Abstract
The relationships among inflammation, oxidative balance, and the severity of alcoholic fatty liver disease (AFLD) remain unknown. The aim of this study is to explore the relationships among tumor necrosis factor alpha (TNF-α), heat shock protein 70 (HSP70), malondialdehyde (MDA), superoxide dismutase (SOD), and the severity of AFLD.
From January 2012 to December 2013, 162 participants were enrolled in this study and divided into 4 groups: 44 cases of mild AFLD (group A), 55 cases of moderate-to-severe AFLD (group B), 44 cases of alcohol consumption without AFLD (group C), and 20 cases of no alcohol consumption without AFLD (group D). A cross-sectional study was conducted by detecting the serum levels of TNF-α, HSP70, MDA, and SOD by enzyme-linked immunosorbent assay.
The median serum levels of TNF-α and HSP70 among the 4 groups were statistically significant (P = 0.000 and 0.001, respectively). The median serum levels of TNF-α in groups A and B were significantly lower than in group C (P = 0.002 and 0.000, respectively), and the median serum level of TNF-α in group B was significantly lower than in group D (P = 0.023). In addition, the median serum level of HSP70 in group B was significantly lower than in groups A and C (P = 0.002 and 0.000, respectively), and the median serum level of HSP70 in group C was significantly higher than in group D (P = 0.044). However, the median serum level of MDA in group B was significantly lower than only group C (P = 0.008).
Chronic alcohol ingestion without AFLD may result in a significant increase in the circulation of certain inflammatory markers; the severity of AFLD is associated with circulating inflammatory markers, and moderate-to-severe AFLD may result in a more significant reduction of these markers. However, moderate-to-severe AFLD may also result in a significant downregulation of oxidative stress products.
INTRODUCTION
The quantity of alcohol consumed in China is enormous, and its consumption is related to multiple health problems. To this point, the clinical diagnostics for fatty liver disease commonly include abdominal ultrasonography (US), computerized tomography (CT), magnetic resonance imaging (MRI), and others. The differences between nonalcoholic steatohepatitis (NASH) and nonprogressive non-alcoholic fatty liver disease (NAFLD) were not apparent with any radiological modality (including US, CT, and MRI). Of the pathologic features important for establishing the diagnosis of NASH, only the severity of steatosis was reflected in these radiological modalities.1 However, because ultrasound is low cost, safe, and accessible, it is the imaging technique of choice for screening for fatty livers in clinical and population settings.2 The utility of US for noninvasive diagnosis and the estimation of hepatic steatosis has been demonstrated in a large prospective pediatric study.3 Ultrasound has a high accuracy in the diagnosis and grading of steatosis and fibrosis in hepatitis C virus (HCV) nonresponders.4 In addition, US allows for reliable and accurate detection of moderate-to-severe fatty liver disease compared with histology.2 Therefore, US is widely used in clinical settings, and alcohol-induced fatty liver disease is a common finding during abdominal ultrasounds at routine health checkups. The liver is the organ that controls ethanol metabolism, and it is susceptible to the toxic effects of alcohol. The metabolism of ethanol generates a number of metabolites, including acetate, reactive oxygen species (ROS), and acetaldehyde, and produces epigenetic changes, which can induce inflammatory responses.5 Growing evidence6–8 illustrates that alcoholic liver disease (ALD) is associated with inflammatory responses, oxidative stress, and the immune response. However, the associations among inflammation, oxidative balance, and the severity of ALD are unknown. For this purpose, enzyme-linked immunosorbent assays (ELISAs) were used to examine serum levels of tumor necrosis factor alpha (TNF-α), heat shock protein 70 (HSP70), malondialdehyde (MDA), and superoxide dismutase (SOD) in order to investigate the associations among inflammation, oxidative balance, and the severity of AFLD.
MATERIALS AND METHODS
Ethics Statement
All participants were enrolled after obtaining informed consent. The protocol for this study was evaluated and approved by the Research Ethics Committee of Taishan Hospital of Shandong Province, China.
