Abstract
To examine the feasibility of using carbon nanoparticles to track nonpalpable breast cancer for breast-conserving surgery.
During breast-conserving surgery, it is often very challenging to determine the boundary of tumor and identify involved lymph nodes. Currently used methods are useful in identifying tumor location, but do not provide direct visual guidance for resection margin during surgery.
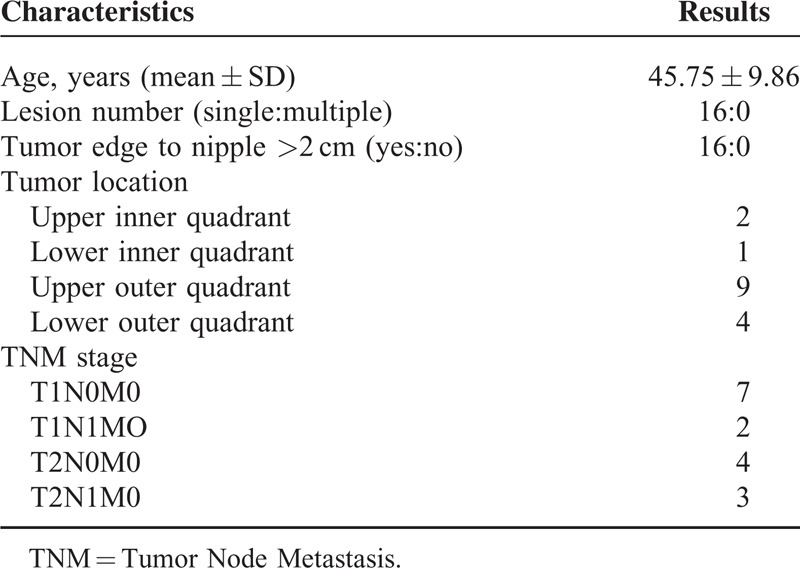
The study was approved by the Institutional Review Board of the Fuzhou General Hospital (Fuzhou, China). The current retrospective analysis included 16 patients with nonpalpable breast cancer receiving breast-conserving surgery under the guidance of preoperative marking using a carbon nanoparticle, as well as 3 patients receiving carbon nanoparticle marking followed by neoadjuvant treatment and then breast-conserving surgery. The Tumor Node Metastasis stage in the 16 cases included: T1N0M0 in 7, T1N1M0 in 2, T2N0M0 in 4, and T2N1M0 in the remaining 3 cases. The nanoparticle was injected at 12 sites at 0.5 cm away from the apparent edge under colored ultrasonography along 6 tracks separated by 60 degrees (2 sites every track). Lymph node status was also examined.
The resection edge was free from cancer cells in all 16 cases (and the 3 cases with neoadjuvant treatment). Cancer cells were identified in majority of stained lymph nodes, but not in any of the unstained lymph nodes. No recurrence or metastasis was noticed after the surgery (2 to 22-month follow-up; median: 6 months).
Tracking nonpalpable breast cancer with carbon nanoparticle could guide breast-conserving surgery.
INTRODUCTION
Since the landmark study by Shaefer,1 breast-conserving surgery has been increasingly used in the treatment of nonpalpable breast cancer. Breast-conserving surgery, however, requires precise location and reasonable estimation of metastasis along the lymph route.
A number of techniques, including ultrasonography,2 molybdenum target,3 CT (computed tomography),4 and MRI (magnetic resonance imaging)5 have been used to locate the lesions. These methods have been proven useful, but limited as direct visual guidance during the surgery.
Carbon nanoparticles have been used to mark thyroid carcinoma to reduce accidental damage of parathyroid glands.6 Carbon nanoparticles have also been used to track lymph drainage route for a variety of other cancers (eg, gastric).7 In this report, we describe the successful use of carbon nanoparticles to track nonpalpable breast cancer in 16 patients. Preliminary results in marking breast cancer before neoadjuvant chemotherapy in 3 cases are also reported.
