Supplemental Digital Content is available in the text
Abstract
A minimum of 15 lymph nodes (LNs) has been recommended as an adequate number for radical gastrectomy for gastric cancer (GC). This study aimed to investigate whether the harvesting of at least 25 LNs was a better criterion for stage N2–3 GC based on the 10-year experience of a high-volume hospital.
A total of 1363 patients who underwent radical gastrectomy for gastric cancer between 2000 and 2010 were included in this study. The relationship between the number of lymph nodes examined during gastrectomy and overall survival (OS) was analyzed.
In multivariate analysis, the numbers of LNs examined (P = 0.001) and N stage were confirmed as 2 of the independent prognostic factors. A larger proportion of N2/N3a/N3b patients was observed in the group with ≥20 LNs examined. The cutoff of ≥25 LNs examined exhibited a significantly lower hazard ratio (HR) than other LN cutoffs among N2–N3 diseases, but the cutoff was not significantly superior to other cutoffs in patients with N0 and N1 disease (HR, 0.64, 0.62, and 0.53 for N2, N3a, and N3b, respectively). The 5-year OS rates were 58.59% and 32.77% for N2 and N3 diseases, respectively, with ≥25 LNs examined, which represents a significant improvement over 15–24 LNs examined (52.48% and 21.67% for N2 and N3 stages, respectively).
Among patients with stage N2–N3 GC, harvesting at least 25 LNs may represent a superior cutoff for radical gastrectomy and could yield better survival outcomes.
INTRODUCTION
Gastric cancer (GC) is a leading cause of cancer-related morbidity and mortality worldwide.1 Currently, surgery with curative intent remains the principal treatment for resectable GC. The depth of primary tumor invasion, lymph node (LN) involvement, and distant metastasis are 3 major predictors of prognosis for patients with GC.2 Therefore, these factors are also considered the 3 basic elements of tumor staging. According to the TNM staging system proposed by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), the N stage is classified into 5 levels (N0, N1, N2, N3a, and N3b) based on the number of metastatic LNs.2 To avoid the stage migration phenomenon, the removal of at least 15 LNs has been recommended as a precondition to ensure reliable N staging.3
In recent years, the number of LNs examined has been considered a potential predictor of prognosis because patients with GC seem to benefit from extended lymphadenectomies with more LNs removed.4 Several analyses have revealed higher 5-year overall survival (OS) rates among patients with more LNs examined.5–7 Nonetheless, to date, a well-accepted criterion has been the harvest of at least 15 LNs by radical gastrectomy for all cases of resectable GC.3,8 However, theoretically, the strategy of surgical treatment could differ between cases of locally advanced GC and cases of relatively early GC with regard to lymphadenectomy. Previously, we hypothesized that the retrieval of >25 LNs might be a superior criterion for locally advanced GC surgery.9
Therefore, this study aimed to investigate whether the harvesting of at least 25 LNs was a better criterion for stage N2–3 GC based on the 10-year experience of a high-volume hospital.
METHODS
Patients
With the approval of the Biomedical Ethics Committee of West China Hospital, a database was established to recruit all patients who underwent gastrectomy in the Gastrointestinal Surgery Department of West China Hospital in China since January 2000. Data including the clinical information, surgical records, pathological findings, and postoperative complications were collected. The follow-up information of the patients was updated at 3-month intervals using the outpatient visit method; however, phone calls and mail interviews were also used as alternative methods. The follow-up duration and survival outcomes were updated through June 2014.
After ethical board approval, the medical records of patients who were diagnosed with GC and who underwent an R0 gastrectomy between January 2000 and December 2010 were selected. Cases were excluded from this analysis if distant metastases were present, if a nonradical resection (ie, R1 or R2 resection) was performed, or if there were no records or follow-up information. The personal information of the patients was redacted during the analyses.
Surgical Techniques
The pattern of resection was decided based on the tumor location and resection margins. The standard procedure for lower-third GC was distal gastrectomy. After distal gastrectomy, Billroth-I gastroduodenostomy, Billroth-II gastrojejunostomy, or Roux-en-Y gastrojejunostomy was performed, according to the surgeon's preference. For middle- or upper-third cases, total gastrectomy with Roux-en-Y esophagojejunostomy was performed. In selected cases, proximal gastrectomy with esophagogastrostomy was performed for upper-third GC without gross involvement of the surrounding structures, particularly in cases of early disease. Lymphadenectomy was classified as D1, D1+, or D2 according to the guidelines of the Japanese Gastric Cancer Association (JGCA) (Ver. 3).8 The extent of LN dissection was decided based on the macroscopic evaluation and the surgeon's training and preferences. After operation, the surgeons selected as many LNs as possible from the surgical specimens. Fluoropyrimidine and platinum regimens comprised the first-line postoperative chemotherapy treatment strategies.
