Abstract
Various studies have shown that irritable bowel syndrome (IBS) is highly associated with other pathologies, including fibromyalgia (FM). The objective of this study was to analyze the differences among risk factors associated with IBS following FM in a nationwide prospective cohort study.
We propose that a relationship exists between FM and IBS. This article presents evidence obtained from a cohort study in which we used data from the Taiwan National Health Insurance Research Database to clarify the relationship between FM and IBS. The follow-up period ran from the start of FM diagnosis to the date of the IBS event, censoring, or December 31, 2011. We analyzed the risk of IBS using Cox proportional hazard regression models, including sex, age, and comorbidities.
During the follow-up period, from 2000 to 2011, the overall incidence of IBS was higher in FM patients than in non-FM patients (7.47 vs 4.42 per 1000 person-years), with a crude hazard ratio = 1.69 (95% confidence interval [CI] 1.45–1.63). After adjustment for age, sex, and comorbidities, FM was associated with a 1.54-fold increased risk for IBS.
Mutually risk factors may influence the relationship between FM and IBS. We recommend that physiologists conduct annual examinations of FM patients to reduce the incidence of IBS progression.
INTRODUCTION
Irritable bowel syndrome (IBS) is a common functional disorder of the gastrointestinal tract that is characterized by chronic abdominal pain or discomfort.1 IBS pathogenesis can involve an altered intestinal motility,2 visceral hypersensitivity,3 altered compliance of the gut wall,4 psychological disturbance,5 or any combination of these conditions. Although the etiology of IBS remains unclear, stressors such as life events and chronic stress, as well as a patient's individual and genetic background, seem to affect intestinal disease activity through complex psychoimmunological mechanisms on both systemic and gut mucosal levels.6
Fibromyalgia (FM) is diagnosed with numerous symptoms of the disease, characterized by widespread musculoskeletal pain accompanied by fatigue,7 sleep disturbance,8 and other symptoms that persist for over 3 months. Female patients are substantially more likely to develop FM than male patients. Currently, effective treatments for FM and IBS are still lacking; only a variety of medications are available for controlling the symptoms.9
The incidence rate of IBS in the general population of Taiwan is ∼10% to 20%, which is lower than that of Western countries.10 The incidence rate of FM in Taiwan's general population is ∼2% to 4%.11 These 2 rare diseases can result from diverse clinical outcomes in patients with multiple chronic conditions, including pain,7 mental health problems,6,12 and hormonal disorders.13,14 Determining whether people with FM have a higher risk for IBS and whether FM and IBS share similar pathophysiological mechanisms is crucial. We conducted a study by using the National Health Insurance Research Database (NHIRD) to examine the risk for IBS, compared with patients with FM and those without FM.
Most of the patients studied were diagnosed with depression and other neuropathological problems and had used specific medications according to their symptoms.1,15 The efficacy of the antidepression drugs16–20 and other drugs used by patients with FM and patients without FM remains undetermined. We examined the risk for IBS associated with tramadol, antidepression drugs (such as amitriptyline, fluoxetine, duloxetine, milnacipran, and moclobemide), tropisetron, pramipexole, and pregabalin.
MATERIALS AND METHODS
Study Design
We constructed a nationwide prospective cohort study, which was based on the healthy insurance claims data of 1 million beneficiaries randomly sampled from the national health insurance system of Taiwan. All personal identification data were encrypted from data released from National Health Research Institutes. We have obtained the Institutional Review Board of China Medical University Hospital's approval (CMU-REC-101-012). Previous articles have already introduced the information regarding NHIRD and verified the diagnostic data accuracy and validity.
Study Population
We used the diagnostic codes of the International Classification of Diseases, Ninth Revision (ICD-9) to identify IBS (ICD-9-CM Code 564.1) and FM (ICD-9-CM Code 729.1) patients, excluding those whose IBS was at the baseline and those for whom information on sex or age was missing. Patients who were diagnosed with FM for at least 3 months and were >20 years were included in the study. We identified 33,729 patients with newly diagnosed FM and 134,915 patients without FM between 2000 and 2011 from the claims data of ambulatory registry and general patients. The IBS diagnosis date was defined as the index date. Each FM patient was randomly frequency-matched with 4 study patients according to age (every 5 years), sex, and index year. The cases were monitored until diagnosis of IBS or until the patients were censored, which followed reasons such as loss to follow-up, withdrawal from the insurance system, or the end of 2011.