METHODS AND MATERIALS
Patients
The study was approved by the Institutional Review Board of the Fuzhou General Hospital (Fuzhou, China). The current report included 16 consecutive cases of nonpalpable breast cancer (all women; age: 24–58 years) receiving breast-conserving surgery under the guidance of carbon nanoparticle marking at the Department of Surgery, Fuzhou General Hospital of Nanjing Military Command. Maximum diameter of the primary lesion, as determined by ultrasound examination before the surgery, ranged from 0.6 to 1.8 cm (average: 1.1). All cases were confirmed as infiltrating ductal breast cancer with biopsy, and met the National Comprehensive Cancer Network (NCCN) indications for breast-conserving resection.8
Preoperative Marking
The lesion was tracked with carbon nanoparticles. Briefly, a suspension of carbon nanoparticles (particle diameter: 150–200 nm; prepared by Pharmaceutical Department, Fuzhou General Hospital of Nanjing Military Command) was injected under ultrasound guidance at 12 points along 6 tracks (2 injection sites per track) using 23-gauge needles (Figure 1). The injection sites were 0.5 cm from the apparent margin of the primary lesion. The tracks were separated by 60 degrees (Figure 2). Each injection contained 0.1-ml 1:1 diluted suspension (0.05-mg carbon nanoparticles).
FIGURE 1.
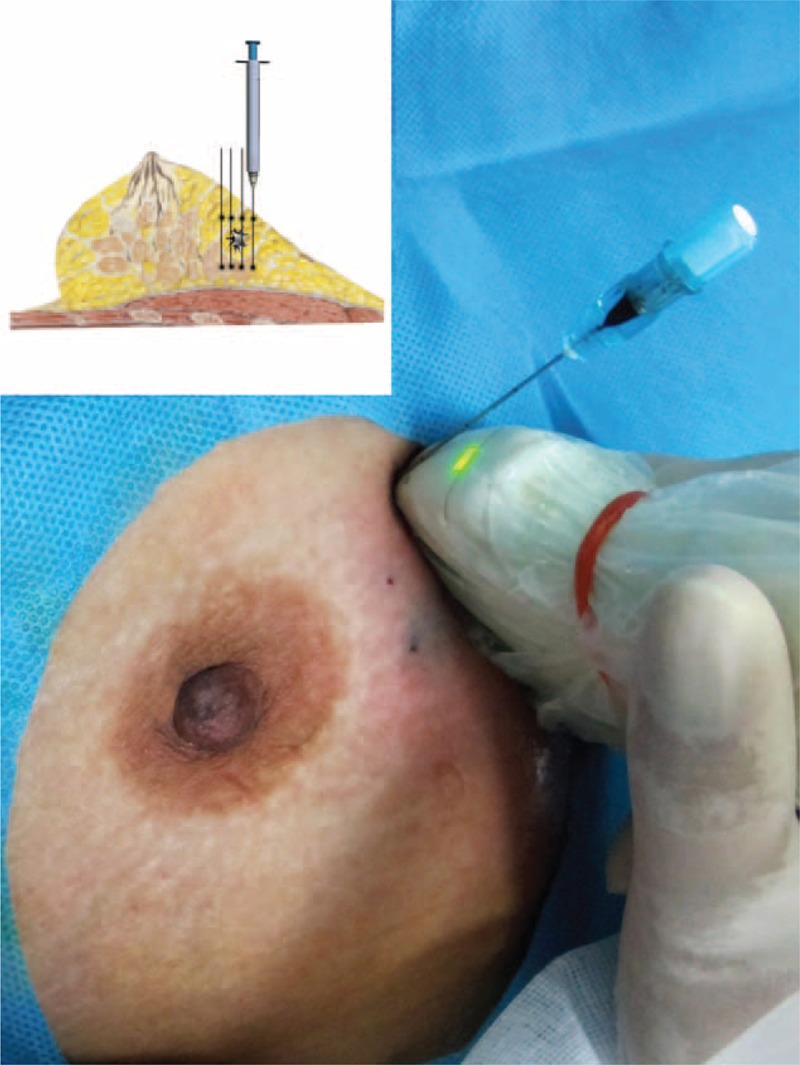
A schematic diagram for the marking. Asterisk: the primary lesion; the outside marking: the injection sites.
