Abstract
There are few studies that have used audiometric testing to gauge the demographic characteristics and associated risk factors for hearing loss at the national-level. Here, we investigated the weighted prevalence and associated factors of hearing impairment in 16,040 Korean adult population. Subjects completed audiometric test and laboratory examination as part of the data from The 2010–2012 Korea National Health and Nutrition Examination Survey (KNHANES). In our respective study, the overall weighted (n = 33,762,584) prevalence of mild hearing impairment among the Korean adult population was 20.5% (95% clearance [CI], 19.6–21.6), whereas moderate-to-profound hearing impairment was 9.2% (95% CI, 8.6–9.9). The weighted prevalence of mild hearing impairment in younger adults (19–39 years’ old) was 4.4% (3.5–5.5), in middle-age adults (40–64 years), it was 21.1% (19.8–22.5), and in older adults (≥65 years’ old), it was 69.7% (67.8–71.6). Logistic regression analyses were performed for low/mid frequency or high-frequency mild hearing impairment with age, sex, tobacco use, heavy alcohol use, educational background, occupational noise exposure, obesity, hypertension, diabetes, total serum cholesterol, and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 as covariates. The analyses revealed independent correlations between increased age, tobacco use, education, hypertension, and eGFR <60 mL/min/1.73m2, and low/mid frequency and high frequency mild hearing impairment. High frequency mild hearing impairment was positively correlated with male sex, diabetes, and an increase in total serum cholesterol. Taken together, hearing impairment in Korea is highly prevalent with approximately one-fifth of Korean adult reporting mild hearing impairment. This study suggests that individuals with cardiovascular risk factors such as hypertension, diabetes, smoking, increased serum cholesterol, or decreased eGFR are at particular risk of developing hearing impairment. As such, these groups may benefit from hearing loss screening in addition to those groups typically considered to be of elevated risk including geriatrics, those of low socioeconomic status, and those with considerable occupational noise exposure.
INTRODUCTION
Hearing impairment is one of the most highly prevalent, chronic conditions after hypertension and arthritis.1 The prevalence of hearing impairment is increasing due to an ever-aging society and the growing use of personal listening devices.2 The number of individuals with impaired hearing more than doubled from 120 million to 275 million from 1995 to 2004.3 The World Health Organization (WHO) reported that 328 million adults and approximately one-third of people over 65 suffer from disabling hearing loss globally.4
There are many causes of hearing loss including a genetic predisposition, maternal rubella or complications at birth, aging, certain infectious diseases such as meningitis, chronic ear infections, use of ototoxic drugs, or exposure to excessive noise. Presbycusis, which is sensorineural hearing loss related to aging, is the most common cause of hearing loss, and is characterized by gradual, bilateral, high-frequency hearing loss.5 The risk of developing hearing impairment is enhanced in the male sex, and in those with less education, or a history of industrial or military service and occupational noise exposure.6,7
Hearing loss can limit meaningful communication and social connectivity8 leading to a lower health-related quality of life and decreased physical and cognitive function.9,10 In respect to public health, hearing impairment is known to be associated with depression, diabetes, and dementia.3,11,12
Despite this concern, there are few studies that have used audiometric testing to gauge the demographic characteristics and associated risk factors for hearing loss at the national-level.2,12–14
As such, we investigated the prevalence and associated risk factors of hearing impairment in the Korean adult population using the data from The 2011–2012 Korea National Health and Nutrition Examination Survey (KNHANES).
METHODS
Study Population and Data Collection
This study is based on data from the 2010–2012 KNHANES, a cross-sectional and nationally representative survey conducted by the Korean Center for Disease Control for Health Statistics. The KNHANES has been conducted periodically since 1998 to assess the health and nutritional status of the civilian, noninstitutionalized population of Korea. Participants were selected using proportional allocation-systemic sampling with multistage stratification. A standardized interview was conducted in the homes of the participants to collect information on demographic variables, family history, medical history, medications used, and a variety of other health-related variables. The Health Interview included an established questionnaire to determine the demographic and socioeconomic characteristics of the subjects including age, education level, occupation, income, marital status, smoking habit, alcohol consumption, exercise, previous and current diseases, and family disease history.
Subjects were asked whether they exercise with an intensity that leaves them sweating or with a slight difficulty in breathing. Subjects who exercised regularly and at a moderate intensity were asked about the frequency at which they exercised per week and the length of time per exercise session. Regular exercise was defined as exercising ≥5 times per week. Alcohol consumption was assessed by questioning the subjects about their drinking behavior during the month before the interview. Heavy alcohol use was categorized as drinking ≥4 times per week during the month before the interview. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications irrespective of blood pressure. Diabetes was defined by fasting plasma glucose (FPG) ≥7.0 mmol/L, current anti-diabetes medication, or a previous diagnosis of diabetes by a physician. Obesity was defined as body mass index (BMI) ≥25 kg/m2 according to the Asia-Pacific obesity classification.15
Height and weight were obtained using standardized techniques and equipment. Height was measured to the nearest 0.1 cm using a portable stadiometer (Seriter, Bismarck, ND, USA). Weight was measured to the nearest 0.1 kg using a Giant-150N calibrated balance-beam scale (Hana, Seoul, Korea). BMI was calculated by dividing weight by the square of their height (kg/m2). Systolic and diastolic blood pressure was measured by standard methods using a sphygmomanometer while the patient was seated. Three measurements were recorded for all subjects at 5-min intervals, and the average of the second and third measurements was used in the analysis.