Variables of Comorbidity and Medication Prescription
Baseline comorbidity history of each patient was examined, and the following diseases were considered potential confounding factors in the inferring of association between FM and IBS: chronic liver disease and cirrhosis (ICD-9-CM Codes 571), chronic kidney disease (ICD-9-CM Codes 585), depression (ICD-9-CM Codes 296.2–296.3, 300.4, 311), anxiety (ICD-9-CM Code 300.00), and sleep disorder (ICD-9-CM Codes 307.4 and 780.5). The medications used were tramadol, antidepression drugs (including amitriptyline, fluoxetine, duloxetine, milnacipran, and moclobemide), tropisetron, and pramipexole.
Statistical Analysis
We demonstrated the difference of distributions of age, sex, and comorbidities between the FM and non-FM groups by conducting the χ2 test. The IBS incidence rates were calculated from the time of follow-up until December 31, 2011, the diagnostic date of IBS, loss to follow-up, or death. We employed the Kaplan–Meier method to plot the cumulative incidence curves of IBS, which were stratified by FM and non-FM groups, and examined whether these curves differed statistically between the FM and non-FM groups using log-rank test. Cox proportional hazard regression analysis was conducted to measure the risk of patients with FM relative to that of patients without FM. The hazard ratio (HR) and 95% confidence intervals (CIs) were also calculated in the model. All of the statistical analyses were performed by using the SAS statistical package (version 9.4 for Windows; SAS Institute, Inc, Cary, NC). The P values <0.05 were shown to have significant difference, and survival curves were graphed using R program (version 2.14.1 for Windows, R Development CT, Vienna, Austria).
RESULTS
FM-Related IBS Risk
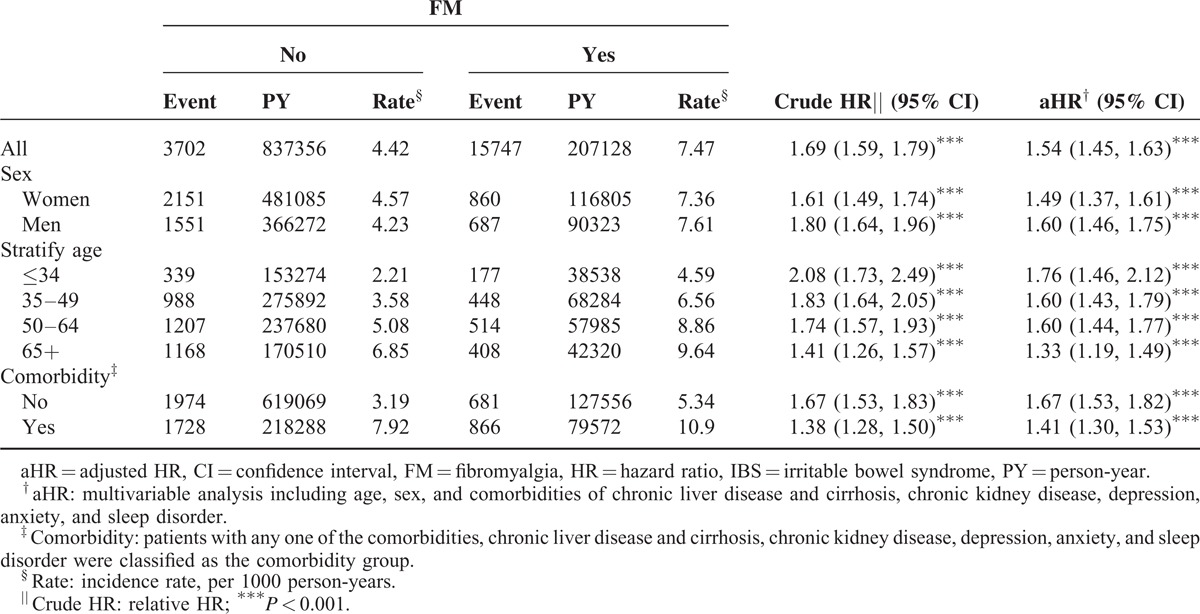