FIGURE 2.
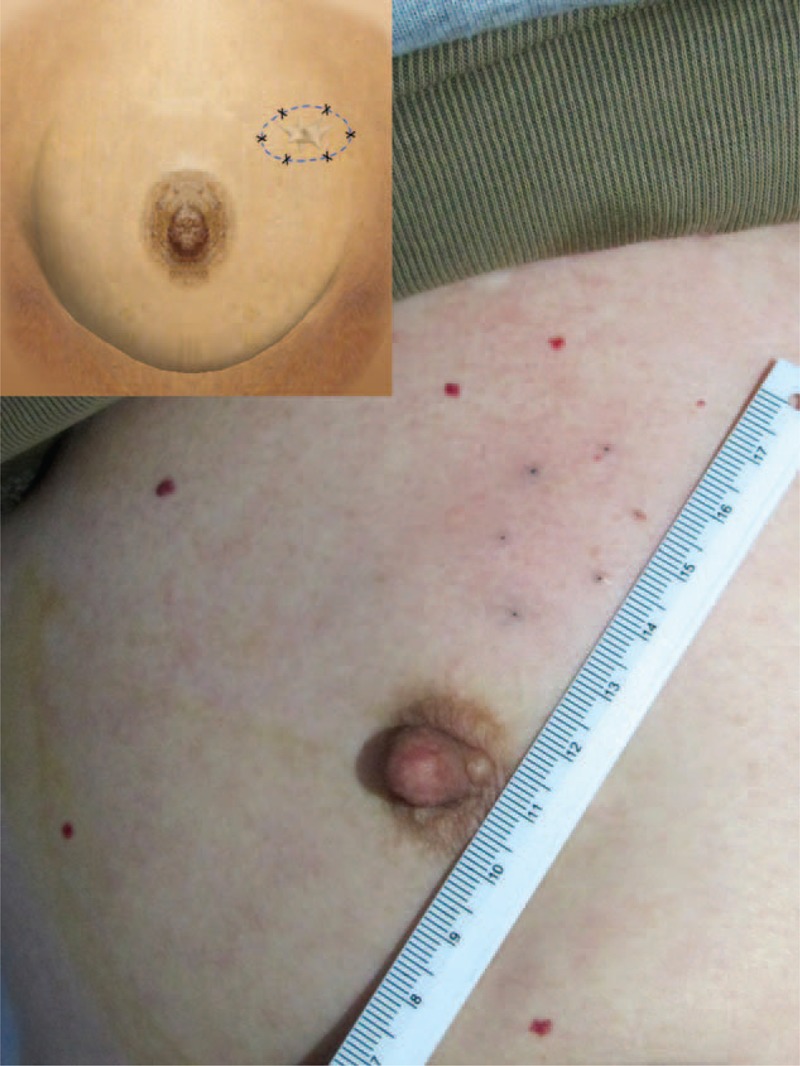
Marking at every 60 degree.
Surgery and Postoperative Treatment
The surgery was carried out under block anesthesia with patients in a supine position. The lesion was removed en block along the outer edge of the staining. The removed tissue block was sent for rapid pathological examination using frozen sections, and then routinely using fixed tissue.
For auxillary lymph node dissection, all stained lymph nodes were removed (Figure 3). Lymph nodes inside the following boundaries were removed regardless of staining: the outside edge of the pectoris minor to latissimus dorsi (level I), outside edge to inside edge of pectoris minor (level II). All removed lymph nodes were examined microscopically at 150-μm interval along the long axis.
FIGURE 3.