Study Population
From January 2012 to December 2013, 142 participants with chronic alcohol ingestion9 and 20 control participants were enrolled in this study. After abdominal ultrasonographic examination according to the criteria for AFLD established by the Fatty Liver and Alcoholic Liver Disease Group, Hepatology Branch, Chinese Medical Association,10 participants were divided into 4 groups. These groups included 44 cases of mild AFLD (group A: 41 males and 3 females with an average age of 46.58 ± 6.56 years); 54 cases of moderate-to-severe AFLD (group B: 50 males and 4 females with an average age of 46.33 ± 6.79 years); 44 cases of chronic alcohol consumption without AFLD (group C: 42 males and 2 females with an average age of 48.08 ± 6.67 years); and 20 cases of no chronic alcohol consumption without AFLD (group D; 18 males and 2 female with an average age of 44.76 ± 5.49 years). There were no significant differences among the groups with regard to average age (P all >0.05). A cross-sectional study was conducted to determine inflammation, oxidative balance, and the severity of AFLD through the separate detection of serum levels of TNF-α, HSP70, MDA, and SOD.
Exclusion criteria for cases included the following factors: age <25 years or >70 years, smoking, fever, pregnancy, women attempting to become pregnant, lactating women, individuals who were HBsAg positive or hepatitis B virus–DNA positive, those infected with HCV as confirmed by serologic tests, and patients with autoimmune hepatitis, cholestatic hepatitis, liver cancer, hepatic encephalopathy, electrolyte and acid-base balance disorders, gastrointestinal bleeding, infection, hepatic decompensation, primary and/or secondary heart, head, endocrine, nervous, or hematological diseases, or mental disorders.
Experimental Apparatus
A color ultrasound system (GEV7 and LOG7; GE) and a standard plate reader (ANTHOS2010; Austria) were utilized for this study. For a detailed description of the reagents listed further, please refer to the comments in our previously published manuscript.
Reagents
The reagents used include a TNF-α kit, a HSP70 kit, a MDA kit, and a SOD kit (provided by Shanghai Enzyme-Linked Immune Co. Ltd, Shanghai, China; all kits were manufactured by R&D companies, USA).
Laboratory Tests
Venous blood was drawn from just above the elbow after all patients had fasted overnight for at least 10 hours. Serum was collected by centrifugation at 3000 rpm for 10 minutes and then preserved at −70°C. Serum levels of TNF-α, HSP70, MDA, and SOD were measured by ELISA. All test items were detected according to the manufacturer's instructions.
Ultrasonic Investigation
After an overnight fast, abdominal ultrasound examinations were performed by an experienced ultrasonographer with a 3.5 to 5 MHz convex probe and a high-resolution B-mode US scanner. The degrees of steatosis at US were divided into 3 grades11: mild, moderate, and severe steatosis.
Statistical Analysis
Quantitative variables are expressed as the medians with ranges. All data analyses were conducted using the SPSS19.0 statistical package (SPSS Inc., Chicago, IL). A Kruskal–Wallis analysis of variance was used for comparisons among the 4 groups, and a Mann–Whitney U test was used for comparisons between groups. P values <0.05 were considered statistically significant.
RESULTS
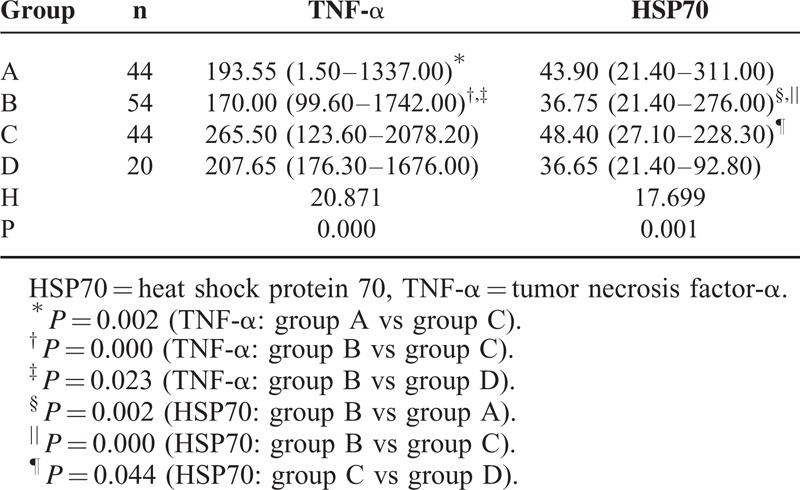
The change in the median serum levels of TNF-α and HSP70 is shown in Table 1. The median serum levels of TNF-α among the 4 groups were significantly different, H = 20.871, P = 0.000. The median serum levels of TNF-α in groups A and B were significantly lower than in group C, P = 0.002 and 0.000, respectively. The median serum level of TNF-α in group B was significantly lower than in group D, P = 0.023. In addition, significant differences were found in the median serum levels of HSP70 among the 4 groups, H = 17.699, P = 0.001. The median serum level of HSP70 in group B was significantly lower than in groups A and C, P = 0.002 and 0.000, respectively, and the median serum level of HSP70 in group C was significantly higher than in group D, P = 0.044.