Pathology and Staging
The surgical specimens and LNs were assessed by 2 pathologists in a peer-review format and were classified according to the 7th Edition of the UICC/AJCC TNM staging system. For nodal staging, LN involvement was defined as follows: N0, no regional LN metastasis; N1, metastasis in 1 to 2 regional LNs; N2, metastasis in 3 to 6 regional LNs; N3a, metastasis in 7 to 15 regional LNs; N3b, metastasis in ≥16 regional LNs.2
Tumors were divided into 2 groups (<4.8 cm or ≥4.8 cm) based on the mean size. The T category (T1–2, T3–4) was used to assess the depth of invasion. The tumors were classified into 5 macroscopic types (Type 0, superficial; Type 1, mass; Type 2, ulcerative; Type 3, infiltrative ulcerative; Type 4, diffuse infiltrative) according to the guidelines of the JGCA (version 3).10 The histologic grade encompassed 4 classes based on the degree of differentiation (ie, well-differentiated, moderately differentiated, poorly differentiated, and undifferentiated).
Statistics
The clinicopathological characteristics included the patient age, sex, length of postoperative stay, resection type, reconstruction type, extent of lymphadenectomy, operation time, blood loss, postoperative complication, tumor site, tumor size, macroscopic type, histological type, number of LNs examined, number of metastatic LNs, T category (T1–2, T3–4), and N stage. All of these factors were analyzed in univariate analyses. The selected factors from the univariate analyses were entered into a multivariate model for analysis. Additionally, the interaction between the number of LNs examined and N stage was tested to confirm the potential causation.
A contour map was plotted to illustrate the relationship among 5-year OS, the number of LNs examined, and the number of metastatic LNs. In brief, patients were categorized into 64 groups based on prespecified ranges of LNs examined (1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, ≥35) and metastatic LNs (0, 1–2, 3–4, 5–6, 7–8, 9–10, 11–15, >15). The 5-year OS of each group was calculated and pooled into a predefined 8 × 8 table to generate the contour map.
The patients were divided into 2 groups based on a series of consecutive cutoff numbers of examined LNs from 15 to 35 (eg, <15 or ≥15). Hazard ratios (HRs) with 95% confidential intervals (CIs) were calculated for each cutoff value. Based on the HR values and the corresponding 95% CIs, an estimated HR curve with CI area for each N stage was then generated using the nonparametric, local regression Loess smoothing method to assess the prognostic trend.