We analyzed the demographic factors and characteristics of the patients in the FM and non-FM groups (Table 1). A significant difference between the FM and non-FM cohorts emerged among comorbidities, including chronic liver disease and cirrhosis, chronic kidney disease, depression, anxiety, and sleep disorder (Table 1). In Table 2, the overall incidence rate of IBS in non-FM and FM cohorts were 4.42 and 7.47 per 1000 person-years. The incidence rate of IBS in men and women were present: 4.23 and 4.57 per 1000 person-years in non-FM cohort, 7.61 and 7.36 per 1000 person-years in FM cohort. With increase in age, the incidence rates were shown 2.21 to 6.85 per 1000 person-years in non-FM cohort and 4.59 to 9.64 per 1000 person-years in FM cohort. The overall crude HR for IBS was 1.69 (95% CI 1.59–1.79), achieving significance, and the adjusted HR (aHR) was 1.54 (95% CI 1.45–1.63) after we adjusted for age, sex, and comorbidities. An elevated significance in the aHR for IBS was observed in the FM when compared with non-FM groups (Table 2). The crude HRs for IBS in women and men were 1.80 (95% CI 1.64–1.96) and 1.61 (95% CI 1.49–1.74), respectively. After we adjusted for age and comorbidities, the aHRs for IBS in men and women were 1.49 (95% CI 1.37–1.61) and 1.60 (95% CI 1.46–1.75), respectively (Table 2). Overall, all age groups had a significantly high risk for IBS in both cohorts; however, those aged ≤34 years, 35 to 49 years, 50 to 64 years, and ≥65 years had 1.41-fold to 2.08-fold increased risks for IBS. After we adjusted for sex and comorbidities, the aHRs indicated that patients aged ≤34 years, 35 to 49 years, 50 to 64 years, and ≥65 years had 1.76-fold (95% CI 1.46–2.12), 1.60-fold (95% CI 1.43–1.79), 1.60-fold (95% CI 1.44–1.77), and 1.33-fold (95% CI 1.19–1.49) increased risks for IBS (Table 2). In patients without comorbidities, a significantly high risk for IBS (1.67, 95% CI 1.53–1.83) emerged and remained high even after we adjusted for age and sex (Table 2). Patients with comorbidities had a significantly high crude HR (1.38, 95% CI 1.28–1.50), and after we adjusted for age and sex, the aHR (1.41, 95% CI 1.30–1.53) remained significant for both the FM and non-FM groups.
TABLE 1.
Comparisons in Demographic Characteristics and Comorbidities in Patient With and Without FM
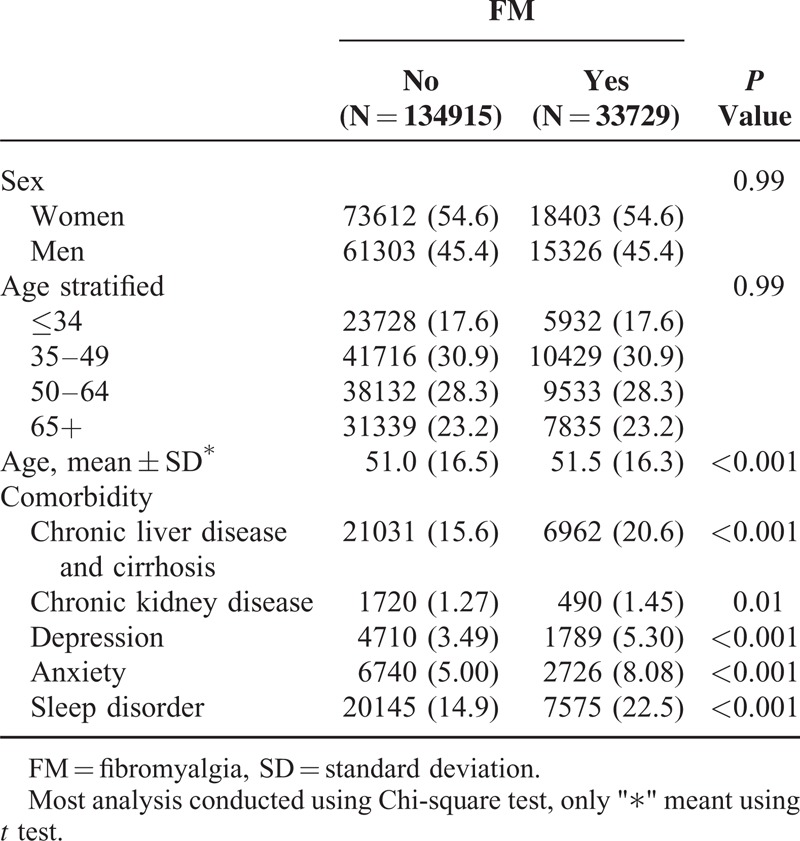
TABLE 2.