TABLE 1.
Change in the Serum Levels of TNF-α and HSP70 Among the 4 Groups (ng/L, Medians With Ranges)
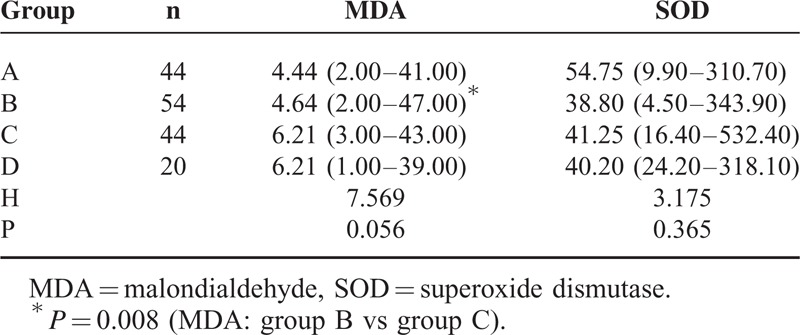
The changes in the median serum levels of MDA and SOD among the 4 groups are shown in Table 2. The median serum levels of MDA and SOD in groups A and B were lower than in groups C and D; however, no significant differences were observed among the 4 groups. Comparisons between the groups showed that the median serum level of MDA in group B was significantly lower than in group C, P = 0.008.
TABLE 2.
Change in the Serum Levels of MDA and SOD Among the 4 Groups (MDA:ng/L, SOD: U/mL, Medians With Ranges)
DISCUSSION
Ethanol (alcohol) is commonly known to alter cytokine levels in the plasma, lung, liver, and brain.12 Growing evidence indicates that chronic alcohol ingestion complicated by ALD is characterized by the activation of inflammatory responses and generates inflammatory cytokines. Inflammatory reactions play an important role in the pathogenesis of ALD. Cytokines are multifunctional proteins that play a critical role in cellular communication and activation and impact a variety of tissues in a complex manner that regulates inflammation, cell death, cell proliferation and migration, as well as healing mechanisms. In addition, TNF-α is a cytokine involved in systemic inflammation during acute-phase reactions.13 A previous study found a significant increase in the spontaneous production of interleukin (IL)1β, IL6, IL12, and TNF-α in peripheral blood monocytes among individuals actively consuming ethanol.14 Conversely, another study found that acute alcohol exposure modestly inhibited TNF-α production. However, by day 6, ethanol consumption significantly upregulated TNF-α production in association with an increased generation of ROS.15 Accumulating evidence suggests that proinflammatory cytokines, such as TNF-α, might cause hepatocellular damage and that hepatoprotective cytokines, such as IL6, have protective effects on hepatocytes.16 In addition, an animal experiment in rats showed that TNF-α and IL6 serum levels may be used as predictive biomarkers for ALD progression.17 HSP70, as an intracellular polypeptide, can be exposed on plasma membranes and/or released into circulation, eliciting immune responses that may contribute to vascular damage.18 A significant increase in HSP70 serum levels is associated with an increasing degree of inflammation.19 Recent studies have identified circulating HSP as an important mediator in inflammation, and the effects of low-grade inflammation on the aging process are overwhelming. The serum concentration of HSP70 decreases with age in the normal population, illustrating that higher levels of HSP70 are associated with inflammation and frailty in elderly patients.20 Meanwhile, HSP70 is a powerful immunogen against the antigenic peptides it chaperones.21 Serum anti-HSP70 antibody levels are independently associated with nascent metabolic syndrome.18 Moreover, plasma HSP70 is associated with the development of arterial calcification.22 A study by Peraçoli et al23 found positive correlations between HSP70 levels and TNF-α, TNFRI, IL1β, IL12, GOT, GPT, and LDH. HSP70 contributes to the inflammation and fibrosis that are present in atherosclerosis and other fibrosis-related diseases.24 Our study found that the median circulating serum levels of HSP70 are significantly increased during chronic alcohol ingestion, but there was no significant change in TNF-α. However, with the aggravation of AFLD, the median serum levels of TNF-α and HSP70 are downregulated, showing that moderate-to-severe fatty liver disease may result in more significantly reduced levels of TNF-α and HSP70. The median serum concentrations of HSP70 in this study are in accordance with those reported by Tarantino et al25 for serum concentrations of HSP70 in patients with NAFLD. The precise mechanism controlling the concentrations of TNF-α and HSP70 remains unknown. In one study, proinflammatory cytokine TNF-α levels, lymphoproliferation, and peripheral mononuclear cell production of the Th1 cytokine IFN-γ decreased as Th2 cytokine IL4 levels increased, which consequently altered the Th1/Th2 equilibrium.26 Another study demonstrated