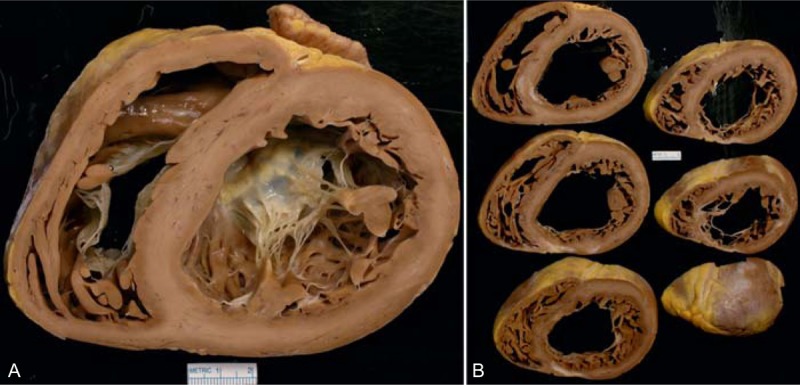
Idiopathic dilated cardiomyopathy. Heart in a 62-year-old woman who developed evidence of heart failure at age 55 years, which progressed thereafter. During the 7 months before cardiac transplant, the heart failure worsened considerably. Coronary angiography showed the major epicardial coronary arteries to be wide open. (a) Basal portion of heart exposing both tricuspid and mitral valves. Both ventricular cavities are considerably dilated. No foci of fibrosis or necrosis were noted in this portion of the ventricular walls. (b) Views of the ventricles caudal to the atrioventricular valves. In this view the posterior wall is thinned by scar tissue as is the most posterior portion of ventricular septum. The right coronary artery, as well as the others, was wide open. The focal scar may have been due to a coronary embolus many years earlier.
FIGURE 19.
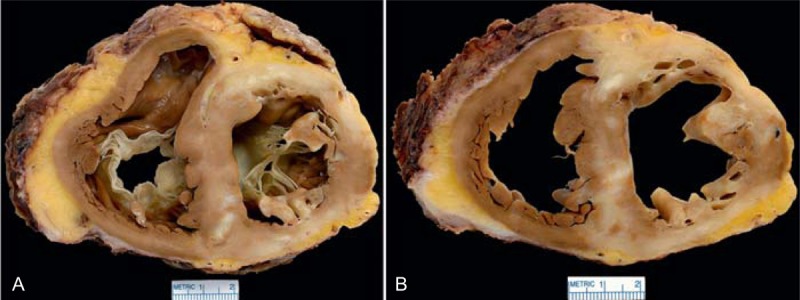
Hypertrophic cardiomyopathy. The heart in a 48-year-old man who developed heart failure at age 41 years and had an intracardiac defibrillator inserted shortly thereafter. A left ventricular assist device was inserted 2.3 years before cardiac transplantation. At age 45 years, he developed atrial fibrillation and had several cardioversion procedures performed. A parent and several cousins had heart disease of unclear type and were either asymptomatic or had died from it. (a) View of the base of the heart exposing both tricuspid and mitral valves. There is considerable scarring in the left ventricular and ventricular septal walls and in both papillary muscles. Both cavities are quite dilated. The thickness of the ventricular septum is greater than that of the left ventricular free wall. (b) Another view more caudal showing extensive scarring. The left anterior descending coronary artery is narrowed on this slice, probably the result of extensive scaring surrounding it. (Figure 19a is reprinted with permission from Elsevier.49)
FIGURE 20.
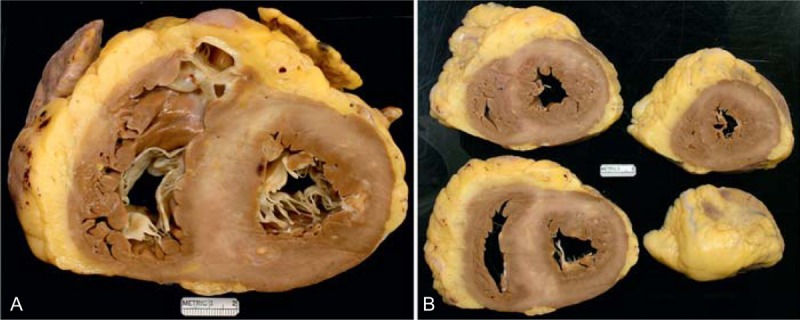
Hypertrophic cardiomyopathy. This 68-year-old man was diagnosed with hypertrophic cardiomyopathy many years earlier. His major problem was recurrent episodes of ventricular tachycardia, and he underwent an ablation procedure for that. He also had atrial fibrillation and other atrial arrhythmias. An implantable cardiac defibrillator was inserted. He also was obese, had obstructive sleep apnea, and chronic renal disease, stage 3. (a) View of the cardiac base exposing tricuspid, pulmonic, and mitral valves. The thickness of the ventricular septum is greater than that of the left ventricular free wall. The 2 ventricular cavities are only mildly dilated. Calcium was present in the mitral annular area. (b) Views of the ventricles caudal to the views shown on the left.
The orifices of the right and left main coronary arteries in the aorta were carefully examined for proper location and degrees of narrowing, if any. Random cuts were made in the 4 major (right, left main, left anterior descending, and left circumflex) coronary arteries, and the arteries were probed (1.5 mm diameter probe) both anterograde and retrograde. The 2 coronary orifices in the aorta were probed to determine any resistance to movement of the probe. Additionally, all incisions into the major coronary arteries were carefully explored for atherosclerotic plaques and luminal narrowing.
Each of the 4 cardiac valves was examined for abnormality.
At least 6 sections of each heart were submitted for preparation of histology slides. They included at least 2 sections of the left ventricular free wall, 1 of ventricular septum, 1 of right ventricular free wall, and 1 each of the walls of left and right atria. If coronary artery bypass grafts were present, sections of them were also prepared. Both hematoxylin-eosin and Masson stains were prepared on all myocardial sections and Movat stains were prepared on all sections of coronary bypass conduits and/or native coronary arteries. If heavy calcific deposits were palpated in the epicardial coronary arteries or in other areas of the heart, usually a radiograph was made of the recipient heart.
The clinical, echocardiographic, hemodynamic, and operative records were sought from the patients’ medical records and/or the BUMC Apollo cardiovascular database. Information regarding death of any patient was obtained from the medical records in deaths during hospitalization at the time of CT and from the Social Security Death Index for post-hospitalization deaths. Survival was assessed as March 31, 2013.
Means, standard deviations (SDs), and percentages were calculated to describe the study cohort. The Kaplan-Meier method was used to construct the survival curve for the whole population. The log-rank test statistic was computed to compare unadjusted survival curves between men and women.
The study was approved by the institutional review board at BUMC.
RESULTS
The main findings in the 314 patients are summarized in Tables 1 and 2. All patients at the time of CT were aged >18 years of age: 230 (73%) were men, and 84 (27%) were women. The patients included had CT only; there were no patients having heart/lung, heart/liver, or heart/bone marrow transplantation. Clinically, the patients were divided into 2 major groups: 1) ischemic cardiomyopathy (IC), 148 patients (47%); and 2) non-ischemic cardiomyopathy (N-IC), 166 patients (53%). The N-IC patients were subdivided into 11 groups, the 2 largest being IDC, 111 patients (66%), and HC, 17 patients (10%). The remaining 9 groups under N-IC consisted of 38 patients (23% of 166).
TABLE 1.
Clinical and Morphologic Diagnoses of the 2 Basic Conditions Leading to Cardiac Transplantation: Ischemic Cardiomyopathy and Non-Ischemic Cardiomyopathy, and Incongruence Found Between the Diagnoses (Number of Patients in Each Category With the Clinical and Morphologic Diagnoses)
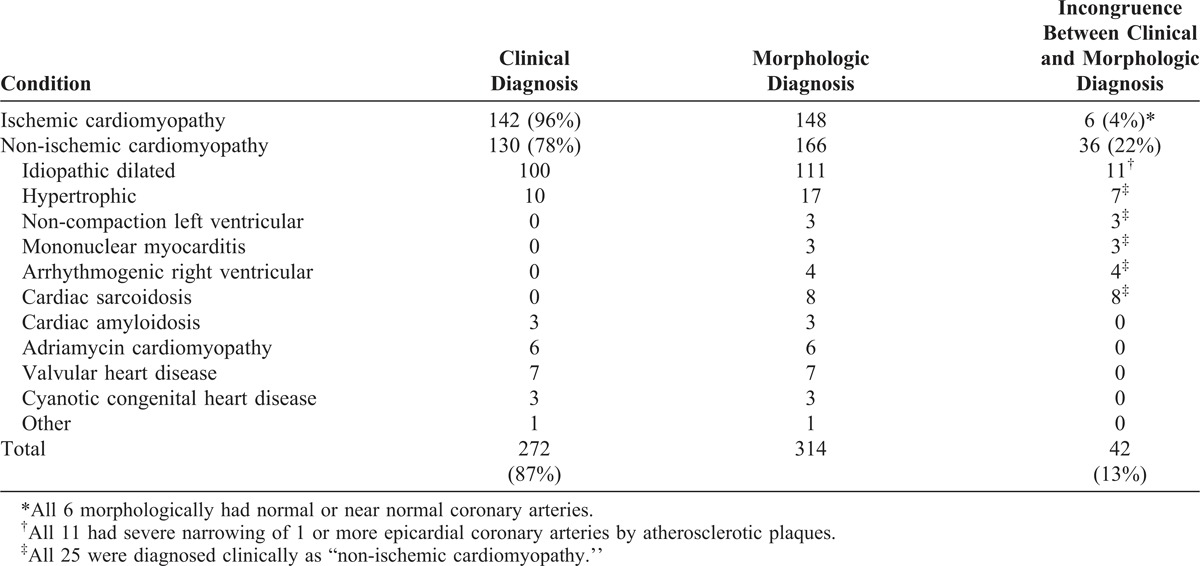
TABLE 2.