Comparison of Incidence Densities of IBS and HR With and Without FM by Demographic Characteristics and Comorbidity
Treatments That Possibly Reduce the Risk for IBS in the FM and Non-FM Cohorts
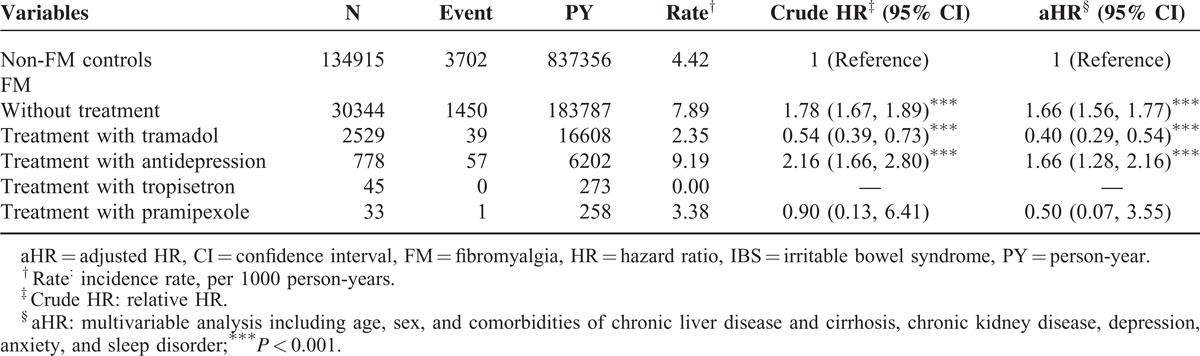
Table 3 was shown the results of an analysis of the effects of FM-related treatments on the risk for IBS compared with the risk among non-FM controls. The patients with FM who did not receive treatment had a significant 1.97-fold (95% CI 1.90–2.05) increased risk for IBS compared with the non-FM controls. After adjustment for age, sex, and comorbidities, the risk for IBS was significantly 1.84-fold higher than that of the control patients (95% CI 1.76–1.91). FM patients who received tramadol exhibited a significant 0.47-fold increased risk (95% CI 0.38–0.59) for IBS; after adjustment, the risk was significantly 0.34-fold higher than that of the control patients (95% CI 0.28–0.43). In the patients receiving antidepression drugs, the risk for IBS was significantly 2.53-fold higher than that of the control patients (95% CI 2.15–2.97), and the risk after adjustment was increased 1.99-fold (95% CI 1.70–2.34). The aHRs in patients receiving tropisetron or pramipexole showed nonsignificant 0.34- to 0.74-fold risks for IBS.
TABLE 3.
Incidence and HR of IBS Compared Among FM Patients With and Without Treatment and Non-FM Controls