Statistical analyses and graphics were completed with the use of R software, version 3.1.2 (R Core Team, Austria), and Stata/SE software version 12.1 (TX: StataCorp LP). The chi-square test was applied to evaluate differences in proportions. The survival analysis was performed as previously reported.11 The survival rates were calculated using the Kaplan–Meier method. Each single prognostic factor was analyzed using the Cox proportional hazards model. The event was defined as death from any cause. The Efron method was used to determine associations. Variables with a P value of ≤0.2 in univariate analysis were considered potential candidates for the final model and were pooled into a multivariate analysis. In multivariate analysis, variables with a P value not <0.05 were discarded in a stepwise procedure for variable selection. P values <0.05 were considered statistically significant.
RESULTS
Patient Selection
In this study, the medical records of 1936 patients were reviewed. Among them, 259 patients were excluded because of nonradical resection, and 78 patients were excluded because of the presence of distant metastasis. In addition, 236 patients with no follow-up records were excluded. Eventually, 1363 patients were analyzed in this study. The mean age of the patients was 56.5 years (range 19–86) with a male-to-female ratio of 2.6:1. The median postoperative follow-up duration was 58.7 months (range 1–165 months).
Univariate and Multivariate Survival Analysis
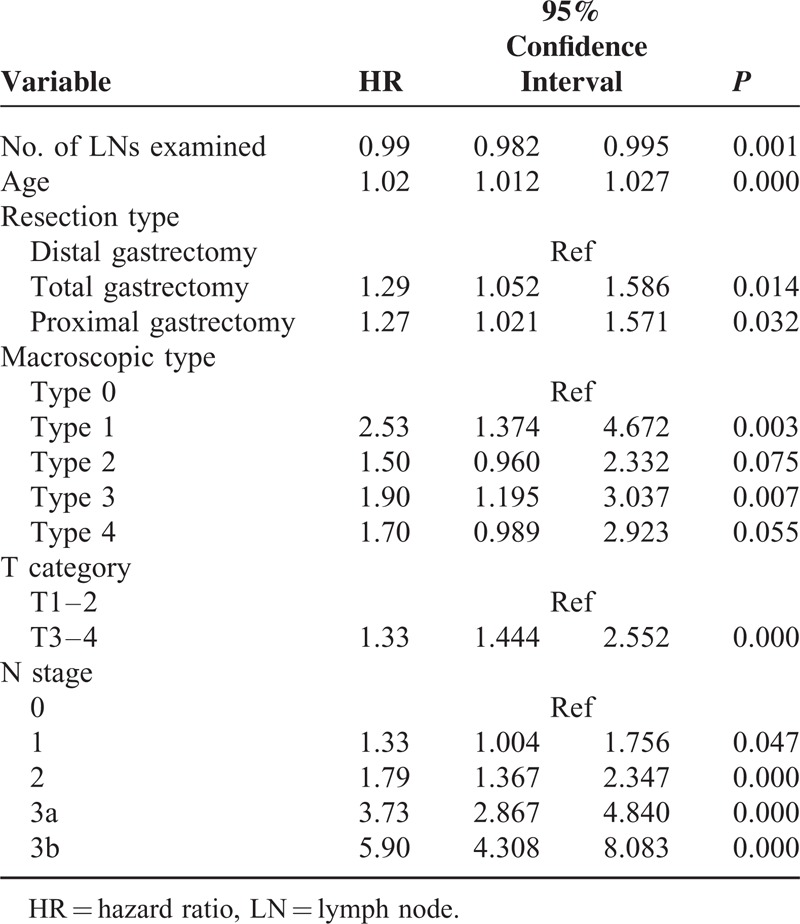
In the univariate analyses, OS was associated with age, resection type, reconstruction type, lymphadenectomy extent, operation time, blood loss, postoperative complications, tumor site, tumor size, macroscopic type, histological grade, number of metastatic LNs, number of LNs examined, T category, and N stage (Table 1). Variables with a P value of ≤0.2 in univariate analysis, including every variable except postoperative days in this particular analysis, were pooled into a multivariate analysis. In multivariate analysis, the number of LNs examined (HR = 0.99, 95% CI 0.982–0.995), age, resection type, macroscopic type, T category, and N stage were identified as independent prognostic factors (Table 2), whereas no significant interaction between N stage and the number of lymph nodes was found (P = 0.710). These significant variables were included as covariates in the subsequent Cox regression analyses.
TABLE 1.
Univariate Analysis of Clinicopathologic Features Using Cox Proportional Hazards Model
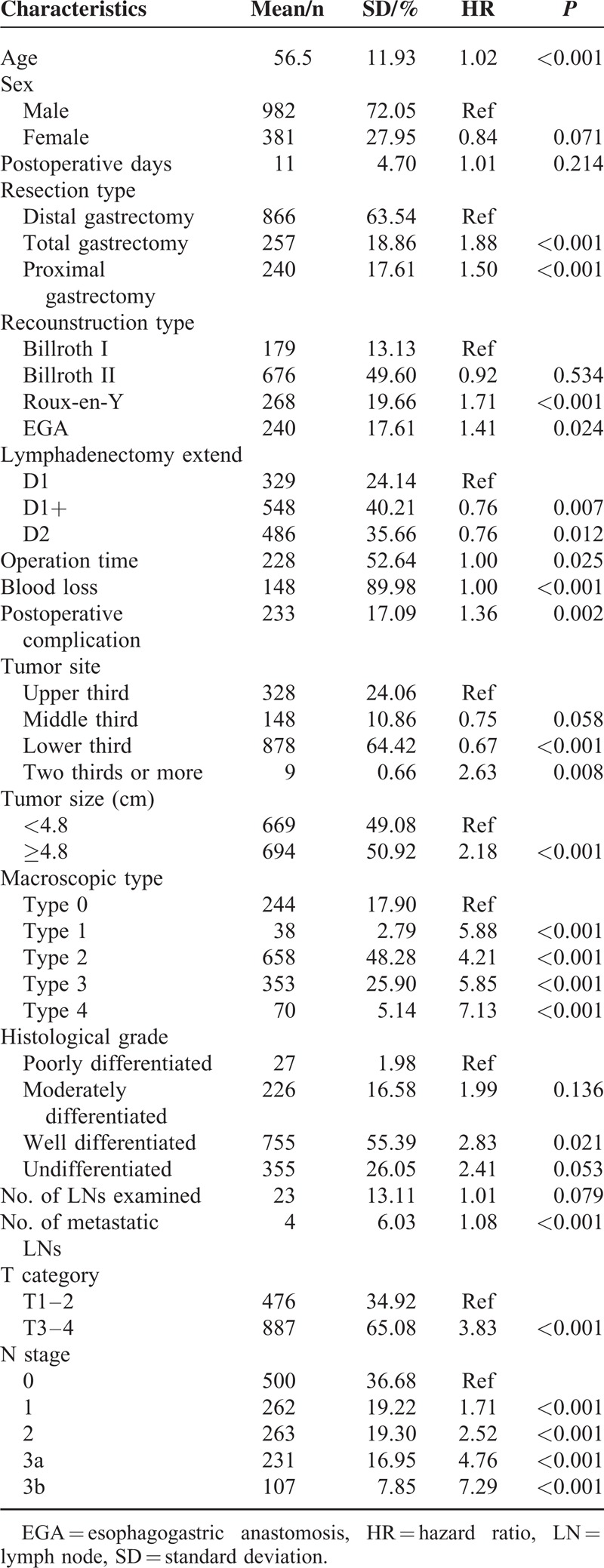
TABLE 2.