Audiometric Measure
In the 2011–2012 KNHANES, the audiometric examination was administered to adults, aged 19 years or older (n = 16,799). Air-conduction pure-tone thresholds were obtained in a soundproof booth using an automatic audiometer (GSI SA-203; Entomed Diagnostics AB, Lena Nodin, Sweden). Trained otolaryngologists collected data independently for each ear at 6 frequencies: 0.5, 1.0, 2.0, 3.0, 4.0, and 6.0 kHz. All audiometric testing was performed under the supervision of an otolaryngologist. To obtain reliable results from the survey, the Epidemiologic Survey Committee of the Korean Society of Otorhinolaryngology-Head and neck surgery carried out the quality control of the survey, which was conducted by periodic education of participating otolaryngologists.
We determined hearing impairment for 2 categories of frequency (low/mid, high) and severity (mild, moderate-to-profound). To produce low/mid frequency pure tone means, we averaged pure tone thresholds measured at 0.5, 1.0, and 2.0 kHz for each ear. To produce high-frequency pure tone means, we averaged pure tone thresholds measured at 3.0, 4.0, and 6.0 kHz for each ear.
Mild-hearing impairment was defined as an unaided pure-tone hearing threshold level for the superior ear of 26 to 40 decibels (Db), and average hearing threshold levels (HLs) for the frequencies of 0.5, 1.0, 2.0, 3.0, 4.0, and 6.0 kHz. Moderate-to-profound hearing impairment was defined as unaided pure-tone hearing threshold level for the superior ear of 40 Db or greater, and HLs for the frequencies of 0.5, 1.0, 2.0, 3.0, 4.0, and 6.0 kHz.
Laboratory Methods
Blood samples were collected in the morning after fasting for at least 8 h. Total cholesterol, FPG, triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, and serum creatinine levels were measured by Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated equation from the Modification of Diet in Renal Disease study: eGFR (mL/min/1.73 m2) = 175 × (SCr/88.4, μmol/l)−1.154 × Age−0.203 × 0.742 (if female).16 HbA1c was measured using a high-performance liquid chromatography method (HLC-723G7, Tosoh, Tokyo, Japan). Detailed methods for comparing and validating the reliability of each survey are described elsewhere.17
Ethics Statement
This study was approved by the institutional review board of Ilsan Paik Hospital, Republic of Korea (IB-1410–043). After approval of the study proposal, the KNHANES dataset was made available at the request of the investigator. Because the dataset did not include any personal information and participants’ consent had already been given for the KNHANES, our study was exempted from participant consent.
Statistical Analyses
The KNHANES participants were not randomly sampled. The survey was designed using a complex, stratified, multistage probability-sampling model; thus, individual participants were not equally representative of the Korean population. To obtain representative prevalence rates from the dataset, it was necessary to consider the power of each participant (sample weight) as representative of the Korean population. Following approval from the Korea Centers for Disease Control and Prevention, we received a survey dataset that included information regarding the survey location, strata by age, sex, and various other factors, and the sample weight for each participant. The survey sample weights, which were calculated by taking into account the sampling rate, response rate, and age/sex proportions of the reference population (2005 Korean National Census Registry), were used in all of the analyses to provide representative estimates of the noninstitutionalized Korean civilian population.
Statistical analyses were performed using SPSS software (ver. 21.0 for Windows; SPSS, Chicago, IL, USA). To compare the weighted prevalence of hearing impairment among groups according to age and sex, the chi-square test was performed. We compared age- and sex-adjusted clinical characteristics by the presence of hearing impairment using an analysis of covariance (ANCOVA). A logistic regression analysis was used to evaluate the odds ratios for hearing impairment as covariates with age, sex, tobacco use, heavy alcohol use, education level, occupational noise exposure, obesity, hypertension, diabetes, total serum cholesterol, and eGFR <60 mL/min/1.73 m2. All of the tests were 2-sided, and P values <0.05 were considered statistical significance.
RESULTS
Demographics and Clinical Characteristics of the Study Population
The weighted demographics and clinical characteristics of study population are presented in Table 1.
TABLE 1.