Morphologic Findings in Patients Having Cardiac Transplantation, by Cardiac Condition (n = 314)
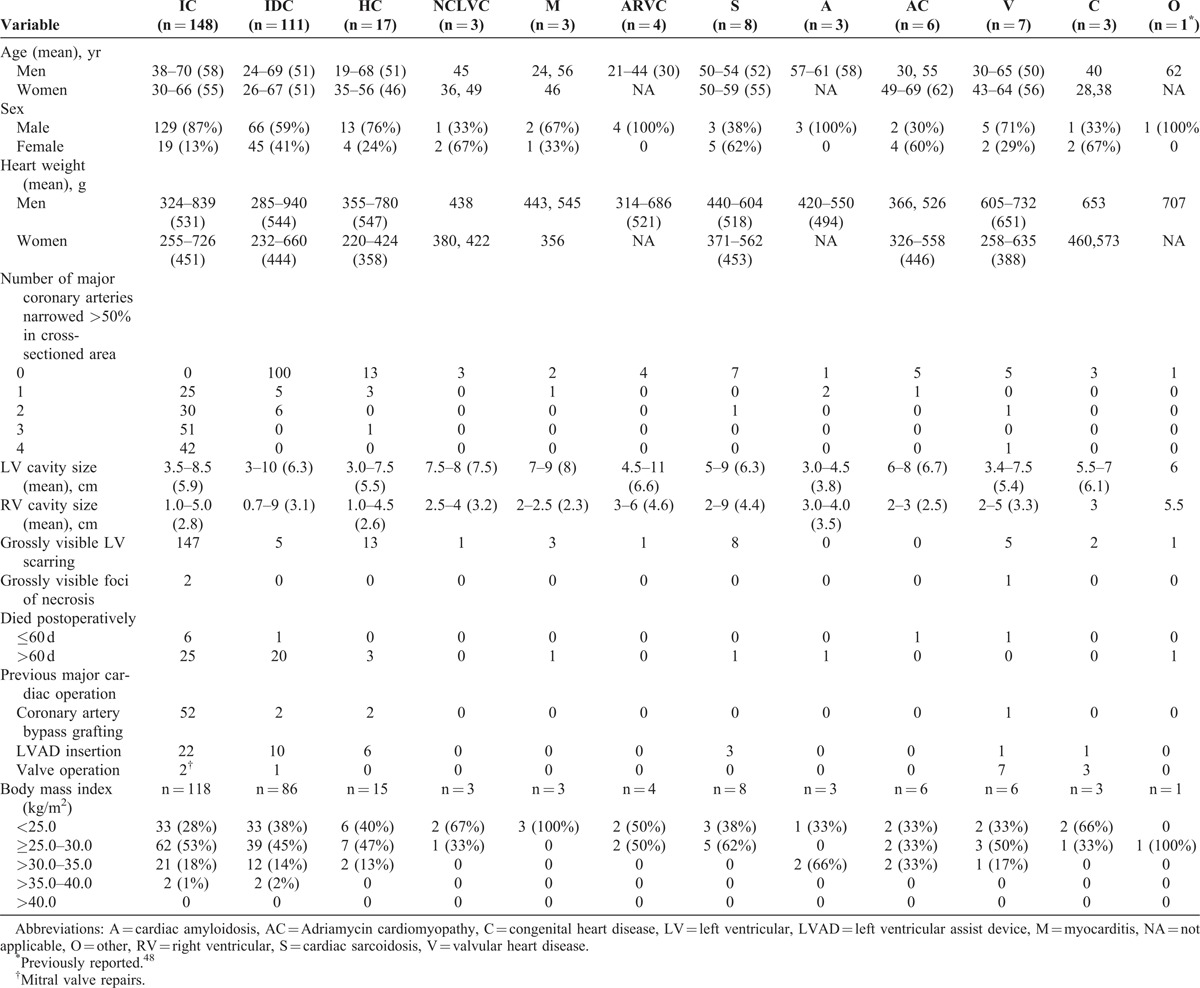
The frequency of congruity between the clinical and morphologic diagnoses is summarized in Table 1 and in Figure 2. Both the clinical and morphologic diagnoses were congruous in 272 (87%) of the 314 patients: in 142 (96%) of the 148 patients with IC and in 130 (78%) of the 166 patients with N-IC. Incongruity between the clinical and morphologic diagnoses was most glaring among the patients with HC (10 of 17 [59%]), non-compaction left ventricular cardiomyopathy (0 of 3); arrhythmogenic right ventricular cardiomyopathy (0 of 4), and cardiac sarcoidosis (0 of 8). Details of individual patients in the non-IC subgroups (excluding IDC) are tabulated in Tables 3–10.
TABLE 3.
Pertinent Data in 17 Patients Having Cardiac Transplantation for Hypertrophic Cardiomyopathy
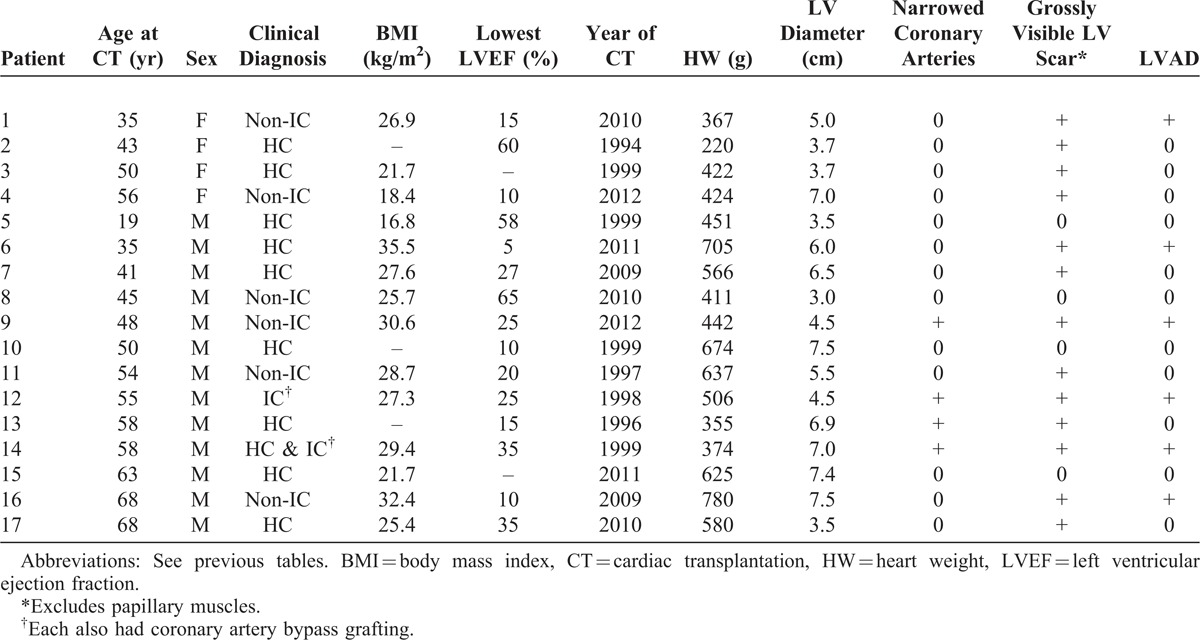
TABLE 10.
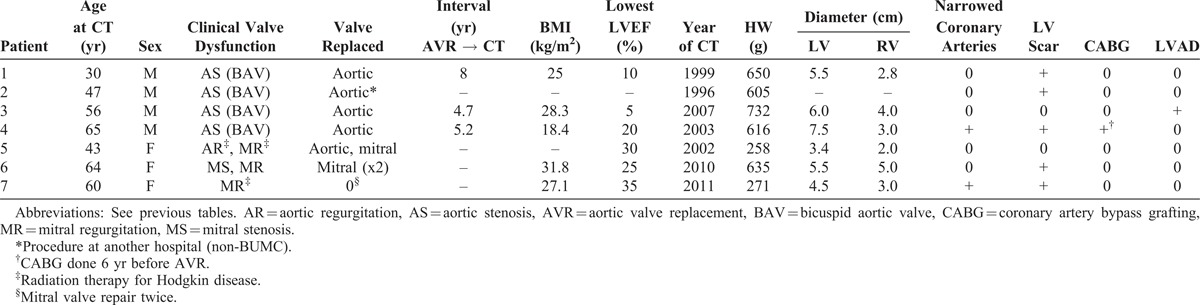
Pertinent Data in 6 Patients Having Cardiac Transplantation for Valvular Disease
Ischemic Cardiomyopathy
The 148 IC patients had hearts with dilated left ventricular cavities (>3.5 cm: range, 3.5–8.5 cm [mean 5.9]); 1 or more major epicardial coronary arteries narrowed >75% in cross-sectional area by atherosclerotic plaque; 1 or more grossly visible transmural (>50% of wall thickness) scars in the left ventricular free wall with or without similar scars in the ventricular septum and right ventricular free wall; normal or nearly normal cardiac valves; and absent inflammatory, giant, neoplastic cell or non-fibrous infiltrates (such as amyloid).56 Additionally, the intramural coronary arteries were normal. Of the total 314 patients, 148 (47%) morphologically fulfilled these criteria. Of these 148 patients, 52 (35%) had had a coronary artery bypass grafting procedure previously and 22 (15%) had a left ventricular assist device. Of these 148 patients, 142 (96%) before CT were diagnosed as IC; the remaining 6 patients were diagnosed clinically as IDC because coronary angiograms, some performed several years earlier, had indicated no significant luminal narrowing, although all 6 had transmural left ventricular scars and severe (>75% cross-sectional area) narrowing of 1 or more major coronary arteries. Examples of IC are shown in Figures 3–13.
FIGURE 3.
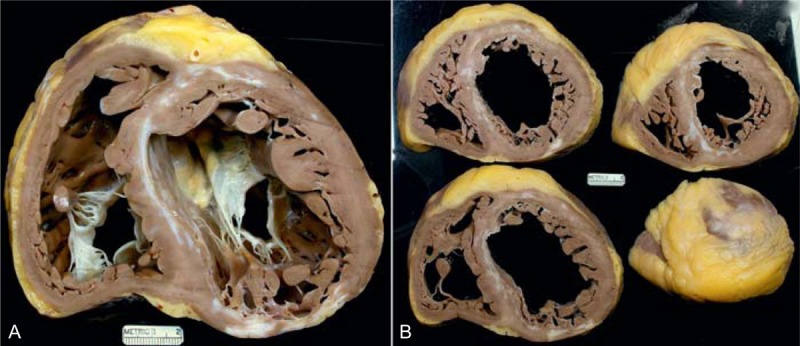
Ischemic cardiomyopathy. The heart in a 48-year-old man who had had at least 2 acute myocardial infarcts in the past. One is located posteriorly and the other anteriorly. The left ventricular ejection fraction was about 5%. (a) View of the heart showing both tricuspid and mitral valves. Both ventricles are greatly dilated. The 2 healed myocardial infarcts are clearly seen, and they are opposite one another. The left ventricular cavity measures up to 8 cm. (b) Views of the cardiac ventricles caudal to the picture shown on the left. These views show both the anterior and posterior scars are present from apex to base.