Interaction between FM and its Comorbidities
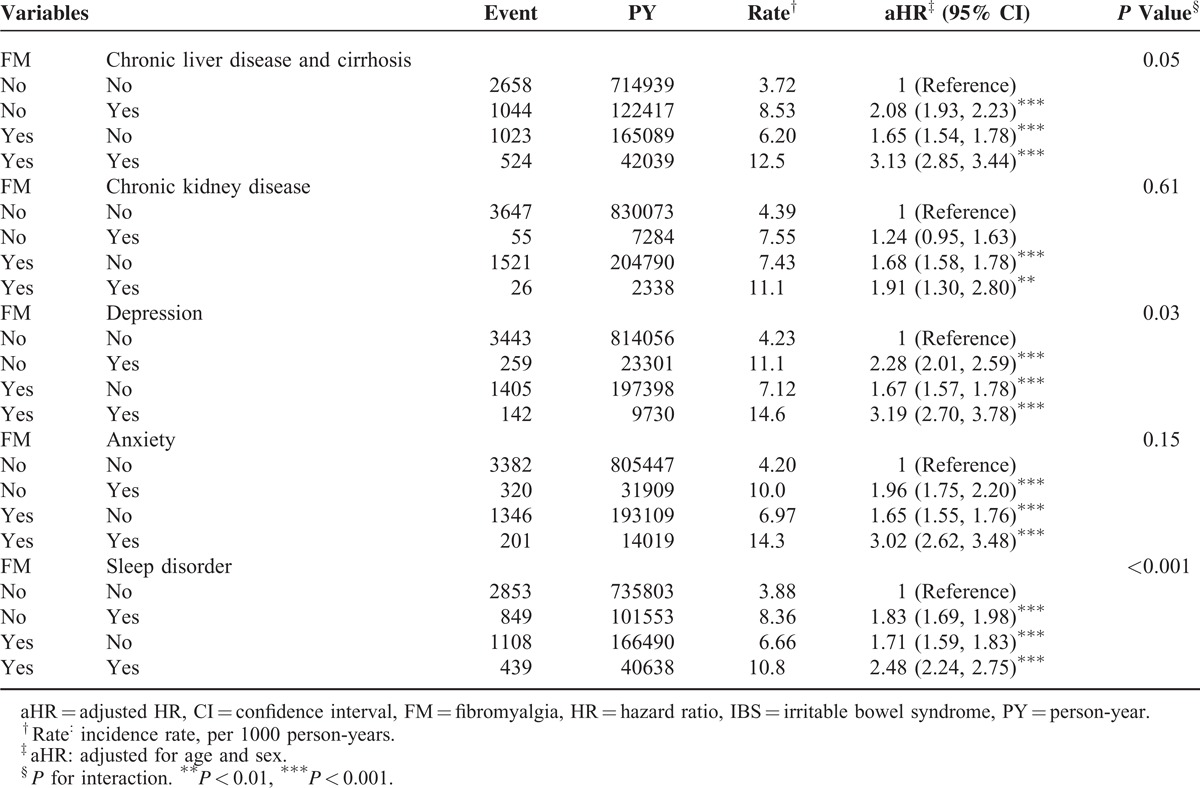
FM was associated with several morbidities such as chronic liver disease and cirrhosis, chronic kidney disease, depression, anxiety, and sleep disorder. Table 4 shows the interactions between FM and these morbidities; significant interactions were evident except for FM and chronic kidney disease. Patients with FM and any other morbidity demonstrated 2.74- to 3.46-fold risks for IBS. Significant syndemic interactions of diseases were evident in patients with FM and any of the following: chronic liver disease and cirrhosis (3.33, 95% CI 3.14–3.54), depression (3.46, 95% CI 3.09–3.87), anxiety (3.24, 95% CI 2.94–3.57), and sleep disorder (2.74, 95% CI 2.56–2.93).
TABLE 4.
Cox Proportional Hazard Regression Analysis for the Risk of IBS-Associated FM With Interaction of Comorbidity
Cumulative Incidence Rate Ratio of FM-Related IBS Risk
The overall incidence rate of IBS in non-FM and FM cohorts were 4.42 and 7.47 per 1000 person-years from 2000 to 2011. We observed a significant difference in the cumulative incidence rate of IBS (log-rank test, P < 0.001) between the FM and non-FM groups, as shown in Figure 1.
FIGURE 1.
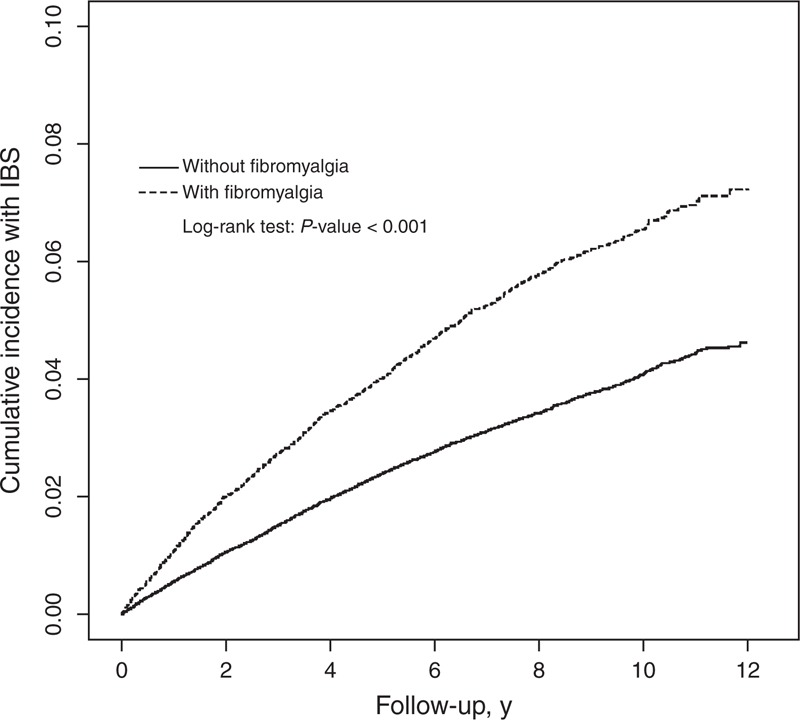
Cumulative incidence of IBS for patients with (dashed line) or without (solid line) FM. FM = fibromyalgia, IBS = irritable bowel syndrome.