Weighted Clinical Characteristics of Study Population (Unweighted n = 16,040; Weighted, n = 33,762,584)
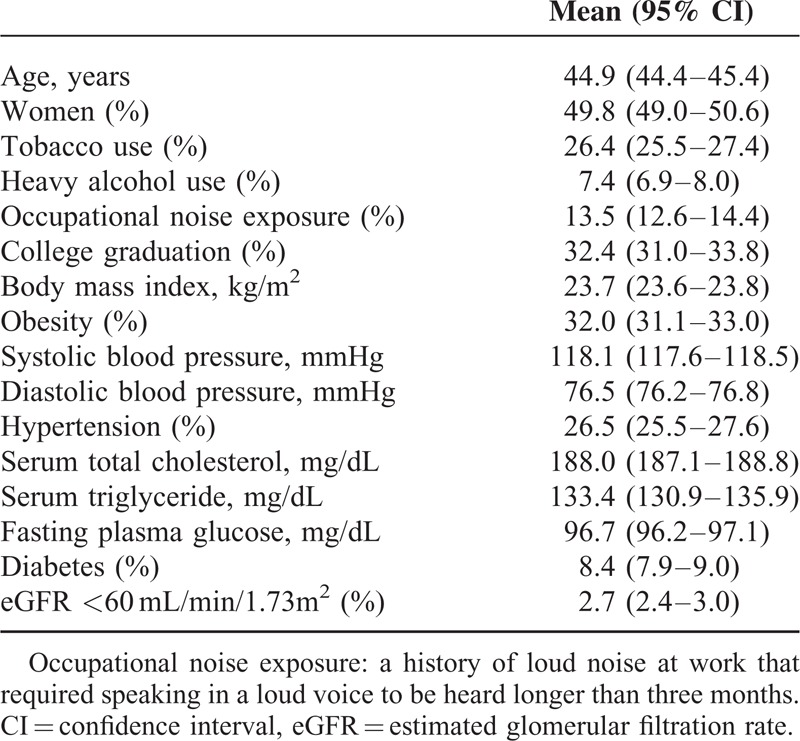
Among 19,599 adults (≥19 years) who participated in the 2010–2012 KNHANES, a total of 16,040 subjects completed audiometric test and laboratory examination and were included in this analysis. The average age of participants was 44.9 years, and the percentage of women was 49.8 %. The prevalence of hypertension was 26.5 %, and 8.4 % of participants were diabetic. Occupational noise exposure and current tobacco use were reported in 13.5% and 26.4 % of the participants, respectively.
Prevalence of Hearing Impairment in the Korean Population by Age and Sex
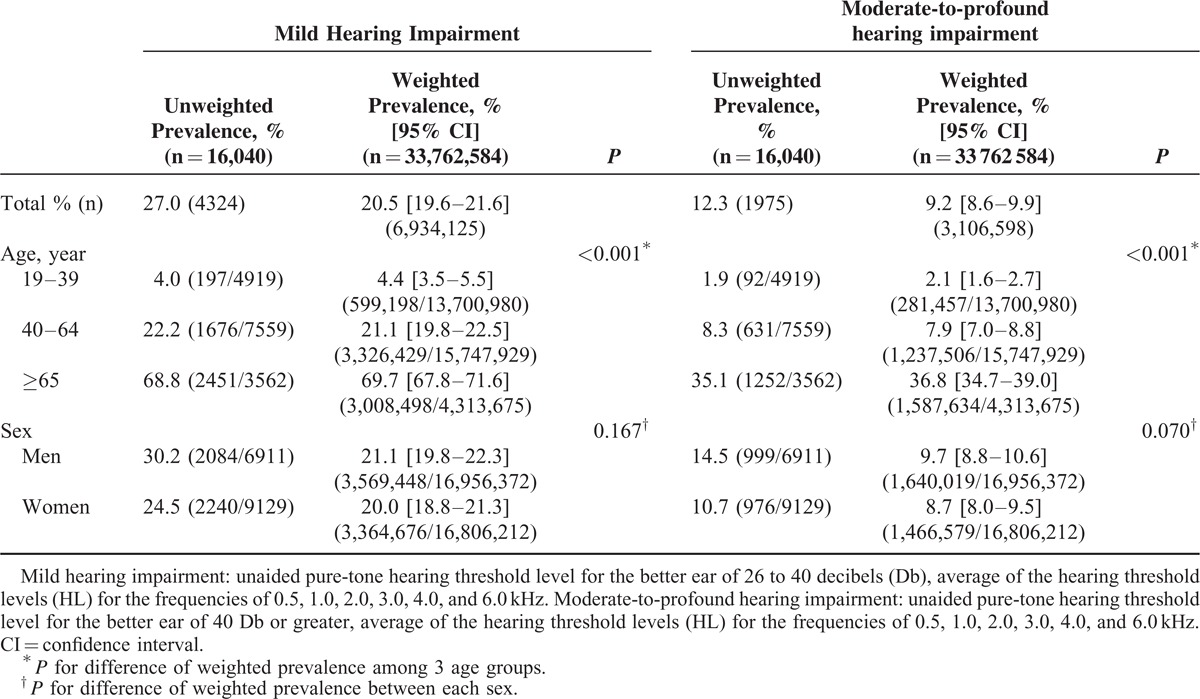
Overall, the weighted prevalence of mild and moderate-to-profound hearing impairment among the Korean population was 20.5% (19.6–21.6) (n = 6,934,125/33,762,584) and 9.2% (8.6–9.9) (n = 3,106,598/33,762,584), respectively. The prevalence of mild and moderate-to-profound hearing impairment increased significantly with age. Among adults older than 65 years, the weighted prevalence of mild and moderate-to-profound hearing impairment was 69.7% (67.8–71.6) (n = 3,008,498/4,313,675) and 36.8 % (34.7–39) (n = 1,587,634/4,313,675), respectively (Table 2).
TABLE 2.