FIGURE 13.
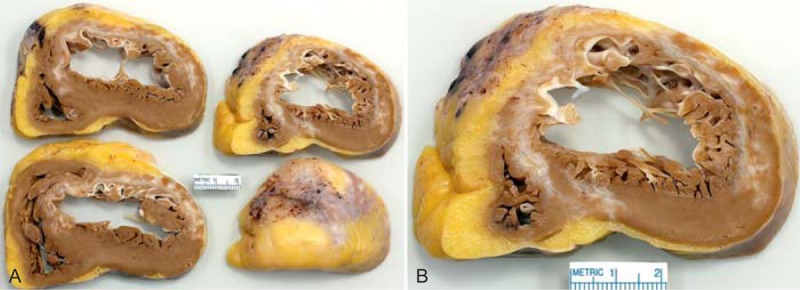
Ischemic cardiomyopathy. Heart in a 69-year-old man who had a large myocardial infarct, involving the anterior, septal, and lateral walls of the left ventricle only 4 months earlier with severe heart failure thereafter. (a) Sections of the ventricular walls caudal to the atrioventricular valves show a healed septal, anterior, and lateral wall infarct from apex to base. (b) A close-up of 1 of the slices showing the extensive scarring.
Idiopathic Dilated Cardiomyopathy
The 111 IDC patients had dilated left ventricular cavities (4.0–10.0 cm [mean 6.3]); normal or nearly normal cardiac valves; normal epicardium; no inflammatory, giant, neoplastic cells or non-fibrous interstitial infiltrates (such as amyloid) in the cardiac walls; and usually normal epicardial coronary arteries (Figures 14–17).20,51,54,71 A few patients had some atherosclerotic plaques but the plaques had narrowed the lumen ≤50% in cross-sectional area in 1 or more of the 4 major epicardial coronary arteries. Eleven patients had narrowing of 1 or more major epicardial coronary arteries >75% in cross-sectional area by plaques, but none of them had grossly visible left ventricular or ventricular septal scars. These 11 patients do not fulfill our definition of IDC because of the coronary narrowing, but none of the 11 had 1 or more grossly visible left ventricular scars. In these patients the coronary angiogram appeared to simply underestimate the degree of coronary narrowing. Their lack of myocardial scarring prevents them from being placed in the IC group. It is likely that these 11 patients actually had IDC, but because they lived in a high-cholesterol environment (United States), their coronary arteries developed significant plaque.55 Five patients with normal epicardial coronary arteries had grossly visible left ventricular free wall and/or ventricular septal scars. The 5 patients were diagnosed morphologically and clinically as IDC.23
FIGURE 14.
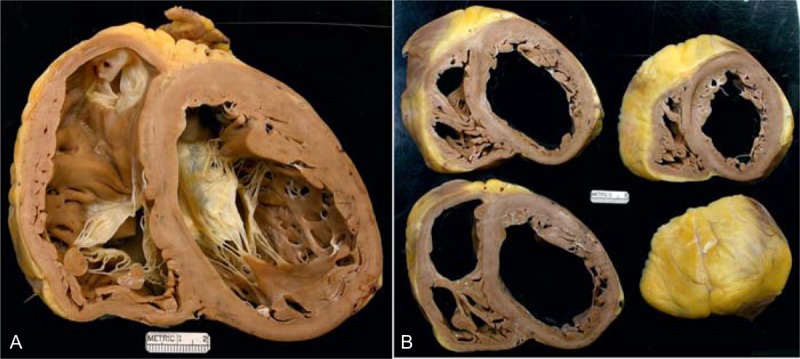
Idiopathic dilated cardiomyopathy. Heart of a 68-year-old man who had had evidence of heart failure since age 54 years. He was known to have severe hypothyroidism and was treated appropriately. He had never had chest pain. He did however have evidence of chronic obstructive lung disease and at 1 time was a habitual alcoholic. He also was known to have a fatty liver. The lowest left ventricular ejection fraction before transplantation was 5%. (a) View of the cardiac ventricles at the base exposing the tricuspid, mitral, and pulmonic valves. Both ventricular cavities are enormously dilated, and the walls of the left ventricle, ventricular septum, and right ventricle are free of foci of fibrosis and necrosis. (b) Views of the ventricles caudal to the atrioventricular valves. The moderator band is prominent in the right ventricle. The right coronary artery wall was quite calcified, and the lumen was quite narrowed. Nevertheless, the patient was classified as having idiopathic dilated cardiomyopathy because there were no lesions in the myocardial walls. It appears that this happened to be a patient who had both a narrowed coronary artery and idiopathic dilated cardiomyopathy.
FIGURE 17.
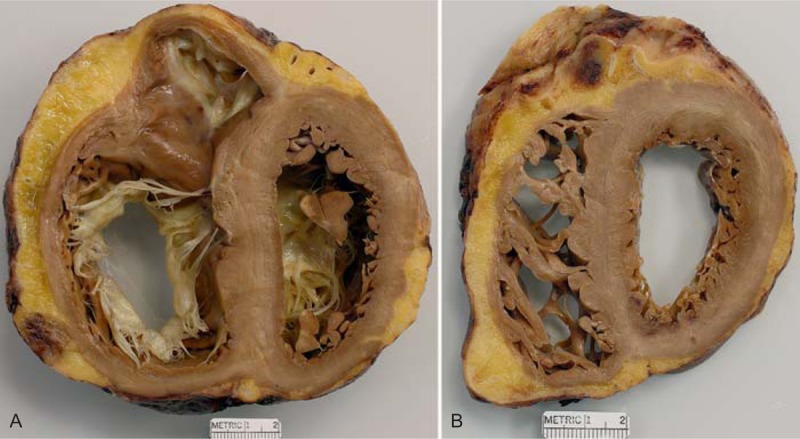
Idiopathic dilated cardiomyopathy. Heart of a 68-year-old man who was found to have heart failure at age 50 years, and, at the time, also to have severe mitral regurgitation. Coronary angiogram showed the coronary arteries to be wide open. At age 50 years, the patient underwent repair of the mitral valve including the insertion of an annular ring. The mitral regurgitation was reduced from severe to mild, but the heart failure continued and progressed. A left ventricular assist device was inserted before the cardiac transplantation. (a) View of the basal portion of the heart showing dilatation of both right and left ventricular cavities. The tricuspid annulus is quite dilated. No lesions are noted in the ventricular walls although the ventricular septum is thicker than the left ventricular free wall. (b) View more caudal showing impressive pectinate muscles within the right ventricular cavity. Again, no myocardial lesions are noted. The quantity of subepicardial adipose tissue is considerably increased.
Hypertrophic Cardiomyopathy
The 17 HC patients (Table 3) had either normal sized (<4 cm) (5 patients) or dilated (>4 cm) (12 patients) left ventricular cavities and past clinical evidence of non-dilated left ventricular cavities (Figures 18–21).5,13,21,24,33,36,37,42,49,61 Transmural scarring involved the ventricular septum and left ventricular free walls in 13 patients (76%) with or without grossly visible scarring of portions of the right ventricular free wall. The ventricular septum at sometime in the past or presently was thicker than the left ventricular free wall; typical myofiber disorganization was present in the ventricular septum, and the intramural coronary arteries on histologic study were abnormal in all 13 patients with ventricular wall scarring.35 None of the 17 HC patients had clinical or hemodynamic evidence of left ventricular outflow obstruction just prior to CT. Four (24%) of the 17 patients also had severe narrowing of 1 or more epicardial coronary arteries, and all 4 had grossly visible left ventricular and ventricular septal scars. Nine other HC patients had left ventricular or ventricular septal scar without narrowing of the epicardial coronary arteries. The clinical diagnosis in 6 of the 17 patients was “non-IC," in 1 patient IC, and in 1 patient both HC and IC. Each of the latter 2 patients had coronary bypass grafting in the remote past before CT. Thus, the clinical and morphologic diagnosis were incongruous in 7 (41%) of these 17 patients.
FIGURE 18.
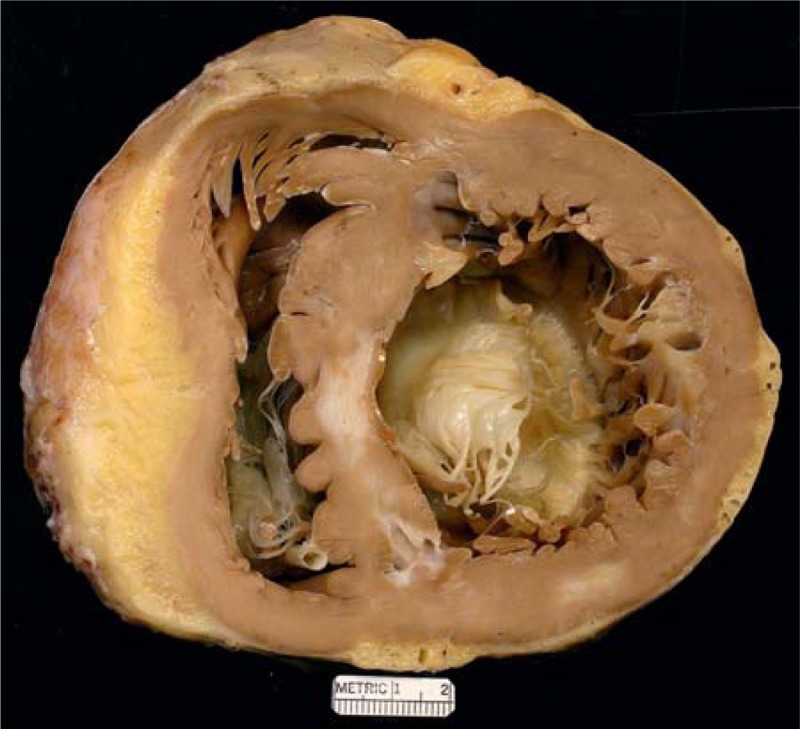
Hypertrophic cardiomyopathy. Heart in a 41-year-old man in whom hypertrophic cardiomyopathy was diagnosed when he was 6 years of age. At age 26 years, atrial fibrillation appeared and during the next 15 years he was cardioverted 98 times. He eventually developed complete heart block, and a pacemaker was inserted. At age 30 years, an intracardiac defibrillator was implanted, and at the same time an alcohol septal ablation procedure was performed. Nevertheless, evidence of heart failure persisted and a heart transplant was performed. View of the basal portion exposing both tricuspid and mitral valves. The left ventricular cavity is quite dilated. The focal scar in the ventricular septum may be the result of the alcohol septal ablation. Another small scar is present in the posterior wall of left ventricle. The thickness of the ventricular septum is greater than that of the left ventricular free wall.