DISCUSSION
Pregabalin21 was listed as a beneficial prescription in the National Health Insurance system in Taiwan from 2012 to the present, which was impossible to survey the effect via coexisting similar conditions, such as subsequent risk for IBS following FM, is indeed efficacy and extending application for these coexisting similar diseases through NHIRD.22
FM and IBS impair patients’ abilities to carry out daily activities and diminish their quality of life for months. These 2 diseases are socioeconomic burdens to Taiwanese health care institutions. Despite considerable progress in recent decades, a major gap in the knowledge on the pathogenesis of FM and IBS remains and has prevented the development of effective treatments.23,24 Moreover, no biological marker of FM and IBS has been identified; therefore, diagnosis is problematic.25–27
We collected the claims data from a nationwide and population-based database: the NHIRD. There are still some study limitations: lack of individual laboratory data and related exposure history; the records in the NHIRD were primarily for insurance requests and not be completely validated for researches; there may have been some uncontrolled confounding factors and potential biases in this retrospective cohort study, which is usually of lower statistical quality than a prospective and double-blinded controlled randomized trial.
However, the advantage of this study are the use of population-based data and NHIRD records with an extra-large sample size including FM and non-FM cohorts. Moreover, NHIRD reflected Taiwan's general population because the reimbursement of health insurance system is all-embracing and operated by single government organization of Taiwan. All insurance claims would be investigated by medical reimbursement specialist physicians and peer-review of relative physicians. Therefore, the ICD-9 codes for the diagnoses of IBS, FM, chronic liver disease, cirrhosis, and chronic kidney disease in the Taiwan's NHIRD should be highly reliable.28–31
This limitation is typical when employing NHIRD data, and further investigation through a large-scale cohort study, such as a multicenter cohort study or a study using the Taiwan Biobank, must be conducted to clarify the relationships between IBS and other similar diseases, such as FM and chronic fatigue syndrome (CFS), even to identify attributed risk factors. FM and IBS might be associated with similar risks and etiological factors in clinics; we recommend defining more detailed criteria to facilitate managing FM/IBS until efficient biomarkers are identified.
FM and IBS were separately defined using numerous symptoms and diagnosed following ruling out of other well-known reasonable disorders, which have persisted for >3 to 6 months. IBS is typically associated with abnormal gastrointestinal tract symptoms that keep for >3 to 6 months. Moreover, most gastrointestinal tract symptoms of FM or IBS patients were abdominal pain and changed bowel habits.32,33 A previous study found out that FM/CFS patients were prone to existing diagnosis of IBS and usually accompanied IBS-related symptoms.34–36 Although a comprehensive pathophysiological mechanism of FM or IBS is yet to be developed, the high frequency of morbidities coexisting with FM or IBS suggests that the underlying mechanisms cannot be confined to a single organ but instead must affect the brain–gut axis37 or autonomic nervous system.38 A previous study indicated that inflammatory bowel disease,39 upregulated the expression of most cytokine genes in colons, whereas electroacupuncture downregulated the expression of trinitrobenzene sulfonic-induced cytokine genes.40
Previous studies have shown that FM and IBS coexist41,42 and that 30% to 40% of patients with FM and IBS have numerous symptoms such as severity, especially regarding pain.42–44 However, these studies had limitations involving small sample sizes and a lack of long-term follow-up. Hence, we used NHIRD to prove that a relationship exists between FM and IBS. The relationship between FM and IBS may partially reflect disorders in gut permeability because altered gut microbiota and a disrupted mucosal barrier were observed in patients with IBS. Further investigation of the Taiwan Biobank is warranted to clarify the association between FM and IBS.45 Moreover, we recommend that physicians annually observe their FM/IBS patients to monitor disease progression.
CONCLUSION
It was generally considered that FM and IBS exist with similar symptoms and without valid biomarkers or effective standard medical procedures for curing. Thus, we considered that to clarify the relationship of these 2 diseases would be conducted for clueing etiologies in the future. We found that FM and IBS are associated, according to data retrieved from the Taiwan NHIRD. FM was associated with a 1.54-fold increased risk for IBS after adjustment for age, sex, and comorbidities; and patients with FM and any comorbidities had a 1.67-fold risk for IBS.
In addition, the results indicated that certain medications, including tramadol and antidepression drugs, might reduce the risks for IBS following FM. These results are consistent with the findings that support increased risk for IBS for FM patients. As mentioned, IBS and FM coexist and elevate the risk for other diseases. This study showed that tramadol was a protective factor; patients who used tramadol had an ∼0.4-fold risk for IBS after adjustment for confounding factors. Users of antidepression drugs had a similar risk for IBS, which was a 1.66-fold higher than that of non-FM controls.