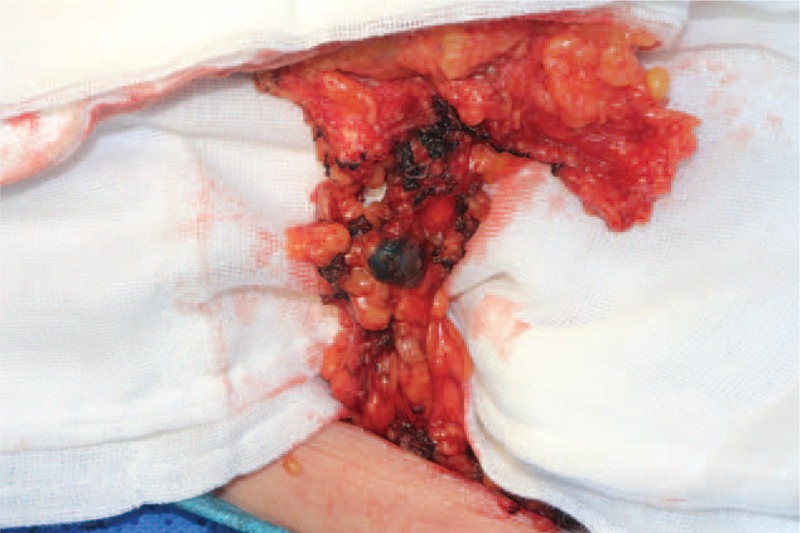
The stained lymph node.
All 16 patients received systemic chemotherapy for 6 cycles after the surgery: 8 with CAF regimen (cyclophosphamide 100 mg/m2, adriamycin 30 mg/m2, fluorouracil 500 mg/m2), 5 with TAC regimen (docetaxel 75 mg/m2, adriamycin 50 mg/m2, cyclophosphamide 500 mg/m2), and the remaining 3 patients with TC regimen (docetaxel 75 mg/m2, cyclophosphamide 600 mg/m2).9–11 The choice of chemotherapeutic regimen was based on physician discretion with reference to the NCCN guideline.
All 16 patients received local radiotherapy, starting at 2 to 4 weeks after the completion of the last chemotherapeutic cycle. Thirteen patients received radiation (6MV X-ray) to the entire breast on the lesion side (1.8–2.0 Gy/time, 5 days a week, for a duration of 5 weeks), plus extra dose directed to the lesion field (10–16 Gy/time, once every 7–10 days, for a total of 5 times). In addition to the radiation to the entire breast and tumor field, 3 patients also received radiation to the supra- and subclavical region (50 Gy, 5 days a week, for duration of 5 weeks). Thirteen of the 16 patients received perpetual hormone treatment: 20-mg/d tamoxifen in 11 cases and 2.5-mg/d letrozole in 2 cases.
Effectiveness Measures
Resected tissue blocks were examined pathologically using a routine procedure. As this is not a clinical trial, we did not set a rigid protocol for the follow-up. Nevertheless, the follow-ups were attempted every 6 months, and included a complete physical, standard blood panel, colored ultrasound examination of the breasts, B-ultrasound examination of the abdomen, and serum carcinoembryonic antigen (CEA) and carbohydrate antigen 15–3 (CA 15–3). Mammography and chest X-ray examination were scheduled every 12 months. Aesthetic appearance of the breast was evaluated immediately before the initiation of radiotherapy using the Rose methods.12 Standard parameters (eg, operation time and blood loss) were recorded during the surgery. The final follow-up was conducted in all 16 subjects in December 2013.
This report also included 3 cases of carbon nanoparticle marking in patients receiving neoadjuvant therapy (standard TAC regimen for three cycles) followed by breast-conserving resection in combination with auxillary lymph node dissection.