FIGURE 21.
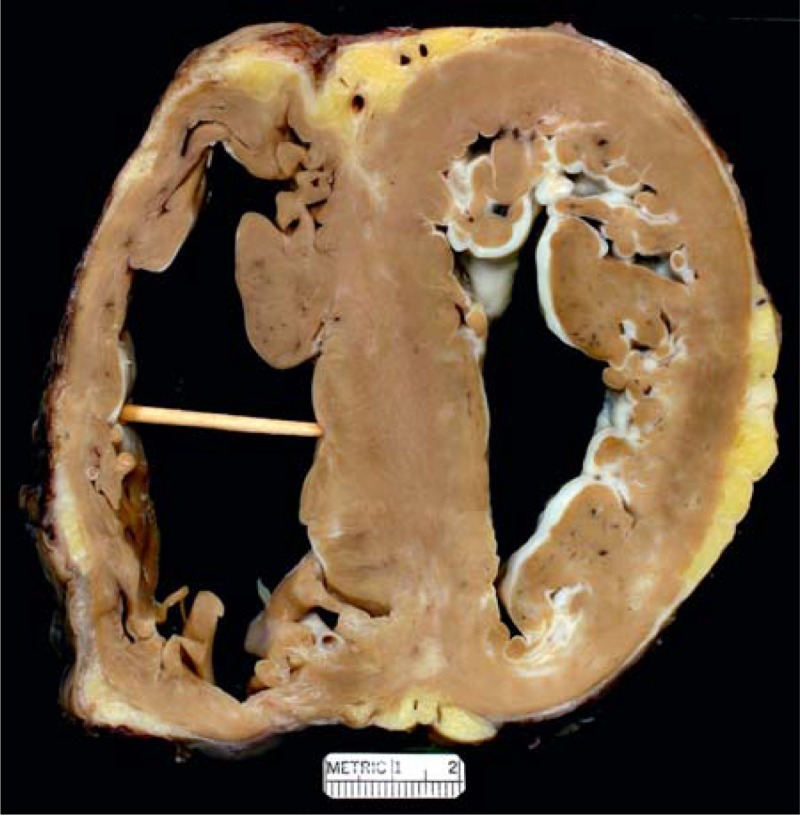
Hypertrophic cardiomyopathy. View of the heart in a 68-year-old man who was known to have some form of heart disease since age 38 years. The problem during the 30 years was various degrees of heart failure which began progressing about age 55 years. In the 2 years before cardiac transplantation he had multiple hospitalizations for decompensated heart failure. He had an intracardiac defibrillator in place for several years. Because of the severity of the heart failure, a left ventricular assist device was inserted 7 months before cardiac transplantation. The heart failure severity, however, continued, and before heart transplantation the left ventricular ejection fraction was approximately 10%. Both cardiac ventricles are dilated, the right more than the left. The ventricular septum is thicker than the left ventricular free wall. The mural endocardium of the left ventricle is thickened by white fibrous tissue. A scar is present in the posterior portion of the left ventricular free wall and also in the posterior portion of the ventricular septum.
Non-Compaction Left Ventricular Cardiomyopathy
Three patients had NCLVC (Table 4, Figures 22 and 23). Each had very dilated ventricular cavities with the non-compacted portion of left ventricular free wall in 1 or more areas being >2 times the thickness of the underlying compacted portion of the wall and unassociated with narrowing of the epicardial coronary arteries.11,45,68 Clinically, each of the 3 patients were diagnosed as “non-IC.” One of the 3 patients had a small grossly visible left ventricular scar.
TABLE 4.
Pertinent Data in 3 Patients Having Cardiac Transplantation for Non-Compaction Left Ventricular Cardiomyopathy∗
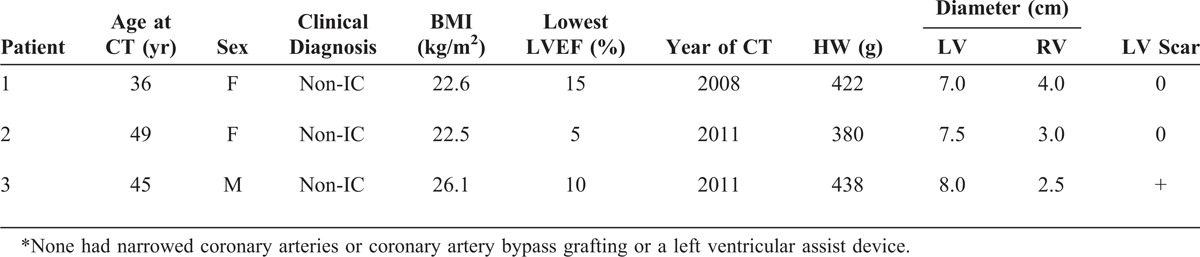
FIGURE 22.
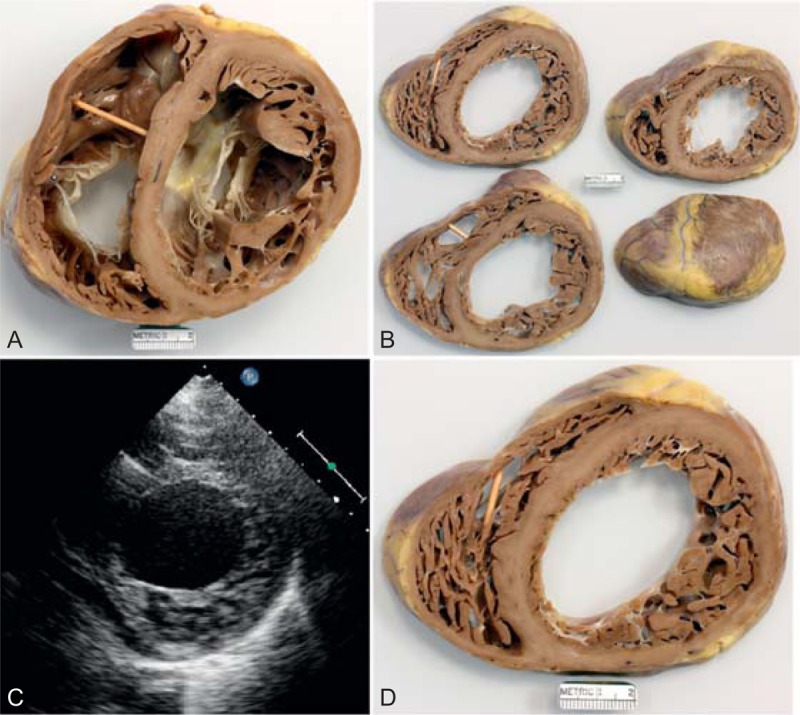
Left ventricular non-compaction cardiomyopathy. Heart in a 49-year-old man who had been well until age 33 years when symptoms of heart failure appeared. At the time, echocardiogram showed both ventricles to be dilated and the left ventricular systolic function to be severely depressed. A defibrillator was inserted at age 39 years, and cardiac resynchronization therapy at age 43 years. He underwent ablation therapy for multiple episodes of ventricular tachycardia. His left ventricular ejection fraction just before cardiac transplantation was 15%. (a) View of both ventricles just caudal to the tricuspid and mitral valves. The thickness of the ventricular septum is much greater than that of the left ventricular free wall. The compacted portion of left ventricular free wall is much thinner than the non-compacted portion. Both ventricular cavities are greatly dilated. The left ventricular free wall posteriorly is focally scarred. (a) View of the basal portion of the heart exposing the tricuspid and mitral valves. Both ventricles are greatly dilated. The posterolateral wall is focally scarred. The compacted portion of left ventricular free wall is much thinner than the trabeculated portion. (b) Views of sections of the ventricular walls caudal to the view shown in the upper left. (c) Echocardiogram showing hypertrabeculation of the left ventricular free wall. (d) Section of the left ventricular free wall corresponding to the echocardiogram. (Reprinted with permission from Elsevier.45).
FIGURE 23.
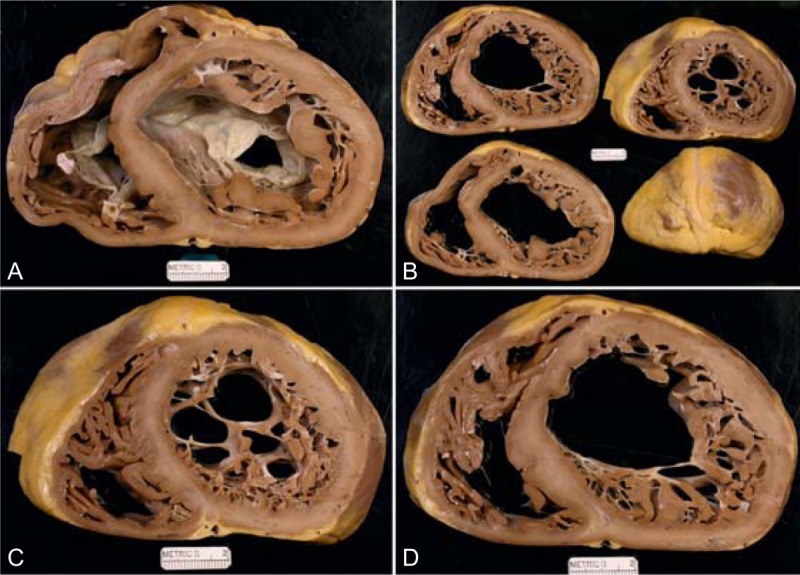
Left ventricular non-compaction cardiomyopathy. Heart of a 36-year-old woman who was well until approximately age 35 years when she developed evidence of heart failure, which slowly but gradually progressed thereafter such that when she was aged 34 years her left ventricular ejection fraction had fallen to 20% and she was in chronic atrial fibrillation. She also developed runs of ventricular tachycardia for which a defibrillator was inserted. (a) View of the heart showing both tricuspid and mitral valves. Both ventricular cavities are enormously dilated, and the ventricular walls are free of foci of fibrosis and necrosis. (b) Views of the ventricles caudal to the view shown in upper left. (c) Close-up view of 1 of the slices in upper right. (d) View showing the hypertrabeculated or non-compacted portion of the left ventricular wall to be much thicker than the compacted portion. (Reprinted with permission from Elsevier.45).