Independent Prognosis Factors at Multivariate Analysis Using Cox Proportional Hazards Model
Number of Lymph Nodes Examined
The frequency distribution of patients in stepwise increments of 5 nodes retrieved is summarized in Supplementary Figure 1, http://links.lww.com/MD/A228. A total of 31,428 nodes were obtained from the patients (mean 23, median 22), of which 5869 (18.67%) nodes contained metastases. Means of 17.4 ± 11.7, 21.3 ± 11.8, and 28.9 ± 13.1 LNs were retrieved by D1 lymphadenectomy, D1+ lymphadenectomy, and D2 lymphadenectomy, respectively. The extent of LN dissection was significantly associated with retrieval of a greater number of LNs (P < 0.001). Among the 1363 patients, 863 patients (63.32%) exhibited LN involvement, with an average of 6.8 metastatic nodes per patient. Based on the number of LNs examined, the patients were divided into 6 groups: 1–14, 15–19, 20–24, 25–29, 30–34, and ≥35 LNs harvested. The N stage distribution according to the number of LNs examined was also described (Figure 1). A larger proportion of patients with N2/N3a/N3b GC was observed in the group with ≥20 LNs examined (P = 0.037). This redistribution of N stage was validated in patients who underwent D2 lymphadenectomies (P = 0.014) (Supplementary Figure 2, http://links.lww.com/MD/A228). The proportion of patients with stage N2–3 GC represented 53.53% of the patients with ≥25 LNs examined, compared with the 50.88% of the patients with ≥15 LNs examined (P < 0.001). Using a linear model, the N stage was significantly associated with more lymph nodes examined (rs = 0.27, P < 0.001).
FIGURE 1.
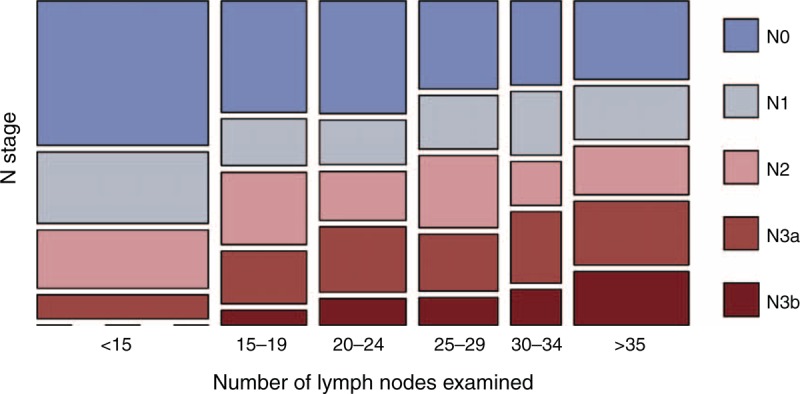
N stage distribution according to the number of lymph nodes examined. Patients in different N stages were stratified into groups according to the number of lymph nodes examined. Each color indicates the specified N stage as shown in the color legend. The length of each box is given based on the proportion in each column or row.
Impact of the Number of Lymph Nodes on the 5-year OS
The 5-year OS rates of the patients in the above-mentioned groups are summarized in Supplementary Table 1, http://links.lww.com/MD/A228 and are stratified by the T and N stages. Overall, the 5-year survival of the patients decreased rapidly from T1 to T4 (86.44%, 74.83%, 66.30%, and 45.78%, respectively), as well as from N0 to N3b (77.95%, 66.42%, 52.80%, 32.74%, and 20.65%, respectively). With the increase in the number of examined LNs, 5-year OS exhibited an upward trend in the patients with stage N2/N3a/N3b GC but not in the patients with stage N0/N1 GC. This same trend was validated with the contour map of 5-year OS (Figure 2). On the contour map, an obvious trend was observed toward improved survival for patients with >6 metastatic LNs, namely those in the N3 stage, when >20 LNs were examined. In the range of 2 to 6 metastatic LNs, there was an upward tendency when 15 LNs were examined. Additionally, a sharp increase in survival was observed when 25 LNs were examined and when there were between 2 and 8 metastatic LNs; the survival was calculated based on 17 patients only.
FIGURE 2.
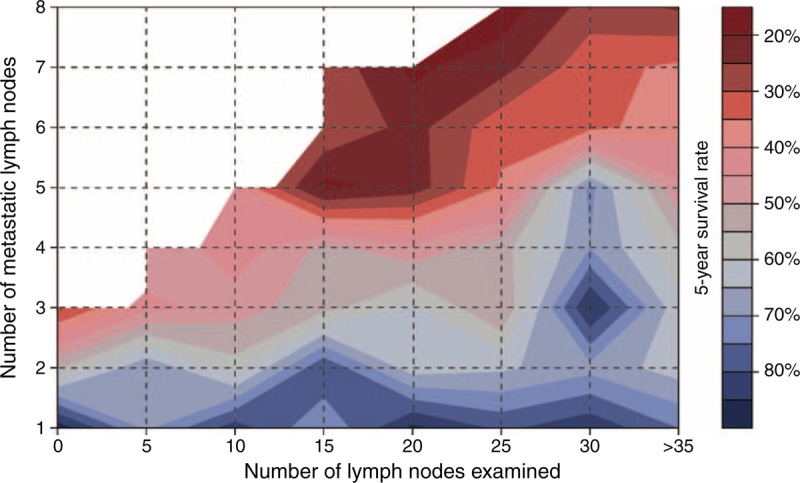
Contour map of the 5-year survival rates after surgery. The red areas depict a low survival rate, blue areas indicate low risk, and white areas indicate a lack of data. The percentage associated with each color indicates the probability of survival of patients diagnosed with a resectable M0 gastric cancer within the first 5 years of follow-up after surgery.