Furthermore, when FM coexisted with any morbidity, including chronic liver disease and cirrhosis, chronic kidney disease, depression, anxiety, and sleep disorder, a significantly high risk for IBS compared with that of patients without FM and other morbidities was observed. The interactions between FM and chronic liver disease and cirrhosis, depression, anxiety, and sleep disorder had significant syndemic interactions of diseases for increasing risks for IBS.
Footnotes
Abbreviations: CFS = chronic fatigue syndrome, CI = confidence interval, FM = fibromyalgia, HR = hazard ratio, IBS = irritable bowel syndrome, ICD-9 = International Classification of Diseases, Ninth Revision, NHIRD = National Health Insurance Research Database.
Conception/design: T-YY and C-HK; provision of study materials: C-HK; collection and/or assembly of data: C-LL; data analysis and interpretation: T-YY and C-HK; manuscript writing and final approval of manuscript: all authors.
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); National Research Program for Biopharmaceuticals Stroke Clinical Trial Consortium (Ministry of Science and Technology 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TD-B-111-03, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002; 122:1140–1156. [DOI] [PubMed] [Google Scholar]
- 2.Dunckley P, Aziz Q, Wise RG, et al. Attentional modulation of visceral and somatic pain. Neurogastroenterol Motil 2007; 19:569–577. [DOI] [PubMed] [Google Scholar]
- 3.Moshiree B, Price DD, Robinson ME, et al. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. Clin J Pain 2007; 23:323–330. [DOI] [PubMed] [Google Scholar]
- 4.Othman M, Aguero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol 2008; 24:11–16. [DOI] [PubMed] [Google Scholar]
- 5.Hart FD. Fibrositis (fibromyalgia). A common non-entity? Drugs 1988; 35:320–327. [DOI] [PubMed] [Google Scholar]
- 6.Feng B, La JH, Schwartz ES, et al. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 302:G1085–G1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vierck CJ, Wong F, King CD, et al. Characteristics of sensitization associated with chronic pain conditions. Clin J Pain 2014; 30:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley LA. Pathophysiology of fibromyalgia. Am J Med 2009; 122:S22–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mease PJ, Choy EH. Pharmacotherapy of fibromyalgia. Rheum Dis Clin North Am 2009; 35:359–372. [DOI] [PubMed] [Google Scholar]
- 10.Lu CL, Chen CY, Lang HC, et al. Current patterns of irritable bowel syndrome in Taiwan: the Rome II questionnaire on a Chinese population. Aliment Pharmacol Ther 2003; 18:1159–1169. [DOI] [PubMed] [Google Scholar]
- 11.Guo HR, Chang YC, Yeh WY, et al. Prevalence of musculoskeletal disorder among workers in Taiwan: a nationwide study. J Occup Health 2004; 46:26–36. [DOI] [PubMed] [Google Scholar]
- 12.Pautex S, Cedraschi C, Allaz AF. Characteristics of elderly patients with fibromyalgia: a pilot retrospective study. Aging Clin Exp Res 2012; 24:490–494. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez del Rio-Gonzalez M. Chronic migraine: pathophysiology. Rev Neurol 2012; 54:S13–S19. [PubMed] [Google Scholar]
- 14.Theoharides TC. Treatment approaches for painful bladder syndrome/interstitial cystitis. Drugs 2007; 67:215–235. [DOI] [PubMed] [Google Scholar]
- 15.Morriss RK, Ahmed M, Wearden AJ, et al. The role of depression in pain, psychophysiological syndromes and medically unexplained symptoms associated with chronic fatigue syndrome. J Affect Disord 1999; 55:143–148. [DOI] [PubMed] [Google Scholar]
- 16.Arnold LM, Keck PE, Welge JA. Antidepressant treatment of fibromyalgia: a meta-analysis and review. Psychosomatics 2000; 41:104–113. [DOI] [PubMed] [Google Scholar]
- 17.O’malley PG, Balden E, Tomkins G, et al. Treatment of fibromyalgia with antidepressants. J Gen Intern Med 2000; 15:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson JL, O’Malley PG, Kroenke K. Antidepressants and cognitive-behavioral therapy for symptom syndromes. CNS Spectr 2006; 11:212–222. [DOI] [PubMed] [Google Scholar]
- 19.Ford AC, Talley NJ, Schoenfeld PS, et al. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 2009; 58:367–378. [DOI] [PubMed] [Google Scholar]