that stimulated Kupffer cells induced oxidative stress and produced proinflammatory cytokines (eg, TNF-α), which resulted in hepatocellular damage.16 However, these data support a mechanism that may rely on ethanol synergizing with LPS to upregulate the induction of TNF gene expression and TNF overproduction by decreasing cellular cAMP levels in monocytes/macrophages.27 Previous studies have shown that when acetaldehyde is administered in drinking water, high levels of protein adducts appear in liver parenchymal cells and in Kupffer cells.28 In ALD, both acetaldehyde and ethanol-induced lipid peroxidation products may also generate protein adducts in Kupffer cells.29 Chronic alcohol ingestion without AFLD causes monocytes/Kupffer cells to become activated. Kupffer cells and monocytes also play a key role in activating other cell types and producing several cytokines, chemokines, and free radicals.30 Alcoholic chronic liver disease is a common form of acquired immunodeficiency. Patients with stable alcoholic chronic liver disease show an attenuation of TLR2-mediated innate immune responses in peripheral blood monocytes, which may represent an important mechanism for acquired immunodeficiency.31 The study also showed that peripheral blood CD4 + CD25hi CD127−/lo regulatory T cells (Tregs) are significantly decreased in patients with alcoholic hepatitis when compared with both healthy individuals and chronic alcoholic patients without liver disease.32 Furthermore, circulating monocytes from actively drinking patients show abnormally low spontaneous and stimulated productions of inflammatory cytokines.14 Further study is needed to clarify these mechanisms.
Oxidative stress is one of the features of alcohol hepatotoxicity and significantly contributes to liver injury.6 ROS induction is involved in ethanol-enhanced TNF-α production by monocytes.15 Moreover, the role of immune responses triggered by oxidative stress was substantiated in the progression of NASH.33 In addition, oxidative stress is also a major contributing factor to the pathogenesis of ALD and NAFLD. In-vivo models of alcohol infusion induce lipid peroxidation because of increased free radical formation and decreased levels of hepatic antioxidants such as GSH.34 Patients with NAFLD show enhanced oxidative stress, which may lead to nonalcoholic steatohepatitis. Reduced paraoxonase-1 activity and increased MDA levels could be considered biochemical markers for lipid peroxidation.35 Previous studies have shown that human NASH is often associated with the presence of circulating antibodies against protein adducted by lipid peroxidation products. The disturbed metabolism of superoxide due to the decreased activities of SOD and catalase seem to be important in the pathogenesis of NASH.36 Sakaguchi et al37 emphasized the important role of gut-derived bacterial toxins, the innate immune system, and oxidative stress in the common pathogenic mechanism of ALD and NASH progression. Oxidative stress plays an important role in the development of liver damage resulting from alcohol consumption.38 The data from Abdelmegeed et al39 indicated that both intestinal and hepatic CYP2E1 induced by binge alcohol consumption appear to be critical in binge alcohol-mediated increases in nitroxidative stress, gut leakage, endotoxemia, altered fat metabolism, and inflammation. Our study reached a different conclusion, namely that only the median serum level of MDA in moderate-to-severe AFLD was significantly lower than in chronic alcohol ingestion without fatty liver disease, suggesting that moderate-to-severe AFLD may result in the downregulation of MDA. At present, the role of MDA downregulation is not entirely clear and requires further research.
CONCLUSIONS
Chronic alcohol ingestion without AFLD may result in a significant increase in the circulation of certain inflammatory markers (eg, HSP70). The severity of AFLD is associated with the circulation of certain inflammatory markers (eg, TNF-α and HSP70), and moderate-to-severe AFLD may result in a more significant reduction of circulating inflammatory markers. In addition, moderate-to-severe AFLD may also result in the significant downregulation of oxidative stress products (eg, MDA).