Cutoff Survival Analysis
To investigate how many LNs should be examined to obtain a survival benefit, patients with different N stages were divided into 2 groups based on a series of consecutive, relative numbers. A number from 15 to 35 was chosen as the cutoff to explore the value more precisely. The HRs were calculated using the Cox proportional hazards model and were adjusted for age, resection type, T category, and macroscopic type. The HRs for the cutoffs of ≥15, ≥20, ≥25, ≥30, and ≥35 LNs are summarized in Table 3, and the estimated HR curves of patients in different N stages were generated (Figure 3).
TABLE 3.
Hazard Ratio of the patients according to the groups composed by different cutoff number of nodes (eg, <25 or ≥25)
FIGURE 3.
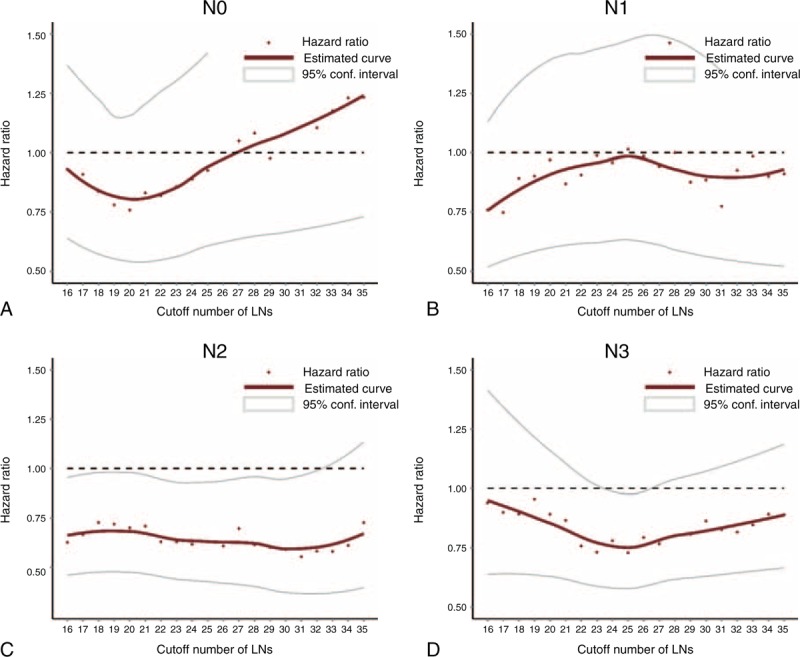
Plots of hazard ratios for patients in AJCC N stages according to the groups distinguished by different cutoff numbers of nodes (eg, <25, or ≥25). The x axes represent different cutoff numbers of lymph nodes, whereas the y axes represent the corresponding hazard ratios. Each red point indicates a specified hazard ratio adjusted by age, resection type, macroscopic type, and T category. (A) N0 stage; (B) N1stage; (C) N2 stage; (D) N3 stage.
A significantly lower HR was not observed in patients with stages N0 and N1 GC at any cutoff, even if the cutoff was ≥15 LNs (Table 3, Figure 3). In patients with N2 GC, the HRs were consistently favorable for cutoffs from 15 to 33 LNs harvested, and cutoffs ≥34 LNs or higher were not optimal for distinguishing 2 groups with different prognoses. This benefit could increase with an increased number of nodes examined and a decrease in the HR from 0.68 to 0.59. In patients with stage N3 GC, the only statistically significant HR was found at ≥25 LNs harvested (HR = 0.62 and 0.53 among patients with stage N3a and N3b GC, respectively), and the HR curve for patients with stage N3 GC in this subset was V-shaped, with the lowest and most significant HR around the cutoff ≥25 LNs (Table 3, Figure 3). To make the results more comparable, we selectively analyzed patients with N2/N3 category disease according to D1/D1+/D2 lymphadenectomy and validated the survival benefit in patients with ≥25 LNs examined (Supplementary Figure 3, http://links.lww.com/MD/A228).