RESULTS
Surgery
Preoperative marking was uneventful in all 16 cases. The baseline characteristics are shown in Table 1. The duration of the procedure was 8 min (range: 5–10). No allergic reaction was observed. Breast-conserving surgery was conducted as planned (12–72 h after the marking) in 15 cases, and 14 days after the marking in 1 patient (based on patient choice).
TABLE 1.
Patient and Baseline Characteristics
The surgery lasted for 42.54±10.26 min. Blood loss during the surgery was 15.65 ± 4.37 mL. No apparent contamination was observed. The maximum diameter of the removed tissue block ranged from 3.0 to 5.5 cm. No cancer cell was noted in the edge of removed tissue in any case. The distance between the edge of the lesion to the edge of the removed block was 1.0 cm (range: 0.8–1.5 cm).
The Tumor Node Metastasis stage in the 16 cases included: T1N0M0 in 7, T1N1M0 in 2, T2N0M0 in 4, and T2N1M0 in the remaining 3 cases. In each of the 16 cases, 1 to 3 stained lymphatic vessel(s) were identified. Stained auxillary lymph nodes (1–3 in each case) were also noted. Cancer cells were identified in stained lymph nodes in 10 cases (1/1 in 8 cases, and 2/2 in 2 cases), and not in the remaining 6 cases (0/1 in 4, 0/2 in 1, and 0/3 in 1 case). No cancer cell was identified in any of the unstained lymph nodes (8–16 per case).
Follow-Ups
The follow-up lasted from 2 to 22 months (median: 14.5 months). No recurrence or distant metastasis was noted in any of the 16 cases. The aesthetic appearance was “good” in 13, “fair” in 2, and “acceptable” in the remaining 1 case.
Preliminary Studies for Neoadjuvant Chemotherapy
The carbon nanoparticle was used to track non-palpable breast cancer before neoadjuvant chemotherapy (standard TAC regimen for three cycles) followed by breast-conserving resection in combination with auxillary lymph node dissection in three cases of infiltrating ductal cancer (BI RADS 5) using identical protocol, with the exception of three cycle of neoadjuvant chemotherapy before the surgery. The surgery was completed in 30–55 minutes. The resection edge was free from cancer cells in all 12 directions in all three cases. Cancer cells were identified in 1/1, 0/1, and 0/2 of stained auxillary lymph nodes.
Unstained lymph nodes were not removed/examined due to ethical consideration (promising results in the 16 cases not receiving neoadjuvant chemotherapy). The follow-up (range: 6–18 months) lasted for 6, 10, and 18 months in the 3 cases, respectively. The last follow-up (in December 2013) included a complete physical, standard blood panel, colored ultrasound examination of the breasts, B-ultrasound examination of the abdomen, mammography, chest X-ray, and serum CEA and CA 15–3, and failed to discover any evidence for local recurrence or distant metastasis.
DISCUSSION
With the increasing popularity of mammography, nonpalpable breast cancer is being increasingly detected. The results from the current study confirmed the feasibility of using carbon nanoparticles to track nonpalpable breast cancer before breast-conserving surgery. The marking around the lesions provided visual guidance for removing the lesions. Cancer cells were identified in stained lymph nodes in 10 of the 16 cases, whereas no cancer cells were identified in unstained lymph nodes upon pathologic examination of the removed lymph nodes. Consistent with the goal of preserving healthy tissue, the maximum distance from the resection edge to the edge of the cancerous tissue was <1.0 cm in majority of the cases (12/16). We believe that such a method is particularly useful for women with small breast (and consequently a need to preserve as much healthy tissue as possible). Apparently, the maximum distance from the resection edge to the edge of the cancerous tissue is not satisfactory in all cases. There is a clear need to fine-tune the method, including optimizing injection dose and identifying factors (eg, advanced age) that could increase the dispersion of the carbon nanoparticles.
Another serendipity finding of the current study is that the marking could still be used 14 days after the injection of carbon nanoparticles.
