Abstract
This article aims to analyze the frequency and clinical characteristics of peripheral neuropathy (PN) in patients with systemic lupus erythematosus (SLE).
A total of 4924 SLE patients admitted to the Peking Union Medical College Hospital, Beijing, China, from January 1995 to September 2013 were included in this retrospective analysis. The individuals designated as control patients were selected from the pool of SLE patients without PN using the systematic sampling method of 1:2 during the same time.
The prevalence of SLE-associated PN (SLE-PN) in SLE patients was 1.5% (73/4924). Seventy-nine cases of PN affected 73 patients and 6 of these patients (8.2%) presented with 2 types of PN. Among the 7 types of PN, polyneuropathy was the most frequent and was diagnosed in 47 cases (59.5%); the remaining patients suffered from mononeuropathy (13.9%), cranial neuropathy (12.7%), myasthenia gravis (10.1%), autonomic neuropathy (2.5%), or acute inflammatory demyelinating polyradiculoneuropathy (1.3%). Five patients developed PN before the onset of SLE (3 out of 5 patients had myasthenia gravis). The most common PN-related symptoms were myasthenia and numbness (50.6%), followed by pain in affected regions (35.9%). PN symptoms were relieved in a majority of the patients (76.7%) after treatment. Compared with non-SLE-PN patients, patients with SLE-PN had a higher frequency of fever (65.8% vs 45.9%, P < 0.01), mucocutaneous involvement (73.9% vs 36.3%, P < 0.01), arthritis (42.5% vs 28.1%, P < 0.05), myositis (17.8% vs 5.5%, P < 0.01), and central nervous system involvement (38.4% vs 21.9%, P < 0.05) as well as being positive for the anti-Sm antibody (31.4% vs 18.8%), immunoglobulin G (IgG) elevation (53.6% vs 37.1%, P < 0.01), and reduction in complement 3 (54.8% vs 36.9%, P < 0.05). A statistically significant difference was found between the Systemic Lupus Erythematosus Disease Activity Index scores in SLE-PN patients compared with the non-SLE-PN patients (P < 0.05). Multivariate logistic regression showed that the only risk factor for PN was IgG elevation (odds ratio = 2.553, 1.224–5.327, P = 0.012).
The prevalence of PN in SLE occurs more frequently in patients with an active form of the disease. IgG elevation is a risk factor for SLE-PN and should be assessed in these patients. Young female patients with myasthenia gravis should be closely monitored for the development of SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multiorgan involvement and the production of multiple autoantibodies. Neuropsychiatric manifestations in SLE patients are frequently reported with a prevalence of 37% to 90%, which is mainly associated with the central nervous system (rarely in the peripheral nervous system).1–3 Previous studies have demonstrated a significant decrease in the age and gender-standardized physical component summary scores (which derived from the SF-36 questionnaire and evaluated the physical condition on health-related quality of life) in SLE patients with peripheral neuropathy (PN) compared with those SLE patients without PN.4 Thus, SLE-associated PN (SLE-PN) may be associated with decreased quality of life in these patients, despite the fact that PN alone rarely impacts the risk of death. To date, no systematic clinical strategies and/or guidelines are available for the clinicians, as a limited number of studies on SLE-PN have been published to date. In this study, we aimed to evaluate the prevalence and clinical manifestations of SLE-PN patients using a large sample size study to investigate characteristics at the terms of onset, during treatment, and disease prognosis.
MATERIALS AND METHODS
Data from a total of 4924 patients with SLE admitted in Peking Union Medical College Hospital, Beijing, China, from January 1995 to September 2013 were retrospectively analyzed. All patients fulfilled the classification criteria for SLE proposed by the American College of Rheumatology (ACR) in 1982.5 Peripheral neuropathies were defined according to the ACR nomenclature and case definitions for neuropsychiatric syndromes in SLE found online at http:///www.rheumatology.org/publications/ar/1999/499.6 The individuals designated as control patients were selected from a pool of SLE patients without PN using the systematic sampling method of 1:2 during the same time. Patients with PN caused by non-SLE disease (eg, diabetes mellitus and heavy alcohol consumption) were excluded. SLE-related demographic, clinical, and laboratory data, as well as the treatment and prognosis, were also recorded. The local institutional review board approved the study. Because the study was based on a review of medical records that had been obtained for clinical purposes, the requirement for written informed consent was waived.