Mononuclear Myocarditis
Three patients had active or healed mononuclear myocarditis (Table 5, Figures 24 and 25). The key identifying mark of this condition was focal subepicardial left ventricular wall lesions.19,25,58,60,62,65 Each had mononuclear-cell infiltrates–-mainly lymphocytes–-in the left ventricular free wall or subepicardial scars with or without mononuclear-cell infiltrates and without granulomas. The hearts were of increased weight (>350 g in women; >400 g in men), and the ventricular cavities were very dilated. One of the 3 patients had significant narrowing by atherosclerotic plaque of 1 or more epicardial coronary arteries. This patient clinically was diagnosed as IC, the other 2 as “non-IC.”
TABLE 5.
Pertinent Data in 3 Patients Having Cardiac Transplantation for Active or Healed Mononuclear Myocarditis∗
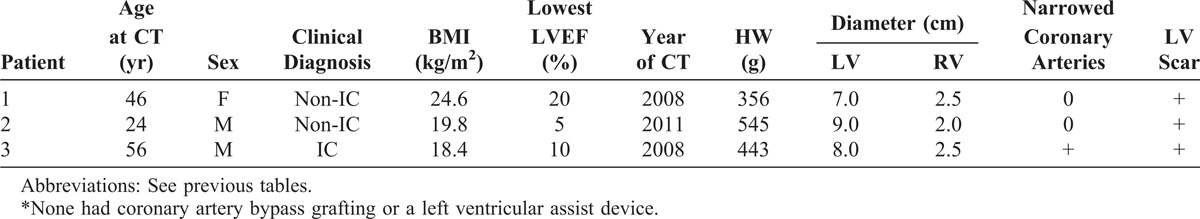
FIGURE 24.
Mononuclear myocarditis. The heart in a 56-year-old man who had been well until age 48 years, when he had a cardiac event that resulted in the appearance of heart failure, which progressed thereafter. He also smoked heavily and had evidence of chronic obstructive lung disease. A defibrillator was inserted when he was 53 years old. (a) Basal portion of the heart showing severe dilatation of the left ventricular cavity with transmural scarring in the posterior and anterior left ventricular free wall and in the anterior portion of the ventricular septum. (b) Sections of the ventricular cavities caudal to the view shown on the left. The ventricular septum in 2 of the slices is completely scarred. Histologic section showed focal collections of mononuclear cells in the walls of left ventricle and ventricular septum. The coronary arteries at necropsy were wide open.
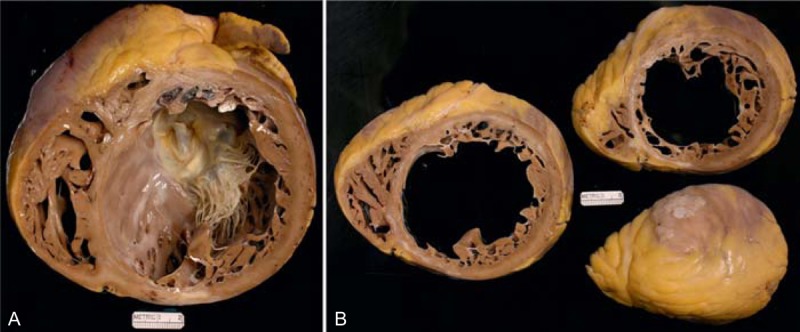
FIGURE 25.
Monocytic myocarditis. Photographs and photomicrographs of the heart in a 24-year-old man who had been well until approximately 2.5 years earlier when evidence of heart failure appeared and not long thereafter his left ventricular ejection fraction was approximately 15%. A defibrillator was implanted. Progressive worsening of the heart failure prompted the CT. The echocardiogram 15 months before cardiac transplantation showed his left ventricular ejection fraction to be as low as 5%. (a) View of the cardiac base exposing the mitral valve. (b) Slice of the ventricles more caudal. The scarring and inflammatory cell infiltrates are mainly subepicardial in location. (c) Inflammatory cell collection in the subepicardial adipose tissue (Movat stain ×20). (d) Close-up of the lymphocytes (hematoxylin/eosin stain, ×100).
Arrhythmogenic Right Ventricular Cardiomyopathy
Four patients had ARVC; pertinent findings for them are summarized in Table 6 and illustrated in Figure 26.2,4,8,12,14,28,31,32,46,64,66,70,72,73 All 4 during life were diagnosed as “non-IC.” All 4 had thinning of the right ventricular free wall with replacement of the myocardial wall focally by adipose tissue and/or scar tissue without focal thinning (except in 1 patient) of the left ventricular free wall. All 4 had very dilated ventricular chambers. Before CT all 4 had documented runs of ventricular tachycardia. Three had other family members with a similar cardiac disease.
TABLE 6.
Pertinent Data in 4 Men Having Cardiac Transplantation for Arrhythmogenic Right Ventricular Cardiomyopathy∗
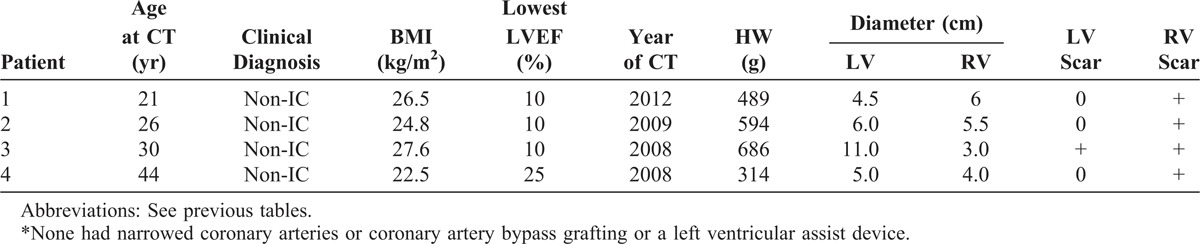
FIGURE 26.
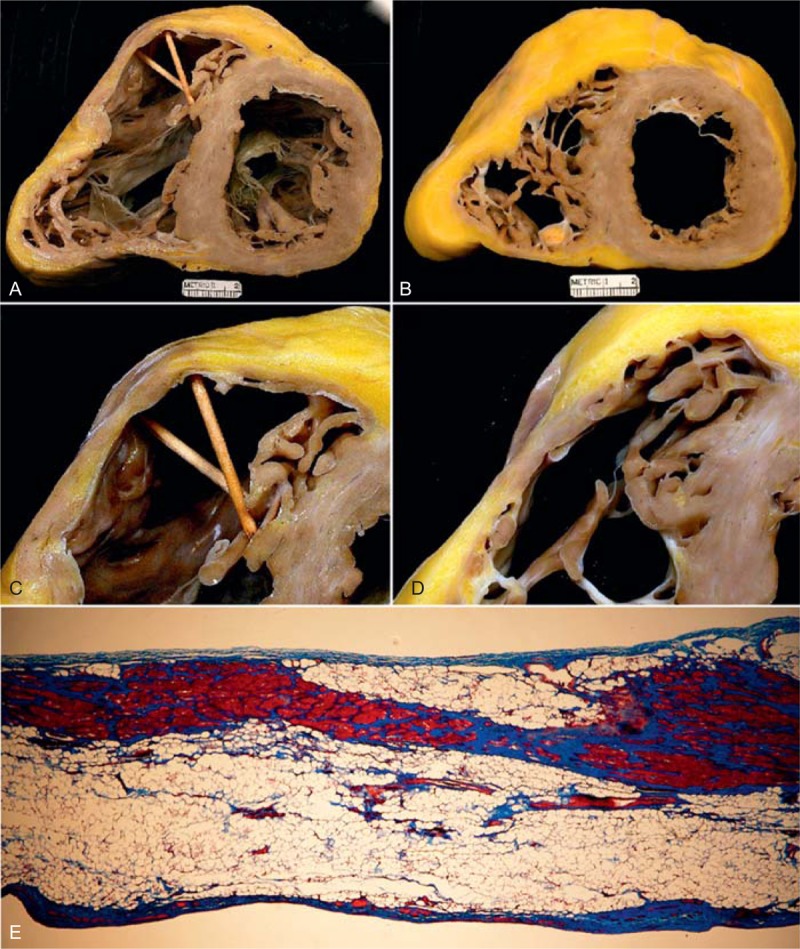
Arrhythmogenic right ventricular cardiomyopathy. The heart in a 26-year-old man who developed symptoms of heart failure at age 12 years, followed later by ventricular arrhythmias and bundle branch block. His left ventricular ejection fraction fell to 15%. (a) View of the base of the heart showing marked dilatation of both ventricles. The right ventricular wall in its outflow tract is thin (b, c, d). (e) Photomicrograph of the right ventricular wall in the right ventricular outflow tract, which consists of adipose tissue, fibrous tissue, and a few myocardial cells (Masson stain, ×20). (Reprinted with permission from Elsevier.46).
Cardiac Sarcoidosis
Pertinent findings in the 8 cardiac sarcoidosis patients are summarized in Table 7 and illustrated in Figure 27.9,15,43,47,52,62,67 All 8 were diagnosed clinically as “non-IC.” Six had focal but extensive scars in the free walls of both right and left ventricles and in the ventricular septum without associated narrowing of the epicardial coronary arteries (except for 1 patient), and the scarring tended to be subepicardial. The ventricular chambers were very dilated in all 8 patients. Non-caseating giant cell granulomas were present in the ventricular walls in 7 of the 8 patients. In 1 patient the typical “hard” granulomas were present in the portion of left ventricular free wall excised to insert a left ventricular assist device but not in the recipient heart sometime later. Another patient had right and left ventricular free wall and ventricular septal scarring typical of cardiac sarcoidosis but no granulomas were found in the histologic sections of left ventricular free wall. This case we believe is an example of “burnt out” sarcoidosis.43
TABLE 7.