Estimated Prevalence of Hearing Impairment in the Korean Adult Population, by Age and Sex
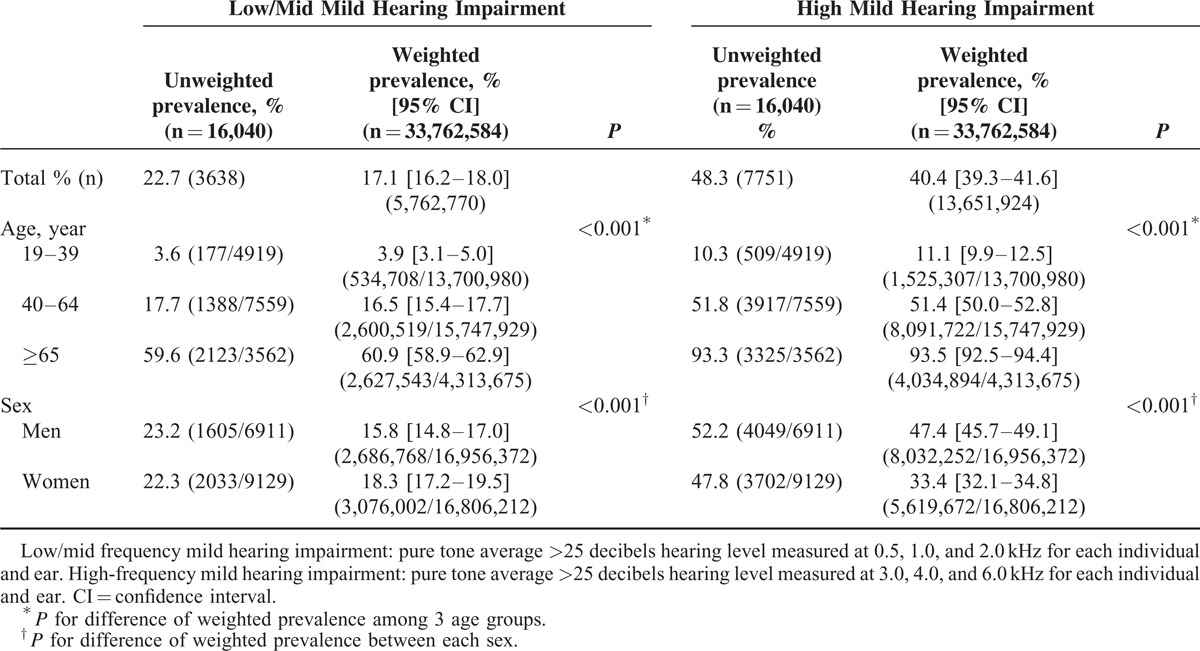
According to the frequency, the weighted prevalence of low/mid frequency and high frequency mild hearing impairment was 17.1% (16.2–18.0) (n = 5,762,770/33,762,584) and 40.4 % (39.3–41.6) (n = 13,651,924/33,762,584), respectively, and increased with age. With regards to low/mid frequency mild hearing impairment, women were more likely to be affected compared with men (18.3% [17.2–19.5] vs 15.8% [14.8–17.0], P < 0.001). In contrast, men demonstrated a higher percentage of high-frequency mild hearing impairments compared with women (47.4% [45.7–49.1] vs 33.4% [32.1–34.8], P < 0.001) (Table 3).
TABLE 3.
Estimated Prevalence of Mild Hearing Impairment (Low/Mid Frequency or High Frequency) in the Korean Adult Population, by Age and Sex
Age- and Sex-adjusted Clinical Characteristics by the Presence of Mild Hearing Impairment
Data for age- and sex-adjusted clinical characteristics in the presence of mild-hearing impairment are presented in Table 4.
TABLE 4.
Age- and Sex-adjusted Clinical Characteristics by the Presence of Mild Hearing Impairment
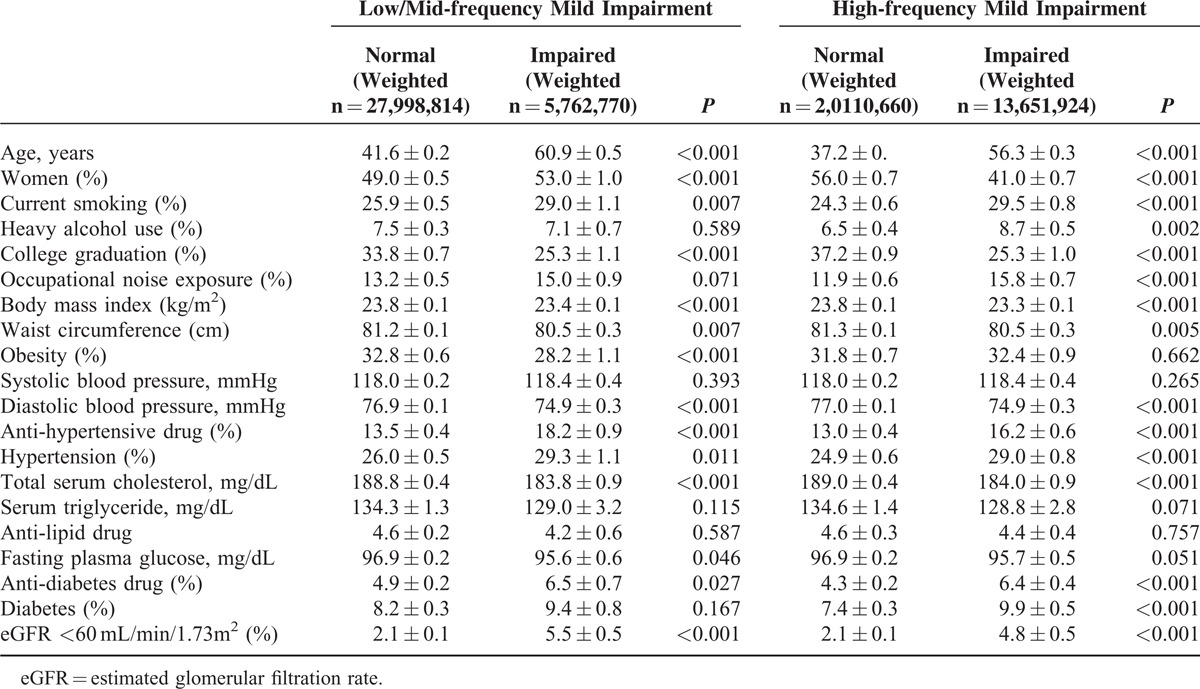
Subjects with mild hearing impairment demonstrated a greater percentage of tobacco use, hypertension, eGFR <60 mL/min/1.73 m2, and anti-diabetes drug use compared with subjects without hearing impairment for both low/mid and high frequencies. However, the level of total serum cholesterol, waist circumference, BMI, and percentage of college graduates were lower in adults with mild hearing impairment compared with adults without hearing impairment.