Using at least 25 LNs as the cutoff, Kaplan–Meier curves demonstrated the survival benefits among patients with stage N2–3 GC with at least 25 LNs harvested compared with those with <25 LNs harvested (Figure 4). Interestingly, no significant difference was observed between the prognosis of 2 adjacent N stages, that is, <25 LNs harvested for a lower N stage and ≥25 LNs harvested for a higher N stage (eg, subset <25 in N3a vs subset ≥25 in N3b, P = 0.967). Patients with stage N2–N3 GC exhibited better 5-year OS rates given at least 25 LNs harvested compared with those with <25 LNs harvested (48.67% vs 58.59%, 32.77% vs 23.81%, 38.44% vs 26.63%, and 23.79% vs 11.90% for N2, N3, N3a, and N3b patients, respectively) (Supplementary Figure 4, http://links.lww.com/MD/A228). Additionally, the 5-year OS rates of N2 and N3 diseases with ≥25 LNs harvested were improved compared with disease with 15 to 24 LNs harvested (58.59%, 32.77% vs 52.48%, 21.67% for N2 and N3 stage, respectively). In the N2 subgroup, patients with ≥25 LNs examined exhibited a significant survival benefit compared with patients with <15 LNs examined (HR = 0.59, 95% CI 0.38–0.91), whereas no significant difference was observed between patients with 15 to 24 LNs examined and patients with <15 LNs examined (HR = 0.78, 95% CI 0.53–1.17). In the N3 subgroup, patients with ≥25 LNs examined exhibited a significant survival benefit compared with patients with 15 to 24 LNs examined (HR = 0.72, 95% CI 0.54–0.96) (Supplementary Figure 5, http://links.lww.com/MD/A228).
FIGURE 4.
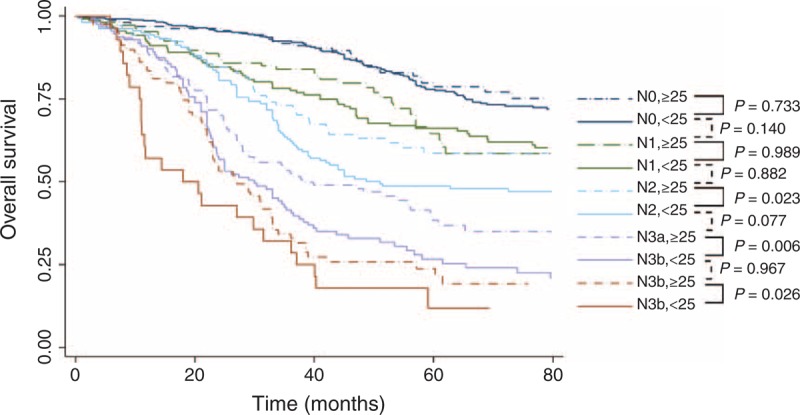
Overall survival according to the AJCC N stage, stratified by the number of lymph nodes retrieved (<25 and ≥25). The comparison between the 2 adjacent subgroups was analyzed using the Cox proportional hazards model, adjusted by age, resection type, macroscopic type, and T category.
Impact of Lymph Node Resection on the Postoperative Complications and OS
D2 lymphadenectomy is associated with higher risk of abdominal complications rather than systematic complication, compared with D1/D1+ lymphadenectomy (Table 4). However, no significant difference was observed between patients with ≥25 LNs harvested and patients with <25 LNs examined regarding both abdominal complications and systemic complications. The Kaplan–Meier curves according to the lymphadenectomy extend (D1/D1+ and D2), stratified by the number of LNs retrieved (<25 or ≥25), were shown in Figure 5. In both D1/D1+ and D2 subgroups, patients with at least 25 LNs harvested exhibited better 5-year OS rates compared with those with <25 LNs harvested (58.62% vs 53.94% in D1/D1+ subgroup; 66.14% vs 58.74% in D2 subgroup, respectively). In patients with ≥25 LNs examined, the 5-year OS of patients following D2 lymphadenectomies remained better than that of patients who underwent D1/D1+ lymphadenectomies (66.14% vs 58.62%).
TABLE 4.
Relative Risk Ratio for Abdominal and Systemic Complications After Gastrectomy
FIGURE 5.
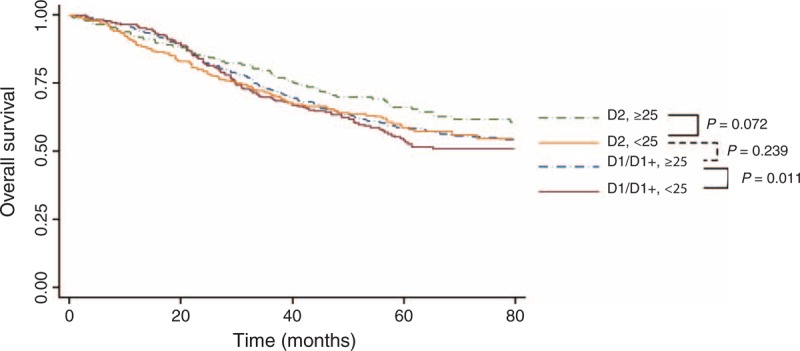
Overall survival according to the lymphadenectomy extends, stratified by the number of lymph nodes retrieved (<25 and ≥25). The comparison between the 2 adjacent subgroups was analyzed using the Cox proportional hazards model, adjusted by age, resection type, macroscopic type, and T category.