Pertinent Data in 8 Patients Having Cardiac Transplantation for Cardiac Sarcoidosis∗
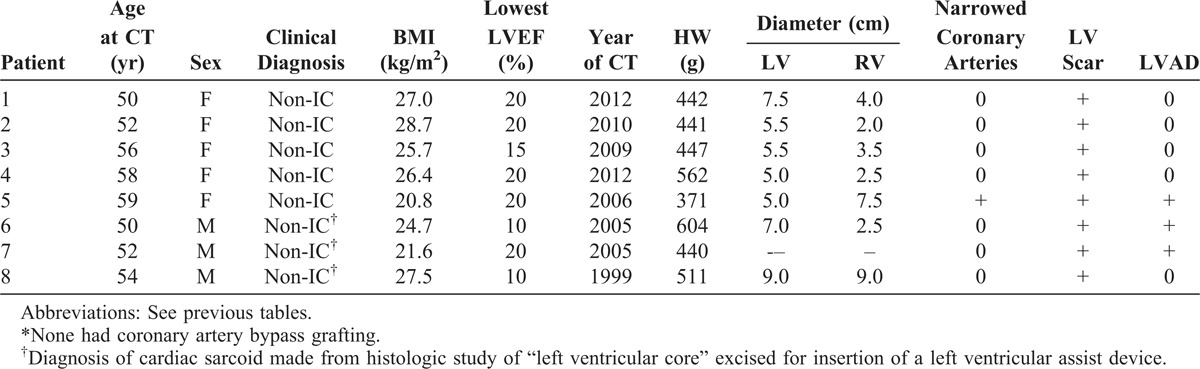
FIGURE 27.
Cardiac sarcoidosis. Heart in a 52-year-old black woman who had developed evidence of heart failure beginning at age 48 years. It progressed, and an implantable cardiac defibrillator/pacemaker was placed. Runs of ventricular tachycardia occasionally were seen. Cardiac catheterization 8 months before cardiac transplantation disclosed normal coronary arteries, severe global left ventricular hypokinesis, and an estimated ejection fraction of 10%. The pressures in mm Hg were as follows: left ventricle, 115/34; right ventricle, 59/15; pulmonary artery wedge a wave 20, v wave 20, mean 18; right atrium a wave 12, v wave 10, mean 9. The cardiac index was 1.7 L/min per m2. A diagnosis of cardiac sarcoidosis was never entertained clinically. (a) Heart at the base showing the greatly dilated ventricular cavities and scarring in the ventricular septum and left ventricular free wall anteriorly. (b) Cross-section of the ventricles showing the extent of the scarring. (c) Close-up of the left ventricular free wall and ventricular septum showing the scarring. (Reprinted with permission from Elsevier.43).
Cardiac Amyloidosis
Pertinent findings in the 3 cardiac amyloidosis patients are summarized in Table 8, and the hearts are illustrated in Figure 28.7,10,16–18,40,53,69 The clinical and morphologic diagnoses were congruous in all 3 patients. The ventricular cavities were either not dilated or dilated to a small degree. The patients had firm—actually rubbery—myocardium, no grossly visible focal myocardial lesions but focal endocardial lesions in either the right atrium or left atrium or both and in the tricuspid valve leaflets. The presence of amyloid was confirmed by Congo-red stains of sections of the heart. Two of the 3 patients had narrowing >75% in cross-sectional area by atherosclerotic plaque, but none had grossly visible ventricular wall scars.
TABLE 8.
Pertinent Data in 3 Men Having Cardiac Transplantation for Cardiac Amyloidosis∗
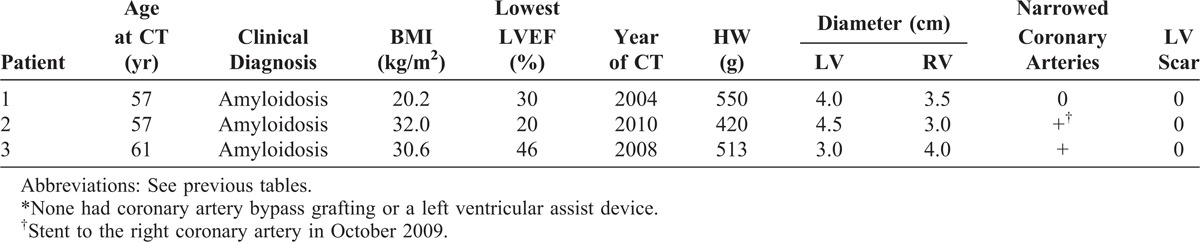
FIGURE 28.
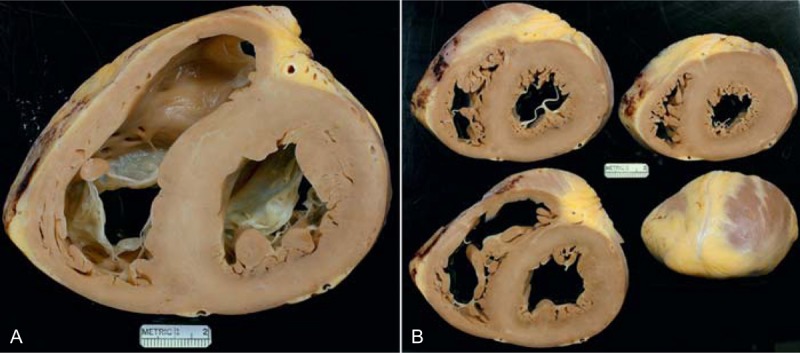
Cardiac amyloidosis. Heart in a 57-year-old man who had been well until 1 year before cardiac transplantation, when evidence of heart failure appeared. On examination 2 months later he was noted to have periorbital ecchymoses and a biopsy of an abdominal fat pad disclosed amyloidosis. During the year before CT, he lost 40 pounds, and the dyspnea and exercise intolerance progressively worsened. Echocardiogram 5 months before CT disclosed an ejection fraction of 55%, a restrictive diastolic filling pattern, and an “ineffective atrial kick.” The heart weighed 420 g and amyloid deposits were extensively present in the walls of all 4 cardiac chambers. The right ventricular cavity was larger than the left ventricular cavity. The epicardial coronary arteries were wide open. Amyloid deposits were extensive in the walls of all 4 cardiac chambers. (a) View of the cardiac base. (b) Views of the more caudal portions of the heart.
Adriamycin Cardiomyopathy
Pertinent findings in the 6 patients with Adriamycin cardiomyopathy are summarized in Table 9 and illustrated in Figure 29.22,26,27,41,44 The clinical and morphologic diagnoses were congruous in each. All 6 had severely dilated ventricular chambers. All had received Adriamycin for cancer treatment.
TABLE 9.
Pertinent Data in 6 Patients Having Cardiac Transplantation for Adriamycin Cardiomyopathy∗
FIGURE 29.
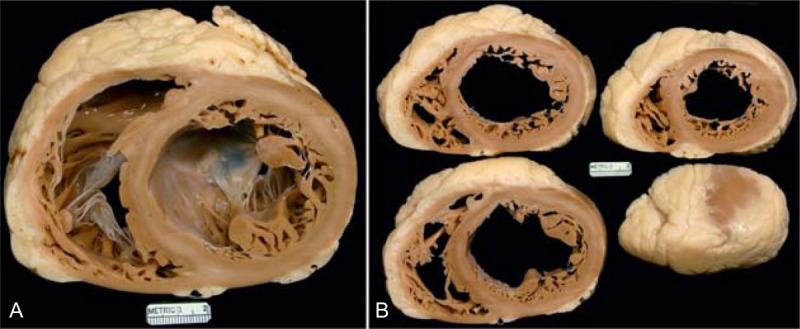
Adriamycin-induced cardiomyopathy. Heart in a 50-year-old woman who had received Adriamycin at age 38 years (12 years earlier) for cancer of the right breast with total mastectomy. She had evidence of heart failure only a couple of months before CT with rapid progression thereafter such that 1 month before CT her left ventricular ejection fraction was only 10%. The pressures in mm Hg were left ventricle 85/23; aorta 85/50. The cardiac output was 1.2 L/min, the ventricular cavities were severely dilated, and the epicardial coronary arteries were wide open. The heart weighed 405 g and the ventricles were dilated. Histologically, no specific cardiac lesions are present. (a) View of the cardiac base. (b) View of the more caudal cardiac slices.
Valvular Heart Disease
Pertinent findings in the 7 patients with valvular heart disease are summarized in Table 10.39,50,57 Four had aortic valve stenosis initially and repeated valve replacements; 1 had mitral valve replacements for mitral stenosis in 1 and for pure mitral regurgitation in the other. The latter patient also had severe tricuspid valve regurgitation, which had not been approached at a previous operation. The clinical and morphologic diagnoses were congruous in all 7 patients.
Cyanotic Congenital Heart Disease
Pertinent findings in the 3 patients with cyanotic congenital heart disease are summarized in Table 11.38,63 One had complex complete transposition of the great arteries, 1 severe right ventricular hypoplasia, and 1 tetralogy of Fallot. All 3 had had more than 1 previous cardiac operation. The clinical and morphologic diagnosis were congruous in all 3.
TABLE 11.
Pertinent Data in 3 Patients Having Cardiac Transplantation for Congenital Heart Disease∗

Other
One patient had CT for ventricular septal defect resulting from a stab wound to the heart many years earlier. Details of this patient are described elsewhere.48
Outcomes
The survival probabilities for all 257 patients are shown in Figure 30. Of the 257 (of 314) patients in whom survival data are available, 66 (26%) have died: 8 (12%) within 60 days of CT and 58 (88%) at later periods. The log-rank test show no significant survival differences between men and women (log-rank = 0.479, p value = 0.4926) (Figure 30).
DISCUSSION
The present study serves 2 major functions: 1) it illustrates by gross photographs of the hearts the variability that occurs morphologically in each of the etiologic categories, and 2) it determines the frequency among the 314 patients of discrepancies between the clinical and morphologic diagnoses. Just like clinical presentations and courses are quite variable among patients with various types of cardiac diseases, the morphologic features in each type of cardiac disease are also quite variable. To properly illustrate that variability a number of photographs of recipient hearts are presented.