Our study has certain limitations. First, the subject study sample was small and biased by a disproportionate number of male individuals. Second, our conclusions are based on a prospective observational study, and the individuals were selected by whether they had chronic alcohol ingestion or neither chronic alcohol ingestion nor AFLD, rather than a completely randomized selection of individuals.
ACKNOWLEDGMENTS
The authors would like to thank Mr Sun Yi-Sheng and Ms Wang Hong for their technical assistance. This article was re-edited by American Journal Experts.
Footnotes
Abbreviations: AFLD = alcoholic fatty liver disease, ALD = alcoholic liver disease, HSP = heat shock protein, MDA = malondialdehyde, NASH = nonalcoholic steatohepatitis, ROS = reactive oxygen species, SOD = superoxide dismutase, TNF-α = tumor necrosis factor alpha.
This research was supported by a grant from the Technology Bureau of Taian City (No. 20123039) and the Health Department of Shandong Province (No. 2013BJYB26).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750. [DOI] [PubMed] [Google Scholar]
- 2.Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection fatty liver: a meta-analysis. Hepatology 2011; 54:1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobili V, Della Corte C, Monti L, et al. The use of ultrasound in clinical setting for children affected by NAFLD: is it safe and accurate? Ital J Pediatr 2011; 37:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohail S, Aziz S, Mirza T. Ultrasound based evaluation of hepatic steatosis and fibrosis in hepatitis C non-responders. J Coll Physicians Surg Pak 2013; 23:548–552. [PubMed] [Google Scholar]
- 5.Wang HJ, Gao B, Zakhari S, et al. Inflammation in alcoholic liver disease. Annu Rev Nutr 2012; 32:343–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 2006; 65:278–290. [DOI] [PubMed] [Google Scholar]
- 7.Viitala K, Makkonen K, Israel Y, et al. Autoimmune responses against oxidant stress and acetaldehyde-derived epitopes in human alcohol consumers. Alcoholism: Clin Exper Res 2000; 24:1103–1109. [PubMed] [Google Scholar]
- 8.Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis 2012; 30:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Quintela A, Dominguez-Santalla MJ, Pérez LF, et al. Influence of acute alcohol intake and alcohol withdrawal on circulating levels of IL-6, IL-8, IL-10 and IL-12. Cytokine 2000; 12:1437–1440. [DOI] [PubMed] [Google Scholar]
- 10.The Group of Chinese Medical Association Branch of Hepatology Fatty Liver and Alcoholic Liver Disease. Guidelines for diagnosis and treatment of alcoholic liver disease. Chin J Liver Dis 2006; 14:164–166. [Google Scholar]
- 11.Ballestri S, Lonardo A, Romagnoli D, et al. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int 2012; 32:1242–1252. [DOI] [PubMed] [Google Scholar]
- 12.Crews FT, Bechara R, Brown LA, et al. Cytokines and alcohol. Alcohol Clin Exp Res 2006; 30:720–730. [DOI] [PubMed] [Google Scholar]
- 13.Zahran WE, Salah El-Dien KA, Kamel PG, et al. Efficacy of tumor necrosis factor and interleukin-10 analysis in the follow-up of nonalcoholic fatty liver disease progression. Indian J Clin Biochem 2013; 28:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laso FJ, Vaquero JM, Almeida J, et al. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom 2007; 72:408–415. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Bagby GJ, Stoltz D, et al. Prolonged ethanol treatment enhances lipopolysaccharide/phorbol myristate acetate-induced tumor necrosis factor-production in human monocytic cells. Alcohol Clin Exp Res 2001; 25:444–449. [PubMed] [Google Scholar]
- 16.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol 2012; 27:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Taukhy MA, Salama SM, Abou-Shousha SA, et al. Effects of chronic ethanol and vitamin C administration on production of tumor necrosis factor-alpha and interleukin-6 in rats. Egypt J Immunol 2006; 13:1–10. [PubMed] [Google Scholar]