Complete removal of the lesions while preserving healthy breast tissue remains often an art rather than science. Staining with dyes (eg, methylene blue, ink, and gentian violet) has been used to mark the lesions and to provide visual guidance during the surgery, but is prone to contamination of surrounding tissues,13 as well as local reaction.14 Tags (eg, guide wire, probe and radionuclide) typically only mark the lesion center, and thus are limited in determining the extent of the lesions.15,16 Also, tags are associated with a variety of adverse effects, such as local hemorrhage and even tumor spreading along the needle route.17 The use of radioactive isotope has inherent danger, and could be disastrous upon leakage.
Another critical issue in surgical treatment of breast cancer is estimation of the lymph node involvement. The methods for identifying sentinel lymph node are less developed than for identifying the primary lesions. A study by Ko et al18 suggested that injecting activated carbon into nonpalpable breast cancer is helpful in identifying sentinel lymph nodes. Also, a comparison study with methylene blue seemed to suggest higher sensitivity of activated carbon particles in detecting sentinel lymph nodes.19 But apparently, dye injection into the lesions is associated with a risk of dissemination of tumor cells along the needle track. In our study, the carbon nanoparticles were injected along the periphery of the lesions under ultrasound guidance, and thus could avoid tumor dissemination.
We did not notice any sign of recurrence or metastasis in any of the 16 cases. Such a finding is encouraging, but needs to be interpreted with caution; the current study is a retrospective analysis with only short-term follow-up.
In a pilot study, we used carbon nanoparticles in 3 patients receiving neoadjuvant chemotherapy before breast-conserving surgery. The marking greatly facilitated the surgery. More importantly, no recurrence was noticed in any of the three cases. These findings suggest that carbon nanoparticles could be effectively used to track the lesions that would have been difficult to identify otherwise after neoadjuvant chemotherapy.
The nanoparticles used in this study ranged from 150 to 200 nm in diameter,20 and thus could readily enter lymph capillary but not blood vessels. The safety profile of this preparation has been established for use in colorectal as well as in thyroid cancer.6,20
In summary, the advantages of this method include:) technical feasibility; clear visual guidance during the surgery, and as a result, minimized risk of cancer spreading due to surgical maneuver to identify the lesions and potentially preservation of more healthy tissue; potential to identify sentinel lymph node. These features are particularly important for Asian women with typically smaller breasts,21 and consequently a need to preserve as much healthy breast tissue as possible.
CONCLUSION
Marking with carbon nanoparticle is a safe and feasible method for localization of nonpalpable breast cancer for breast-conserving surgery. The potential to identify sentinel lymph node needs to be further explored.
Footnotes
Abbreviations: BI RADS = breast imaging reporting and data system, CA 15–3 = carbohydrate antigen 15–3, CAF = cyclophosphamid, adriamycin, fluorouracil, CEA = carcinoembryonic antigen, CT = computed tomography, MRI = magnetic resonance imaging, NCCN = national comprehensive cancer network, TAC = docetaxel, adriamycin, cyclophosphamide, TC = docetaxel cyclophosphamide.
YJ and NL worked equally to this study.
This study was supported by the Fujian Provincial Social Development Fund (#2014Y0035).
YJ, NL, SH, CL, NJ, and JZ participated in the marking and surgery, and collected the data. ZZ, JK performed the statistical analysis. YW conceived of the study and drafted the manuscript. All authors read and approved the final manuscript.
All authors declare no conflicts of interest.