DISCUSSION
Based on the survival data of 1363 patients, this study investigated the prognostic value of the number of examined LNs in patients with GC who underwent radical gastrectomies. The number 25 was eventually chosen as the cutoff for stage N2–N3 disease based on the multivariate analysis. In particular, this value suggests that the removal of at least 25 LNs is a favorable prognostic factor for survival in patients with stage N2–N3 GC with no distant metastases. This result might provide some insight into the relationship between the number of LNs examined and the prognoses of patients with GC, and it could also be recommended as a superior criterion for lymphadenectomy for stage N2–N3 resectable GC.
In recent years, several studies have suggested that the number of LNs examined is associated with better OS in patients with GC.5–7 The guidelines of the NCCN recommend that no <15 LNs should be examined for pathological assessment.3 Thus far, different cutoff numbers of LNs have been proposed. However, the majority of studies in the literature have focused on the role of the number of examined LNs in node-negative patients with GC.5,12,13 Some of these studies might have noted the prognostic effect of the number of examined LNs, but it would be illogical to translate their conclusions, which were drawn from node-negative patients, to patients with metastatic LNs. To date, a uniform consensus has insisted that 15 is an adequate number by which to determine the N stage.
In the present study, because of the exclusion of patients with distant metastases, we only included patients who underwent radical gastrectomy with curative intent. After the stratification of patients into different N stages, a survival benefit based on more LNs examined was found in patients with stage N2/N3 GC with respect to 5-year OS. This finding supported the number of LNs examined as a predictor of survival of patients with advanced GC without distant metastases (Table 3). In consecutive numbers from 15 to 35, the cutoff of 25 was found to have the lowest HR value for patients with stage N2–N3 GC (Figure 3), and a similar tendency was also validated in subgroup analysis based on lymphadenectomy extent (Supplementary Figure 3, http://links.lww.com/MD/A228). Overall, for patients with stage N2/N3 GC, the examination of at least 25 LNs indicated that the patients had a 60% to 65% probability of a better prognosis compared with patients with <25 LNs examined (5-year survival 48.67% vs 58.59%, 23.81 vs 32.77% for N2 and N3, respectively) (Supplementary Figure 4, http://links.lww.com/MD/A228). Because preoperative staging of LN metastasis has been reported with an accuracy from 54% to 76.6%,14–16 the removal of ≥25 LNs in patients with stage N2/N3 GC might be a practical and effective strategy in the near future.
Of all the reasons why the examination of more LNs might provide patients with a survival benefit, stage migration might be the most important. It is reasonable to assume that patients might be understaged if only a few randomly removed LNs are examined, and with more LNs examined, there is a greater likelihood for the identification of metastatic LNs. As one form of stage migration, the redistribution of N stage was observed with the increase in the number of examined LNs in this study (Figure 1, Supplementary Figure 2, http://links.lww.com/MD/A228). With <25 LNs examined, the prognosis of patients in the N3a stage was similar to that of patients in the N3b stage with ≥25 LNs examined (Figure 4). Therefore, the improved survival of patients with more nodes examined might partially be explained by more accurate staging. Previous studies by Smith et al and Schwarz et al6,7 recommended that at least 10 negative LNs should be retrieved to determine the N stage accurately. This finding indicated that at least 25 LNs were required to fully stage a patient with stage N3b disease who had >15 metastatic LNs. For patients with stage N2/N3 GC who have multiple metastatic LNs, accurate staging could eventually translate into a more effective adjuvant therapy. This theory might explain why patients with stage N0/N1 GC, who have no or a few metastatic nodes, cannot benefit from extended lymphadenectomy; the current suggestion of 15 LNs is sufficient to stage patients in this subtype accurately. At the same time, in the absence of therapeutic strategy adjustment, accurate staging might not necessarily provide patients without LN metastasis with a significant survival benefit. Based on these considerations, the current criterion of 15 LNs appears insufficient to assess LN involvement, particularly for N3 patients. Such a convention could provide misleading information with respect to the treatment strategy and prognosis. Thus, a higher cutoff, such as 25, as found in this study, should be considered for more accurate N staging.
The number of LNs examined was significantly affected by the extent of the LN dissection. Although the criterion of at least 25 nodes harvest could be achieved in either D1/D1+ lymphadenectomy or D2 lymphadenectomy, more extended lymphadenectomy of gastric cancer surgery is capable of harvesting more lymph nodes compared with limited lymphadenectomy (Supplementary Figure 1, http://links.lww.com/MD/A228). The 15-year follow-up of Dutch trial has documented that D2 lymphadenectomy could reduce gastric cancer-related death against D1 lymphadenectomy.4 Thus, it is reasonable to perform more extended lymphadenectomy (D2) to harvesting more lymph nodes and gaining better survival outcome. However, at present, the extent of lymph node dissection varies geographically. In Eastern countries with a high incidence of GC, such as Japan, the Republic of Korea, and China, a more aggressive strategy of lymphadenectomy has been used that entails the removal of more LNs to achieve a more curative resection.17 In contrast, many surgeons in Western countries still prefer limited lymphadenectomy when they perform radical gastrectomy.18 Numerous studies have found a higher risk of postoperative complications in D2 dissection group than that in D1 group and failed to demonstrate a survival benefit over D1 dissection.19,20 This conclusion was questioned because D2 lymphadenectomy requires a certain degree of specialist training and extensive experience. A relationship between case volume and mortality has been noted in terms of D2 lymphadenectomy, emphasizing the expertise needed for this aggressive procedure.21 In the present study, the examination of at least 25 lymph nodes provides proven survival benefit for patients following either D1/D1+ or D2 lymphadenectomies (Figure 5). Despite a higher risk of abdominal complications (Table 4), D2 lymphadenectomy maintained a significant survival advantage over D1 lymphadenectomy (Table 1). In patients with ≥25 LNs examined, the 5-year OS of patients following D2 lymphadenectomies remained better than that of patients who underwent D1/D1+ lymphadenectomies. Furthermore, the cutoff of 25 seemed to render D2 dissection an ideal procedure for lymphadenectomy because a retrieval of 25 LNs used to be classified as standard D2 lymphadenectomy.22 Therefore, LN resection of at least 25 LNs for D2 lymphadenectomy could be one promising option to improve the outcomes of patients with locally advanced resectable GC.