Heavy alcohol drinking (8.7% vs 6.5%, P = 0.002), occupational noise exposure (15.8% vs 11.9%, P < 0.001), and diabetes (9.9% vs 7.4%, P < 0.001) were more common for high-frequency hearing impairment, but not for low/mid frequency impairment. On the contrary, the percentage of obese subjects was lower in low/mid frequency mild impairment (28.2% vs 32.8%, P < 0.001), and not in high-frequency impairment.
Factors Associated With Hearing Impairment at Low/Mid and High Frequency
In the logistic regression analysis for mild hearing impairment, age, current smoking, education level, occupational noise exposure, hypertension, and eGFR <60 mL/min/1.73 m2 were associated with low/mid and high frequency mild hearing impairment (Table 5).
TABLE 5.
Logistic Regression Analyses for Hearing Impairment
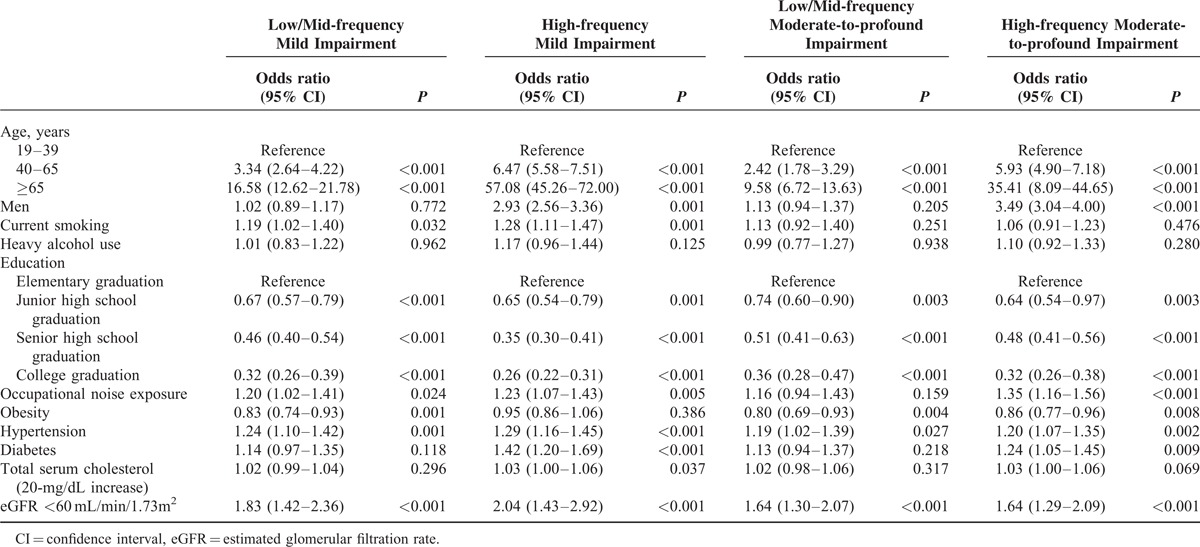
Subject age of 40 to 64 years (low/mid frequency: odds ratio [OR] 3.34, 95% confidence interval [CI] 2.64–4.22, P < 0.001; high frequency: OR 6.47, 95% CI 5.57–5.71, P < 0.001) and ages >65 years (low/mid frequency: OR 16.58, 95% CI 12.62–21.78, P < 0.001; high frequency: OR 57.08, 95% CI 45.26–72.00, P < 0.001) were correlated with mild hearing impairment, respectively. Subjects aged 19 to 39 years were used as a control group.
Smoking tobacco (low/mid-frequency: OR 1.19, 95% CI 1.02–1.40, P = 0.032; high-frequency: OR 1.28, 95% CI 1.11–1.47, P = 0.001) and occupational noise exposure (low/mid-frequency: OR 1.20, 95% CI 1.02–1.41, P = 0.024; high-frequency: OR 1.23, 95% CI 1.07–1.43, P = 0.005) both increased the likelihood of a hearing impairment.
Educational level was inversely correlated with the prevalence of hearing loss. People who had only completed elementary graduation were about 3 times more likely to have experience hearing loss than those with a college education (low/mid-frequency: OR 0.32, 95% CI 0.26–0.39, P < 0.001) and 2 times more likely compared with those with a senior high school graduation (low/mid-frequency: OR 0.46, 95% CI 0.40–0.54, P < 0.001).