- 18.Gruden G, Barutta F, Pinach S, et al. Circulating anti-Hsp70 levels in nascent metabolic syndrome: the Casale Monferrato Study. Cell Stress Chaperones 2013; 18:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Njemini R, Smitz J, Demanet C, et al. Circulating heat shock protein 70 (Hsp70) in elderly members of a rural population from Cameroon: association with infection and nutrition. Arch Gerontol Geriatr 2011; 53:359–363. [DOI] [PubMed] [Google Scholar]
- 20.Njemini R, Bautmans I, Onyema OO, et al. Circulating heat shock protein 70 in health, aging and disease. BMC Immunol 2011; 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ménoret A. Purification of recombinant and endogenous HSP70s. Methods 2004; 32:7–12. [DOI] [PubMed] [Google Scholar]
- 22.Krepuska M, Szeberin Z, Sótonyi P, et al. Serum level of soluble Hsp70 is associated with vascular calcification. Cell Stress Chaperones 2011; 16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peraçoli JC, Bannwart-Castro CF, Romao M, et al. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol 2013; 100:129–134. [DOI] [PubMed] [Google Scholar]
- 24.González-Ramos M, Calleros L, López-Ongil S, et al. HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int J Biochem Cell Biol 2013; 45:232–242. [DOI] [PubMed] [Google Scholar]
- 25.Tarantino G, Finelli C, Colao A, et al. Are hepatic steatosis and carotid intima media thickness associated in obese patients with normal or slightly elevated gamma-glutamyl-transferase? J Transl Med 2012; 10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchi S, Sacerdote P, Moretti S, et al. The effects of alcoholism pharmacotherapy on immune responses in alcohol-dependent patients. Int J Immunopathol Pharmacol 2010; 23:847–855. [DOI] [PubMed] [Google Scholar]
- 27.Gobejishvili L, Barve S, Joshi-Barve S, et al. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2006; 291:G681–G688. [DOI] [PubMed] [Google Scholar]
- 28.Jokelainen K, Parkkila S, Salaspuro M, et al. Covalent adducts of proteins with acetaldehyde in the liver as a result of acetaldehyde administration in drinking water. J Hepatol 2000; 33:926–932. [DOI] [PubMed] [Google Scholar]
- 29.Niemelä O, Parkkila S, Bradford B, et al. Effect of Kupffer cell inactivation on ethanol induced protein adducts in the liver. Free Radic Biol Med 2002; 33:350–355. [DOI] [PubMed] [Google Scholar]
- 30.Saito H, Ishii H. Recent understanding of immunological aspects in alcoholic hepatitis. Hepatol Res 2004; 30:193–198. [DOI] [PubMed] [Google Scholar]
- 31.Pimentel-Nunes P, Roncon-Albuquerque R, Jr, Gonçalves N, et al. Attenuation of toll-like receptor 2-mediated innate immune response in patients with alcoholic chronic liver disease. Liver Int 2010; 30:1003–1011. [DOI] [PubMed] [Google Scholar]
- 32.Almeida J, Polvorosa MA, Gonzalez-Quintela A, et al. Decreased peripheral blood CD4+/CD25+ regulatory T cells in patients with alcoholic hepatitis. Alcohol Clin Exp Res 2013; 37:1361–1369. [DOI] [PubMed] [Google Scholar]
- 33.Sutti S, Jindal A, Locatelli I, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014; 59:886–897. [DOI] [PubMed] [Google Scholar]
- 34.Iimuro Y, Bradford BU, Yamashina S, et al. The glutathione precursor L-2-Oxothia-zolidine-4-carboxylic acid protects against liver injury due to chronic enteral ethanol exposure in the rat. Hepatology 2000; 31:391–398. [DOI] [PubMed] [Google Scholar]
- 35.Samy W, Hassanian MA. Paraoxonase-1 activity, malondialdehyde and glutathione peroxidase in non-alcoholic fatty liver disease and the effect of atorvastatin. Arab J Gastroenterol 2011; 12:80–85. [DOI] [PubMed] [Google Scholar]
- 36.Park KS, Jang BK, Kwon KM, et al. Antioxidant status in nonalcoholic steatohepatitis. Korean J Hepatol 2005; 11:135–143. [PubMed] [Google Scholar]
- 37.Sakaguchi S, Takahashi S, Sasaki T, et al. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet 2011; 26:30–46. [DOI] [PubMed] [Google Scholar]
- 38.Galicia-Moreno M, Gutiérrez-Reyes G. The role of oxidative stress in the development of alcoholic liver disease. Rev Gastroenterol Mex 2014; 79:135–144. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmegeed MA, Banerjee A, Jang S, et al. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med 2013; 65:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]