REFERENCES
- 1.Schaefer FK, Eden I, Schaefer PJ, et al. Factors associated with one step surgery in case of non-palpable breast cancer. Eur J Radiol 2007; 64:426–431. [DOI] [PubMed] [Google Scholar]
- 2.Weber WN, Sickles EA, Callen PW, et al. Nonpalpable breast lesion localization: limited efficacy of sonography. Radiology 1985; 155:783–784. [DOI] [PubMed] [Google Scholar]
- 3.Khan S, Mitha N, Kazi F, et al. Needle localization of non-palpable breast lesions. J Pak Med Assoc 1996; 46:149–152. [PubMed] [Google Scholar]
- 4.Ueda T, Takano H, Suzuki M, et al. Dynamic helical CT-guided needle localization of non-palpable and mammographically occult breast lesions. Acta Radiol 2001; 42:383–385. [DOI] [PubMed] [Google Scholar]
- 5.Kubota K, Gomi N, Wakita T, et al. Magnetic resonance imaging of the metal clip in a breast: safety and its availability as a negative marker. Breast Cancer 2004; 11:55–59. [DOI] [PubMed] [Google Scholar]
- 6.Huang K, Luo D, Huang M, et al. Protection of parathyroid function using carbon nanoparticles during thyroid surgery. Otolaryngol Head Neck Surg 2013; 149:845–850. [DOI] [PubMed] [Google Scholar]
- 7.Bartels SA, van der Zaag ES, Dekker E, et al. The effect of colonoscopic tattooing on lymph node retrieval and sentinel lymph node mapping. Gastrointest Endosc 2012; 76:793–800. [DOI] [PubMed] [Google Scholar]
- 8.NCCN. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 2. In edition 2013. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 9.Bull JM, Tormey DC, Li SH, et al. A randomized comparative trial of adriamycin versus methotrexate in combination drug therapy. Cancer 1978; 41:1649–1657. [DOI] [PubMed] [Google Scholar]
- 10.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US oncology research trial 9735. J Clin Oncol 2009; 27:1177–1183. [DOI] [PubMed] [Google Scholar]
- 11.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005; 352:2302–2313. [DOI] [PubMed] [Google Scholar]
- 12.Rose MA, Olivotto I, Cady B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic results. Arch Surg 1989; 124:153–157. [DOI] [PubMed] [Google Scholar]
- 13.Burnside ES, Sohlich RE, Sickles EA. Movement of a biopsy-site marker clip after completion of stereotactic directional vacuum-assisted breast biopsy: case report. Radiology 2001; 221:504–507. [DOI] [PubMed] [Google Scholar]
- 14.Salhab M, Al Sarakbi W, Mokbel K. Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. Int Semin Surg Oncol 2005; 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasrinossadat A, Ladan F, Fereshte E, et al. Marking non-palpable breast masses with injected methylene blue dye, an easy, safe and low cost method for developing countries and resource-limited areas. Asian Pac J Cancer Prev 2011; 12:1189–1192. [PubMed] [Google Scholar]
- 16.Köhler J, Krause B, Grunwald S, et al. Ultrasound and mammography guided wire marking of non-palpable breast lesions: analysis of 741 cases. Ultraschall Med 2007; 28:283–290. [DOI] [PubMed] [Google Scholar]
- 17.De Roos MA, Welvaart WN, Ong KH. Should we abandon wire-guided localization for nonpalpable breast cancer? A plea for wire-guided localization. Scand J Surg 2013; 102:106–109. [DOI] [PubMed] [Google Scholar]
- 18.Ko K, Han BK, Jang KM, et al. The value of ultrasound-guided tattooing localization of nonpalpable breast lesions. Korean J Radiol 2007; 8:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge J, Yan B, Cao XC. Comparison of sentinel lymph node detection by methylene blue and carbon nanoparticle suspension injection in early breast cancer. Zhonghua Zhong Liu Za Zhi 2011; 33:226–228. [PubMed] [Google Scholar]
- 20.Wang W, Wang R, Wang Y, et al. Preoperative Colonic Lesion Localization with Charcoal Nanoparticle Tattooing for Laparoscopic Colorectal Surgery. J Biomed Nanotechnol 2013; 9:2123–2125. [DOI] [PubMed] [Google Scholar]
- 21.Thurfjell E, Hsieh CC, Lipworth L, et al. Breast size and mammographic pattern in relation to breast cancer risk. Eur J Cancer Prev 1996; 5:37–41. [PubMed] [Google Scholar]