Hypertension (low/mid frequency: OR 1.24, 95% CI 1.10–1.42, P = 0.001; high-frequency: OR 1.29, 95%CI 1.16–1.45, P < 0.001) and eGFR <60 mL/min/1.73 m2 (low/mid frequency: OR 1.83, 95% CI 1.42–2.36, P < 0.001; high-frequency: OR 2.04, 95% CI 1.43–2.92, P < 0.001) also increased the risk of hearing impairment. Being male and having diabetes also increased the risk of developing a hearing impairment at high-frequency hearing impairment, but not at low/mid frequencies.
We obtained similar results for moderate-to-profound hearing impairment. Age, level of education, obesity, hypertension, and eGFR <60 mL/min/1.73 m2 were associated with low/mid and high-frequency hearing impairment. Being male, occupational noise exposure, and diabetes each increased the likelihood of having a high frequency hearing impairment. In contrast with mild hearing impairments, smoking did not increase the risk of moderate-to-profound hearing impairment.
DISCUSSION
Using data from the KNHANES 2010–2012 study, we demonstrate in our present study that the weighted prevalence of hearing impairments in the Korean population aged 19 years or older is 20.5%. This prevalence is similar to that previously reported for national estimates derived from adults of all ages, despite some potential differences in the method used to assess hearing loss. The prevalence of hearing impairment in a representative adult population in southern Taiwan was previously reported to be 21.4%.18 In Norway,19 the United Kingdom,20 and Australia,21 the proportion of the population with hearing impairment is reported to be 27%, 35%, and 22%, respectively. Based on data from US NHANES (1999–2004) study, the prevalence of hearing impairment within adult US Population was 16.1%, which was lower than that might have been expected.14 However, data from a recent US NHANES (2005–2010) study demonstrated an estimated prevalence of self-reported hearing impairment of 21.7%.12
Among adults older than 65 years, the weighted prevalence of hearing impairment increases to 69.7 %. In previous studies that reported the prevalence of hearing impairment in older adults, values ranged from 30% for people aged 65 to 74 years (US NHANES conducted in the 1970s)22 to 49% for adults aged 60 to 69 years (US NHANES 1999–2004).2 According to the most recent study data (US NHANES 2005–2006), the prevalence of hearing loss for adults aged 70 years and older is 63%.23
In the present study, we uncovered a frequency-specific difference between men and women in weighted prevalence of hearing impairment. At high frequency, mild hearing impairment was more common in men, which corresponds with previous results.1,2,24 In contrast, women were more likely to be affected by low/mid-frequency hearing impairment than men. It is likely that men have a tendency to experience more occupational noise exposure, which typically affects hearing impairment at a higher frequency. However, being male was still independently associated with high-frequency mild hearing impairment, even after logistic regression. Therefore, further evaluation will be needed to understand why men are more vulnerable to mild hearing impairment at high frequency.
We also explored the effects of lifestyle parameters, including smoking, heavy alcohol use, and occupational noise exposure, education level, cardiovascular risks such as hypertension, total serum cholesterol, serum TG, diabetes, and decreased eGFR as well as age and sex on hearing impairment.
Our data indicate that current smokers are significantly more likely to have hearing impairment compared with nonsmokers. Several studies have reported an association between smoking and hearing loss.24–26 A meta-analysis study showed that the overall risk ratios were 1.33 (95% CI, 1.24–1.44) for 5 cross-sectional studies, 1.97 (95% CI, 1.44–2.70) for 4 cohort studies, and 2.89 (95% CI, 2.26–3.70) for 1 case-control study.27 The mechanism responsible for the effect of smoking on hearing loss is unclear; however, some studies have suggested that direct ototoxic effects of nicotine or reactive oxygen species could induce necrotic and apoptotic hair cell death.28,29,30 Indeed, recent studies have reported that antioxidant treatment reduced cochlear damage and hearing loss.31,32
Occupational noise exposure is known to contribute up to 37% of all adult causes of hearing loss, and remains a significant contributor to employment-related morbidity internationally.33 Professions associated with an increase in noise exposure include mining, the armed forces, manufacturing, construction work, farming, piloting, engineering, and working in night club.33 Our analysis showed that occupational noise exposure was significantly associated with mild hearing impairment across the frequency spectrum. However, in the case of moderate-to-profound hearing impairment, occupational noise exposure increased the likelihood of having an impairment in the high frequency range only. Ahmed et al34suggested that noise might initially induce damage at frequencies >8 kHz and hence high frequency audiometry may be useful as an early indicator of hearing loss.