As part of the second purpose of this report, we determined the frequency of congruity or incongruity between the clinical and morphologic diagnoses among the 314 patients. As shown in Table 1, most (96%) of the patients with IC at morphologic study had been diagnosed with that condition clinically. The discrepancy occurred when 6 patients with IC anatomically had insignificant coronary narrowing by angiography before CT, but nevertheless severe narrowing of 1 or more major epicardial coronary arteries morphologically and extensive left ventricular wall scarring. We defined IC as the presence of severe (>75% in cross-sectional area) coronary narrowing accompanied by 1 or more transmural left ventricular wall scars. The reverse occurred in the case of IDC. Of the 111 patients with IDC anatomically (no left ventricular wall lesion grossly), 11 had severe narrowing of 1 or more major epicardial coronary arteries such that these patients in actuality did not fulfill our definitions for either IC or IDC. We suspect, however, that these 11 patients actually had IDC but resided in a high-cholesterol environment (United States), where coronary atherosclerosis is so prevalent.
Most of the incongruity between clinical and morphologic diagnoses occurred in the non-IC subgroups other than IDC. Thus, among the 56 patients with non-IC, excluding the patients with IDC, only 31 (55%) had congruity between the clinical and morphologic diagnoses. Specifically, among the 17 patients with HC, the 3 with NCLVC, the 3 with mononuclear myocarditis, the 4 with ARVC, and the 8 with cardiac sarcoidosis, a total of 35 patients, only 11 (31%) had the specific diagnosis made clinically. One could argue that including the single case without cardiac granulomas in the sarcoid group is improper. The type and distribution of the myocardial scarring, however, is absolutely typical of the other cases of cardiac sarcoidosis, a type of scarring not seen by us in any other condition.43 Additionally, documented sarcoid granulomas in the lungs have been shown to disappear as the scarring increases.59 Obviously, it is not sufficient to stop clinically with the diagnosis of non-IC cardiomyopathy. Ideally, of course, the specific subgroup needs to be sought even though therapy and outcome might not be altered by that knowledge.
The present study is not the first to compare the frequencies of discrepancies between the clinical and morphologic diagnoses in patients having CT. Bortman and colleagues6 in 1994 examined the congruity-incongruity issue in 112 patients who underwent their first orthotopic CT at the authors’ institution (University of Texas Southwestern Medical Center at Dallas). Among their 64 patients diagnosed with IC clinically, all had severe coronary narrowing morphologically; of their 48 patients with IDC clinically, 12 (25%) morphologically had severe coronary disease, and 3 had acute myocarditis. Thus, of their 112 patients a discrepancy between pre- and post-transplant diagnosis occurred in 15 (13%). Neither gross photographs nor definitions of the entities were provided.
In 1999, Angelini and colleagues1 from Padua, Italy, studied the agreement or lack thereof between pre- and post-CT diagnoses in 257 patients having CT, and found a discrepancy between clinical and pathologic diagnoses in 20 patients (8%). Among their 87 patients with a clinical diagnoses of IC, 3 had IDC and 1 granulomatous myocarditis; among their 126 patients whose clinical diagnosis was non-IC, 7 morphologically had IC, 5 myocarditis, 1 ARVC, and 1 with NCLVC; among their 10 patients with a clinical diagnosis of HC, 1 had ARVC, and 1 had a cardiac fibroma.1 No gross photographs of the heart or definitions of the conditions were provided.
In 2009, Luk and colleagues29 from Toronto, Canada, studied the problem in 296 patients who had had CT from 1987 to 2006 (Table 12). The frequency of the diagnosis made clinically and after examination of the recipient heart is shown in Table 12. It is apparent that the incongruity between the clinical and morphologic diagnoses was relatively frequent. Three gross photographs of the heart were presented. Definitions of the various conditions were not provided.
TABLE 12.
Previously Reported Data on Congruity vs Incongruity Among 296 Patients Having Cardiac Transplantation (From Luk et al29
It is apparent from the present and previous studies that the largest incongruity between clinical and morphologic diagnosis in patients having CT is among the non-IC subtypes, particularly the dilated (end-stage) form of HC, mononuclear myocarditis, cardiac sarcoidosis, ARVC, and NCLVC. Would the use of additional diagnostic “instruments of precision” have allowed more precise pre-CT diagnosis among the non-IC group? Possibly so, particularly more use of magnetic resonance imaging among the patients with HC and sarcoidosis.35 Would a more accurate pre-CT diagnosis among the non-IC group have led to a re-evaluation of the need for CT in the first place? Or, if clinicians had actually known the specific non-IC diagnosis, would treatment have been different and might CT have been avoided? Among the 314 patients included in this study, the morphologic and clinical diagnoses were incongruent in 42 (13%), including 7 of 17 patients with HC, 3 of 3 with NCLVC, 3 of 3 with mononuclear myocarditis, 4 of 4 with ARVC, and 8 of 8 with cardiac sarcoidosis. This incongruity certainly leaves open the possibility that, if immunosuppressive or immunomodulatory treatment had been used in certain cases, such as specific antiarrhythmic agents for ARVC or intracardiac defibrillator for non-obstructive HC with normal or near-normal left ventricular ejection fraction, this therapy might have made a difference with respect to treatment, outcome, and even potentially the need for CT.
The positive features of the present study include the fact that all hearts were examined and described and all morphologic diagnoses were provided by the same person (WCR), who has been studying cardiovascular diseases for several decades. Had many of the non-IC cases been “grossed” by a first or second year trainee, which is the modus of operation in the surgical pathology laboratory at BUMC–except for the cardiovascular cases–it is unlikely that the cases of burnt-out or end-stage HC, or ARVC, or NCLVC would have been recognized grossly, and histologic examination in these cases would unlikely have provided the proper morphologic diagnoses.
Footnotes
Abbreviations: ARVC = arrhythmogenic right ventricular cardiomyopathy, CT = cardiac transplantation, HC = hypertrophic cardiomyopathy, IC = ischemic cardiomyopathy, IDC = idiopathic dilated cardiomyopathy, NCLVC = non-compaction left ventricular cardiomyopathy.
Financial support and conflicts of interest: Support for this investigation was provided by the Baylor Health Care System Foundation. The authors have no conflicts of interest to disclose.
References
- 1.Angelini A, Boffa GM, Livi U, et al. Discordance between pre and post cardiac transplant diagnosis: implications for pre- and postoperative decision making. Cardiovasc Pathol. 1999; 8:17–23. [DOI] [PubMed] [Google Scholar]
- 2.Avella A, d’Amati G, Zachara E, et al. Comparison between electro- anatomic and pathologic findings in a patient with arrhythmogenic right ventricular cardiomyopathy/dysplasia treated with orthotopic cardiac transplant. Heart Rhythm. 2010; 7:828–831. [DOI] [PubMed] [Google Scholar]
- 3.Barnard CN. The Operation. A human heart transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S AFr Med J. 1967; 41:1271–1274. [PubMed] [Google Scholar]
- 4.Basso C, Corrado D, Marcus FI, et al. Arrhythmogenic right ventricular cardiomyopathy. Lancet. 2009; 373:1289–1300. [DOI] [PubMed] [Google Scholar]
- 5.Biagini E, Spirito P, Leone O, et al. Heart transplantation in hypertrophic cardiomyopathy. Am J Cardiol. 2008; 101:387–392. [DOI] [PubMed] [Google Scholar]
- 6.Bortman G, Sellanes M, Odell DS, et al. Discrepancy between pre- and post-transplant diagnosis of end-stage dilated cardiomyopathy. Am J Cardiol. 1994; 74:921–924. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw SH, Veinot JP. Cardiac amyloidosis: what are the indications for transplant?. Curr Opin Cardiol. 2012; 27:143–147. [DOI] [PubMed] [Google Scholar]
- 8.Burke AP, Farb A, Tashko G, et al. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases?. Circulation. 1998; 97:1571–1580. [DOI] [PubMed] [Google Scholar]
- 9.Chang TI, Chi NH, Chou NK, et al. Isolated cardiac sarcoidosis in heart transplantation. Transplant Proc. 2012; 44:903–906. [DOI] [PubMed] [Google Scholar]
- 10.Conner R, Hosenpud JD, Norman DJ, et al. Heart transplantation for cardiac amyloidosis: successful one-year outcome despite recurrence of the disease. J Heart Transplant. 1988; 7:165–167. [PubMed] [Google Scholar]
- 11.Conraads V, Paelinck B, Vorlat A, et al. Isolated non- compaction of the left ventricle: A rare indication for transplantation. J Heart Lung Transplant. 2001; 20:904–907. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D, Basso C, Thiene G, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997; 30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 13.Coutu M, Perrault LP, White M, et al. Cardiac transplantation for hypertrophic cardiomyopathy: a valid therapeutic option. J Heart Lung Transplant. 2004; 23:413–417. [DOI] [PubMed] [Google Scholar]
- 14.Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005; 112:3823–3832. [DOI] [PubMed] [Google Scholar]
- 15.Donsky AS, Escobar J, Capehart J, et al. Heart Transplantation for undiagnosed cardiac sarcoidosis. Am J Cardiol. 2002; 89:1447–1450. [DOI] [PubMed] [Google Scholar]
- 16.Dubrey SW, Burke MM, Hawkins PN, et al. Cardiac transplantation for amyloid heart disease: the United Kingdom experience. J Heart Lung Transplant. 2004; 23:1142–1153. [DOI] [PubMed] [Google Scholar]
- 17.Dubrey SW, Burke MM, Khaghani A, et al. Long term results of heart transplantation in patients with amyloid heart disease. Heart. 2001; 85:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011; 124:1079–1085. [DOI] [PubMed] [Google Scholar]
- 19.Garner WL, Starling C, Kuiper JJ, et al. Lymphocytic myocarditis as a cause of fulminant fatal heart failure. Proc (Bayl Univ Med Cent). 2006; 19:122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gungor H, Oguz E, Ayik MF, et al. Comparison of heart transplantation patients with ischemic and idiopathic dilated cardiomyopathy. Transplant Proc. 2011; 43:3847–3850. [DOI] [PubMed] [Google Scholar]