Our study had some limitations. With its retrospective design, this study provided limited power for clinicians to reference. Although prospective trials can provide strong references for guidelines, we believed that by obtaining fully recorded pathological information of LNs retrospectively, our study could be helpful in the generation of some new hypotheses for future studies. Another limitation was that this was a single-institution study from China. In China, GC is characterized by its high incidence and advanced stage. Thus, the results from this study should be validated in both Eastern and Western countries before they are applied for the refinement of any guidelines. However, as a national surgical training center with a large number of surgeries each year, our data could indicate reliable and concordant surgical outcomes with other high-volume medical centers with respect to extended lymphadenectomies.
In conclusion, the present study suggests that the number of nodes examined is correlated with OS in patients with resectable GC. Among patients with stage N2–N3 GC, harvesting at least 25 LNs could be a superior cutoff for radical gastrectomy and may yield better survival outcomes.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CIs = confidential intervals, GC = gastric cancer, HR = hazard ratio, JGCA = Japanese Gastric Cancer Association, LNs = lymph nodes, OS = overall survival, UICC = Union for International Cancer Control
Funding: Domestic support from National Natural Science Foundation of China (No. 81372344); National Natural Science Foundation of China (No. 81301866); New Century Excellent Talents in University support program, Ministry of Education of China (2012SCU-NCET-11–0343).
H-NC and X-ZC contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 14/August/2014. [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th ed.New York, Dordrecht, Heidelberg, London: Springer; 2010. [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Gastric Cancer. Version 1.2014. 2014. Accessed August 8, 2014, 2014. [Google Scholar]
- 4.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. [DOI] [PubMed] [Google Scholar]
- 5.Baiocchi GL, Tiberio GA, Minicozzi AM, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 2010; 252:70–73. [DOI] [PubMed] [Google Scholar]
- 6.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005; 23:7114–7124. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007; 14:317–328. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14:113–123. [DOI] [PubMed] [Google Scholar]
- 9.Chen XZ, Yang K, Zhang B, et al. Is retrieval of >25 lymph nodes a superior criterion for locally advanced gastric cancer surgery? Ann Surg 2011; 254:834–835.author reply 835. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 11.Bruin J. Survival Analysis with Stata, UCLA: Statistical Consulting Group. Available from: http://www.ats.ucla.edu/STAT/stata/seminars/stata_survival/ Accessed August 14, 2014. [Google Scholar]
- 12.Biffi R, Botteri E, Cenciarelli S, et al. Impact on survival of the number of lymph nodes removed in patients with node-negative gastric cancer submitted to extended lymph node dissection. Eur J Surg Oncol 2011; 37:305–311. [DOI] [PubMed] [Google Scholar]
- 13.Hirabayashi S, Kosugi S, Isobe Y, et al. Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol 2014; 25:1179–1184. [DOI] [PubMed] [Google Scholar]
- 14.Saito T, Kurokawa Y, Takiguchi S, et al. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer. Eur Radiol 2015; 25:368–374. [DOI] [PubMed] [Google Scholar]
- 15.Chen CY, Hsu JS, Wu DC, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT-correlation with surgical and histopathologic results. Radiology 2007; 242:472–482. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Kim AY, Oh ST, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology 2005; 236:879–885. [DOI] [PubMed] [Google Scholar]
- 17.Hu JK, Yang K, Zhang B, et al. D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today 2009; 39:207–213. [DOI] [PubMed] [Google Scholar]
- 18.Chen XZ, Hu JK, Zhou ZG, et al. Meta-analysis of effectiveness and safety of D2 plus para-aortic lymphadenectomy for resectable gastric cancer. J Am Col Surg 2010; 210:100–105. [DOI] [PubMed] [Google Scholar]
- 19.Memon MA, Subramanya MS, Khan S, et al. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg 2011; 253:900–911. [DOI] [PubMed] [Google Scholar]
- 20.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999; 79:1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg 2007; 245:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siewert JR, Bottcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998; 228:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]