Although a person with a lower level of education (eg, elementary graduation) might be more likely to work in a poor-quality environment with more noise exposure, such as in the laboring or manufacturing fields, a reverse association between the education level and hearing loss was observed across the frequency spectrum following logistic regression analysis. People with a higher level of education may have enhanced access to health care or may be better nourished or resilient to the biological effects of stress and infection.35,36
We also identified diabetes as a factor that increases the risk of high-frequency hearing impairment. Recent meta-analysis showed that overall pooled OR of hearing impairment for diabetic participants was 2.15 (1.72–2.68) compared with nondiabetic participants.3 Another meta-analysis identified type 2 diabetic patients as experiencing a significantly greater incidence of mild degree of hearing loss compared with controls.37 Mean pure tone audiometry thresholds were greater in diabetics for all frequencies, but were more clinically relevant at 6000 and 8000 Hz, or high frequency, which is analogous to our study. Cochlear microangiopathy, degeneration of the stria vascularis, and loss of cochlear outer hair cells have been suggested to cause hearing loss in patients with type 2 diabetes mellitus.38
There have been a few studies showing a correlation between cardiovascular risk factors, including hypertension, hypercholesterolemia, and hypertriglycemia, and an increased risk of developing hearing impairment. These studies reported that hypercholesterolemia was associated with age-related hearing-loss, possibly by a mechanism involving atherosclerosis.39 In contrast, another study demonstrated that raised total fasting cholesterol was associated with significantly better hearing threshold levels.40 Chang et al41 found that individuals with hypertriglycemia are at a greater risk for noise-induced hearing loss. A large-scale study to investigate the relationship between hearing loss and cardiovascular risk factors was performed by Gates et al42 In their study, hypertension and systolic blood pressure were linked to hearing thresholds in both men and women. High-density lipoprotein levels were inversely related to low-frequency hearing thresholds in women only. No relationship was observed between hearing impairment and total cholesterol, LDL-C, or TG levels. In this study, hypertension was associated with low/mid and high-frequency mild hearing impairment. However, neither hypercholesterolemia nor hypertriglycemia had any significant effect on hearing impairment.
Decreased renal function was also associated with low/mid and high-frequency hearing impairment in this study. Indeed, a few studies have observed a high incidence of hearing loss (up to 78%) among patients with chronic renal failure.43,44 The cochlea and kidney have similar physiological mechanisms, namely, the active transport of fluid and electrolytes by the stria vascularis and glomerulus, respectively.45 Several etiological factors seem to have cumulative effects on the deterioration of hearing in renal failure, including electrolyte disturbances, proteinuria, and hypertension, even after exclusion of known risk factors, such as noise exposure, ototoxic drugs, and head injuries.46
The major strength of our study is the large, nationally representative sample of adult Koreans analyzed. To the best of our knowledge, there are few other studies describing a national-level assessment of the demographic characteristics and associated risk factors for hearing loss using audiometric testing. Nevertheless, our study had some limitations. Although we adjusted for many confounding factors, residual or hidden confounding variables cannot be excluded, similar to other cross-sectional studies. We also cannot draw an inference as to causality due to the cross-sectional design of the study.
In conclusion, hearing impairment in Korea is extremely prevalent, and about one-fifth of Korean adults experience mild hearing impairment. This study suggests that individuals with cardiovascular risk factors, such as smoking, hypertension, diabetes, increased total serum cholesterol, and decreased eGFR are particularly at risk of hearing impairment, and may benefit from hearing loss screening. These risk factors are exacerbated in groups like those of advanced age, low socioeconomic status, and/or occupational noise exposure. An improved understanding of the etiologic risk factors related to hearing impairment can result in significant public health benefits.
Acknowledgments
Nothing to disclose.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, eGFR = Estimated glomerular filtration rate, FPG = fasting plasma glucose, KNHANES = Korea National Health and Nutrition Examination Survey, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, TG = triglyceride.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am J Epidemiol 1998; 148:879–886. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999-2004. Arch Intern Med 2008; 168:1522–1530. [DOI] [PubMed] [Google Scholar]
- 3.Horikawa C, Kodama S, Tanaka S, et al. Diabetes and risk of hearing impairment in adults: a meta-analysis. J Clin Endocrinol Metab 2013; 98:51–58. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Deafness and hearing loss. 2014; http://www.who.int/mediacentre/factsheets/fs300/en/#. [Google Scholar]
- 5.Yueh B, Shapiro N, MacLean CH, et al. Screening and management of adult hearing loss in primary care: scientific review. JAMA 2003; 289:1976–1985. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg 2003; 129:1041–1046. [DOI] [PubMed] [Google Scholar]
- 7.Muhr P, Mansson B, Hellstrom PA. A study of hearing changes among military conscripts in the Swedish Army. Int J Audiol 2006; 45:247–251. [DOI] [PubMed] [Google Scholar]
- 8.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014; 150:378–384. [DOI] [PubMed] [Google Scholar]
- 9.Dalton DS, Cruickshanks KJ, Klein BE, et al. The impact of hearing loss on quality of life in older adults. Gerontologist 2003; 43:661–668. [DOI] [PubMed] [Google Scholar]
- 10.Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA 1989; 261:1916–1919. [PubMed] [Google Scholar]