- 21.Hamada T, Kubo T, Kitaoka H, et al. Clinical features of the dilated phase of hypertrophic cardiomyopathy in comparison with those of dilated cardiomyopathy. Clin Cardiol. 2010; 33:E24–E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isner JM, Ferrans VJ, Cohen SR, et al. Clinical and morphologic cardiac findings after anthracycline chemotherapy. Analysis of 64 patients studied at necropsy. Am J Cardiol. 1983; 51:1167–1174. [DOI] [PubMed] [Google Scholar]
- 23.Isner JM, Virmani R, Itscoitz SB, et al. Left and right ventricular myocardial infarction in idiopathic dilated cardiomyopathy. Am Heart J. 1980; 9:235–242. [DOI] [PubMed] [Google Scholar]
- 24.Kato TS, Takayama H, Yoshizawa S, et al. Cardiac transplantation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2012; 110:568–574. [DOI] [PubMed] [Google Scholar]
- 25.Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol. 2012; 59:779–792. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Marfatia R, Tannenbaum S, et al. Doxorubicin-induced cardiomyopathy 17 years after chemotherapy. Tex Heart Inst J. 2012; 39:424–427. [PMC free article] [PubMed] [Google Scholar]
- 27.Lennenman AJ, Wang L, Wigger M, et al. Heart transplant survival outcomes for adriamycin-dilated cardiomyopathy. Am J Cardiol. 2013; 111:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobo FV, Silver MD, Butany J, et al. Left ventricular involvement in right ventricular dysplasia/cardiomyopathy. Can J Cardiol. 1999; 15:1239–1247. [PubMed] [Google Scholar]
- 29.Luk A, Metawee M, Ahn E, et al. Do clinical diagnoses correlate with pathological diagnoses in cardiac transplant patients? The importance of endomyocardial biopsy. Can J Cardiol. 2009; 25:e48–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010; 122:173–183. [DOI] [PubMed] [Google Scholar]
- 31.Marcus FI, Zareba W, Calkins H, et al. Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm. 2009; 6:984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus GM, Glidden DV, Polonsky B, et al. Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. 2009; 54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maron BJ, Maron MS, Wigle ED, et al. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009; 54:191–200. [DOI] [PubMed] [Google Scholar]
- 34.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013; 381:242–255. [DOI] [PubMed] [Google Scholar]
- 35.Maron BJ, Wolfson JK, Epstein SE, et al. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1986; 8:545–557. [DOI] [PubMed] [Google Scholar]
- 36.Maron BJ. Hypertrophic cardiomyopathy. Lancet. 1997; 350:127–133. [DOI] [PubMed] [Google Scholar]
- 37.Maron MS, Kalsmith BM, Udelson JE, et al. Survival after cardiac transplantation in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010; 3:574–579. [DOI] [PubMed] [Google Scholar]
- 38.Parisi F. Heart transplantation in congenital heart disease. Curr Cardiol Rev. 2011; 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavie A. Heart transplantation for end-stage valvular disease: indications and results. Curr Opin Cardiol. 2006; 21:100–105. [DOI] [PubMed] [Google Scholar]
- 40.Pelosi F, Capehart JE, Roberts WC. Effectiveness of cardiac transplantation for primary (AL) cardiac amyloidosis. Am J Cardiol. 1997; 79:532–535. [DOI] [PubMed] [Google Scholar]
- 41.Ried M, Rupprecht L, Hirt S, et al. Sequential therapy of primary cardiac lymphoma with cardiectomy, total artificial heart support, and cardiac transplantation. J Heart Lung Transplant. 2010; 29:707–709. [DOI] [PubMed] [Google Scholar]
- 42.Roberts CS, Roberts WC. Morphologic features (of hypertrophic cardiomyopathy). Progress in Cardiology 2/2. In: Zipes DP, Rowlands DJ. editor Philadelphia: Lea & Febiger, 1989; pp. 3–32. [Google Scholar]
- 43.Roberts WC, Chung MS, Ko JM, et al. Morphologic features of cardiac sarcoidosis in native hearts of patients having cardiac transplantation. Am J Cardiol. 2014; 113:706–712. [DOI] [PubMed] [Google Scholar]
- 44.Roberts WC, Glancy DL, DeVita VT. Heart in malignant lymphoma (Hodgkin’s disease, lymphosarcoma, reticulum cell sarcoma and mycosis fungoides). A study of 196 autopsy cases. Am J Cardiol. 1968; 22:85–107. [DOI] [PubMed] [Google Scholar]
- 45.Roberts WC, Karia SJ, Ko JM, et al. Examination of isolated ventricular non-compaction (hypertrabeculation) as a distinct entity in adults. Am J Cardiol. 2011; 108:747–752. [DOI] [PubMed] [Google Scholar]
- 46.Roberts WC, Ko JM, Kuiper JJ, et al. Some previously neglected examples of arrhythmogenic right ventricular dysplasia /cardiomyopathy and frequency of its various reported manifestations. Am J Cardiol. 2010; 106:268–274. [DOI] [PubMed] [Google Scholar]
- 47.Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the Heart. A clinicopathologic study of 35 necropsy patients (Group I) and review of 78 previously described necropsy patients (Group II). Am J Med. 1977; 63:86–108. [DOI] [PubMed] [Google Scholar]
- 48.Roberts WC, Phillips SD, Escobar JM, et al. Cardiac transplantation 40 years after a stab wound to the heart. Proc (Bayl Univ Med Cent). 2001; 14:241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts WC, Roberts CC, Ko JM, et al. Dramatically different phenotypic expressions of hypertrophic cardiomyopathy in male cousins undergoing cardiac transplantation with identical disease-causing gene mutation. Am J Cardiol. 2013; 111:1818–1822. [DOI] [PubMed] [Google Scholar]
- 50.Roberts WC, Roberts CC, Ko JM, et al. Cardiac transplantation in adults with aortic valve disease with focus on the bicuspid aortic valve. Am J Cardiol. 2012; 109:1212–1214. [DOI] [PubMed] [Google Scholar]
- 51.Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: Analysis of 152 necropsy patients. Am J Cardiol. 1987; 60:1340–1355. [DOI] [PubMed] [Google Scholar]
- 52.Roberts WC, Vowels TJ, Ko JM, et al. Cardiac transplantation for cardiac sarcoidosis with initial diagnosis by examination of the left ventricular apical “core” excised for insertion of a left ventricular assist device for severe chronic heart failure. Am J Cardiol. 2009; 103:110–114. [DOI] [PubMed] [Google Scholar]
- 53.Roberts WC, Waller BF. Cardiac amyloidosis causing cardiac dysfunction: analysis of 54 necropsy patients. Am J Cardiol. 1983; 52:137–146. [DOI] [PubMed] [Google Scholar]
- 54.Roberts WC. Defining idiopathic dilated cardiomyopathy: A courtroom discussion. Am J Cardiol. 1989; 63:893–896. [DOI] [PubMed] [Google Scholar]
- 55.Ross EM, Roberts WC. Severe atherosclerotic coronary arterial narrowing and chronic congestive heart failure without myocardial infarction; analysis of 18 patients studied at necropsy. Am J Cardiol. 1986; 57:51–56. [DOI] [PubMed] [Google Scholar]
- 56.Ross EM, Roberts WC. Severe atherosclerotic coronary artery disease, healed myocardial infarction and chronic congestive heart failure; analysis of 81 patients studied at necropsy. Am J Cardiol. 1986; 57:44–50. [DOI] [PubMed] [Google Scholar]
- 57.Rotela Samaniego JA, Castells E, Manito N, et al. Clinical evolution of heart transplantation in patients with previous valvular cardiomyopathy. Transplant Proc. 2007; 39:2355–2356. [DOI] [PubMed] [Google Scholar]
- 58.Sagar S, Liu PP, Cooper LT. Myocarditis. Lancet. 2012; 379:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scadding JG. A ‘burnt-out’ case of sarcoidosis. Postgrad Med J. 1968; 4:105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy. Am J Cardiol. 1993; 72:952–957. [DOI] [PubMed] [Google Scholar]
- 61.Shirani J, Maron BJ, Cannon RO, et al. Clinicopathologic features of hypertrophic cardiomyopathy managed by cardiac transplantation. Am J Cardiol. 1993; 72:434–440. [DOI] [PubMed] [Google Scholar]
- 62.Shirani J. Subepicardial myocardial lesions. Am Heart J. 1993; 125:1346–1352. [DOI] [PubMed] [Google Scholar]
- 63.Siân Pincott E, Burch M. Indications for heart transplantation in congenital heart disease. Curr Cardiol Rev. 2011; 7:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tedford RJ, James C, Judge DP, et al. Cardiac transplantation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2012; 9:289–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Theleman KP, Kuiper JJ, Roberts WC. Acute myocarditis (predominately lymphocytic) causing sudden death without heart failure. Am J Cardiol. 2001; 88:1078–1083. [DOI] [PubMed] [Google Scholar]
- 66.Tops LF, Prakasa K, Tandri H, et al. Prevalence and pathophysiologic attributes of ventricular dyssynchrony in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2009; 54:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valantine HA, Tazelaar HD, Macoviak J, et al. Cardiac sarcoidosis: response to steroids and transplantation. J Heart Transplant. 1987; 6:244–250. [PubMed] [Google Scholar]
- 68.Val-Bernal JF, Nistal JF, Martino M, et al. Isolated non-compaction of the left ventricular myocardium in an adult treated with heart transplantation. Pathol Int. 2006; 56:35–39. [DOI] [PubMed] [Google Scholar]
- 69.Varr BC, Liedtke M, Arai S, et al. Heart transplantation and cardiac amyloidosis: approach to screening and novel management strategies. J Heart Lung Transplant. 2012; 31:325–331. [DOI] [PubMed] [Google Scholar]
- 70.Waller BF, Smith ER, Blackbourne BD, et al. Congenital hypoplasia of portions of both right and left ventricular myocardial walls. Clinical and necropsy observations in two patients with parchment heart syndrome. Am J Cardiol. 1980; 46:885–891. [DOI] [PubMed] [Google Scholar]
- 71.Waller TA, Hiser WL, Capehart JE, et al. Comparison of clinical and morphologic cardiac findings in patients having cardiac transplantation for ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, and dilated hypertrophic cardiomyopathy. Am J Cardiol. 1998; 81:884–894. [DOI] [PubMed] [Google Scholar]
- 72.Xu T, Yang Z, Vatta M, et al. Multidisciplinary Study of Right Ventricular Dysplasia Investigators. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2010; 55:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoda M, Minami K, Fritzsche D, et al. Three cases of orthotopic heart transplantation for arrhythmogenic right ventricular cardiomyopathy. Ann Thorac Surg. 2005; 80:2358–2360. [DOI] [PubMed] [Google Scholar]