- 20.Perrot S. Fibromyalgia syndrome: a relevant recent construction of an ancient condition? Curr Opin Support Palliat Care 2008; 2:122–127. [DOI] [PubMed] [Google Scholar]
- 21.Gale JD, Houghton LA. Alpha 2 Delta (α(2)δ) ligands, gabapentin and pregabalin: what is the evidence for potential use of these ligands in irritable bowel syndrome. Front Pharmacol 2011; 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhadra P, Petersel D. Medical conditions in fibromyalgia patients and their relationship to pregabalin efficacy: pooled analysis of Phase III clinical trials. Expert Opin Pharmacother 2010; 11:2805–2812. [DOI] [PubMed] [Google Scholar]
- 23.Fietta P. Fibromyalgia: state of the art. Minerva Med 2004; 95:35–47.47-52. [PubMed] [Google Scholar]
- 24.Lucas HJ, Brauch CM, Settas L, et al. Fibromyalgia—new concepts of pathogenesis and treatment. Int J Immunopathol Pharmacol 2006; 19:5–10. [PubMed] [Google Scholar]
- 25.Baraniuk JN, Casado B, Maibach H, et al. A Chronic Fatigue Syndrome-related proteome in human cerebrospinal fluid. BMC Neurol 2005; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buskila D, Neumann L, Press J. Genetic factors in neuromuscular pain. CNS Spectr 2005; 10:281–284. [DOI] [PubMed] [Google Scholar]
- 27.Bazzichi L, Giacomelli C, De Feo F, et al. Antipolymer antibody in Italian fibromyalgic patients. Arthritis Res Ther 2007; 9:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng CL, Yang YHK, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 29.Yu YB, Gau JP, Liu CY, et al. A nation-wide analysis of venous thromboembolism in 497,180 cancer patients with the development and validation of a risk-stratification scoring system. Thromb Haemost 2012; 108:225–235. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh CY, Chen CH, Li CY, et al. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc 2013. [DOI] [PubMed] [Google Scholar]
- 31.Cheng CL, Lee CH, Chen PS, et al. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol 2014; 24:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperber AD, Dekel R. Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. J Neurogastroenterol Motil 2010; 16:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simsek I. Irritable bowel syndrome and other functional gastrointestinal disorders. J Clin Gastroenterol 2011; 45:S86–88. [DOI] [PubMed] [Google Scholar]
- 34.Romano TJ. Coexistence of irritable bowel syndrome and fibromyalgia. W V Med J 1988; 84:16–18. [PubMed] [Google Scholar]
- 35.Wolfe F. Fibromyalgia: the clinical syndrome. Rheum Dis Clin North Am 1989; 15:1–18. [PubMed] [Google Scholar]
- 36.Triadafilopoulos G, Simms RW, Goldenberg DL. Bowel dysfunction in fibromyalgia syndrome. Dig Dis Sci 1991; 36:59–64. [DOI] [PubMed] [Google Scholar]
- 37.Nugraha B, Karst M, Engeli S, et al. Brain-derived neurotrophic factor and exercise in fibromyalgia syndrome patients: a mini review. Rheumatol Int 2012; 32:2593–2599. [DOI] [PubMed] [Google Scholar]
- 38.Tougas G. The autonomic nervous system in functional bowel disorders. Can J Gastroenterol 1999; 13:15A–17A. [DOI] [PubMed] [Google Scholar]
- 39.Scully P, McKernan DP, Keohane J, et al. Plasma cytokine profiles in females with irritable bowel syndrome and extra-intestinal co-morbidity. Am J Gastroenterol 2010; 105:2235–2243. [DOI] [PubMed] [Google Scholar]
- 40.Ho TY, Lo HY, Chao DC, et al. Electroacupuncture improves trinitrobenzene sulfonic acid-induced colitis, evaluated by transcriptomic study. Evid Based Complement Alternat Med 2014; 2014:942196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veale D, Kavanagh G, Fielding J, et al. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. Br J Rheumatol 1991; 30:220–222. [DOI] [PubMed] [Google Scholar]
- 42.Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol 1999; 94:3541–3546. [DOI] [PubMed] [Google Scholar]
- 43.Chang L, Mayer EA, Johnson T, et al. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain 2000; 84:297–307. [DOI] [PubMed] [Google Scholar]
- 44.Sivri A, Cindaş A, Dincer F, et al. Bowel dysfunction and irritable bowel syndrome in fibromyalgia patients. Clin Rheumatol 1996; 15:283–286. [DOI] [PubMed] [Google Scholar]
- 45.Fan CT, Lin JC, Lee CH. Taiwan Biobank: a project aiming to aid Taiwan's transition into a biomedical island. Pharmacogenomics 2008; 9:235–246. [DOI] [PubMed] [Google Scholar]