- 11.Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol 2011; 68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Zhang X, Hoffman HJ, et al. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005-2010. JAMA Otolaryngol Head Neck Surg 2014; 140:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge KE, Hoffman HJ, Cowie C. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med 2008; 149:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol 2009; 30:139–145. [DOI] [PubMed] [Google Scholar]
- 15.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 2004; 363:157–163. [DOI] [PubMed] [Google Scholar]
- 16.Lamb EJ, Tomson CR, Roderick PJ. Estimating kidney function in adults using formulae. Ann Clin Biochem 2005; 42 (Pt 5):321–345. [DOI] [PubMed] [Google Scholar]
- 17.Korea Centers for Disease Control and Prevention (KCDC). Korea National Health and Nutrition Examination Survey. Available from http://knhanes.cdc.go.kr Accessed June 27, 2013. [Google Scholar]
- 18.Lin C, Yang Y, Guo YL, et al. Prevalence of hearing impairment in an adult population in Southern Taiwan. Int J Audiol 2007; 46:732–737. [DOI] [PubMed] [Google Scholar]
- 19.Borchgrevink HM, Tambs K, Hoffman HJ. The Nord-Trøndelag Norway Audiometric Survey 1996-98: unscreened thresholds and prevalence of hearing impairment for adults > 20 years. Noise Health 2005; 7:1–15. [DOI] [PubMed] [Google Scholar]
- 20.Davis AC. The prevalence of hearing impairment and reported hearing disability among adults in Great Britain. Int J Epidemiol 1989; 18:911–917. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DH, Walsh PG, Sanchez L, et al. The epidemiology of hearing impairment in an Australian adult population. Int J Epidemiol 1999; 28:247–252. [DOI] [PubMed] [Google Scholar]
- 22.Ries PW. Hearing ability of persons by sociodemographic and health characteristics: United States. Vital Health Stat 10 1982; 10:1–60. [PubMed] [Google Scholar]
- 23.Lin FR, Thorpe R, Gordon Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011; 66:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruickshanks KJ, Klein R, Klein BE, et al. Cigarette smoking and hearing loss: the epidemiology of hearing loss study. JAMA 1998; 279:1715–1719. [DOI] [PubMed] [Google Scholar]
- 25.Sung JH, Sim CS, Lee C, et al. Relationship of cigarette smoking and hearing loss in workers exposed to occupational noise. Ann Occup Environ Med 2013; 25:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao L, Davis R, Heyer N, et al. Effect of cigarette smoking on noise-induced hearing loss in workers exposed to occupational noise in China. Noise Health 2013; 15:67–72. [DOI] [PubMed] [Google Scholar]
- 27.Nomura K, Nakao M, Morimoto T. Effect of smoking on hearing loss: quality assessment and meta-analysis. Prev Med 2005; 40:138–144. [DOI] [PubMed] [Google Scholar]
- 28.Henderson D, Bielefeld EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006; 27:1–19. [DOI] [PubMed] [Google Scholar]
- 29.Guth PS, Norris CH. The hair cell acetylcholine receptors: a synthesis. Hear Res 1996; 98:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Le Prell CG, Yamashita D, Minami SB, et al. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res 2007; 226:22–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heman Ackah SE, Juhn SK, Huang TC, et al. A combination antioxidant therapy prevents age-related hearing loss in C57BL/6 mice. Otolaryngol Head Neck Surg 2010; 143:429–434. [DOI] [PubMed] [Google Scholar]
- 32.Ewert DL, Lu J, Li W, et al. Antioxidant treatment reduces blast-induced cochlear damage and hearing loss. Hear Res 2012; 285:29–39. [DOI] [PubMed] [Google Scholar]
- 33.Kurmis AP, Apps SA. Occupationally-acquired noise-induced hearing loss: a senseless workplace hazard. Int J Occup Med Environ Health 2007; 20:127–136. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed HO, Dennis JH, Badran O, et al. High-frequency (10-18 kHz) hearing thresholds: reliability, and effects of age and occupational noise exposure. Occup Med (Chic Ill) 2001; 51:245–258. [DOI] [PubMed] [Google Scholar]
- 35.Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation,;1; noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res 2010; 264:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan W, Cruickshanks KJ, Klein BE, et al. Modifiable determinants of hearing impairment in adults. Prev Med 2011; 53:338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinpelu OV, Mujica Mota M, Daniel SJ. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope 2014; 124:767–776. [DOI] [PubMed] [Google Scholar]
- 38.Fukushima H, Cureoglu S, Schachern PA, et al. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg 2006; 132:934–938. [DOI] [PubMed] [Google Scholar]
- 39.Martin Villares C, San Roman Carbajo J, Dominguez Calvo J, et al. Lipid profile and hearing-loss aged-related. Nutr Hosp 2005; 20:52–57. [PubMed] [Google Scholar]
- 40.Jones NS, Davis A. A retrospective case-controlled study of 1490 consecutive patients presenting to a neuro-otology clinic to examine the relationship between blood lipid levels and sensorineural hearing loss. Clin Otolaryngol Allied Sci 2000; 25:511–517. [DOI] [PubMed] [Google Scholar]
- 41.Chang NC, Yu ML, Ho KY, et al. Hyperlipidemia in noise-induced hearing loss. Otolaryngol Head Neck Surg 2007; 137:603–606. [DOI] [PubMed] [Google Scholar]
- 42.Gates GA, Cobb JL, D’Agostino RB, et al. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg 1993; 119:156–161. [DOI] [PubMed] [Google Scholar]
- 43.Pandey S, Gore G, Valame D, et al. Audiometric profile in patients with chronic renal failure. J Otolaryngol Head Neck Surg 2011; 40:131–136. [PubMed] [Google Scholar]
- 44.Bazzi C, Venturini CT, Pagani C, et al. Hearing loss in short- and long-term haemodialysed patients. Nephrol Dial Transplant 1995; 10:1865–1868. [PubMed] [Google Scholar]
- 45.Arnold W. Inner ear and renal diseases. Ann Otol Rhinol Laryngol Suppl 1984; 112:119–124. [DOI] [PubMed] [Google Scholar]
- 46.Meena RS, Aseri Y, Singh BK, et al. Hearing loss in patients of chronic renal failure: a study of 100 cases. Indian J Otolaryngol Head Neck Surg 2012; 64:356–359. [DOI] [PMC free article] [PubMed] [Google Scholar]