DATA COLLECTION
Disease duration was defined as the time from onset of SLE to admission. Mucocutaneous involvement included malar rash and other rashes related with SLE, aphthous ulcers, alopecia, and photosensitivity. Myositis was defined as muscle weakness as well as the elevation of creatine kinase, with or without myogenic damage in electromyogram, and muscle damage caused by non-SLE disease (eg, hyperthyroidism and application of glucocorticoid) was excluded. Digestive system involvement was defined as gastrointestinal manifestations related with SLE, including intestinal pseudoobstruction, protein-losing enteropathy, and others. Respiratory system involvement was defined as lung manifestations related with SLE, including interstitial lung disease, alveolar hemorrhage, and others. Kidney involvement was defined as renal impairments due to SLE, including proteinuria, microscopic hematuria, renal tubule acidosis, and others. Kidney insufficiency was defined as creatinine higher than normal value or creatinine clearance rate lower than normal value. Hematological involvement was defined as white blood cell or platelet lower than normal value and hemolytic anemia due to SLE. Central nerve systemic involvement was defined according to the ACR nomenclature and case definitions for neuropsychiatric syndromes in SLE.6
Statistical Analysis
Data analysis was performed using the statistical packages for SPSS 15.0 software (SPSS Inc., Chicago, IL). Quantitative results were presented as mean ± standard deviation, or median, interquartile range. The Student t test was used for the intergroup comparison of data normally distributed. For the data that was nonnormally distributed, the Mann–Whitney test was performed. The χ2 test was used for the analysis of enumeration data. P < 0.05 was considered to be statistically significant. Multivariate logistic regression was used to evaluate an association of presence versus absence of PN using those covariates that were statically significant based on the univariate analysis.
RESULTS
Patient Characteristics
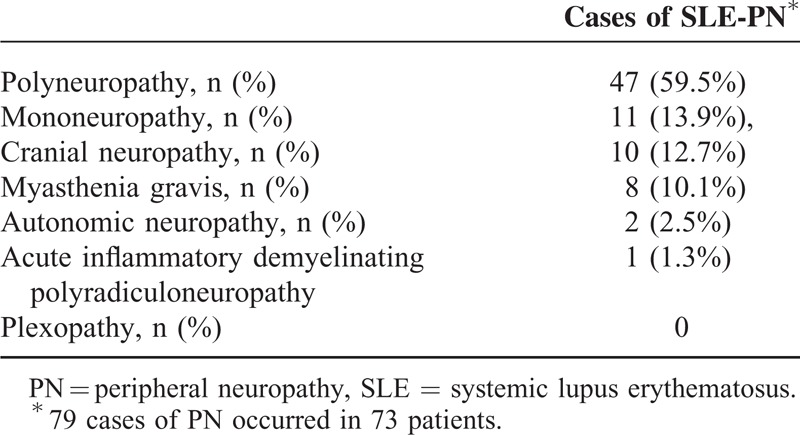
The frequency of SLE-PN was 1.5% (73 of 4924 patients). A total of 79 cases of PN were observed in 73 patients, and 6 patients presented with 2 types of PN. The different subtypes of PN are summarized in Table 1. Among the 7 subtypes of PN identified in this study, polyneuropathy was the most common form and occurred in 47 cases (59.5%). The remaining patients were reported to suffer from mononeuropathy (13.9%), cranial neuropathy (12.7%), myasthenia gravis (10.1%), autonomic neuropathy (2.5%), or acute inflammatory demyelinating polyradiculoneuropathy (1.3%); no plexopathy was observed. Among the 73 SLE-PN patients, 5 patients developed PN (3 manifested by myasthenia gravis) before the onset of SLE, 13 patients developed PN simultaneously with SLE, and the remaining 55 patients developed PN approximately 46.9 ± 83.5 months after the onset of SLE.
TABLE 1.
Subtypes of Peripheral Neuropathy Identified in This Study
Clinical Manifestations and Prognosis of PN
The most common PN-related symptoms were myasthenia and numbness in affected regions (50.6%). Twenty-eight patients (38.4%) experienced pain in the affected regions. Five patients (6.8%) were confirmed to have PN according to the neuroelectrophysiological tests, although no clinical symptoms of PN were observed. A majority of patients were symmetrically affected by PN (63.7%), which involved the sensory (67.5%) and motor nerves (49.3%). Most of the PN-related symptoms (76.7%) were relieved after treatment during a follow-up of 5.2 ± 16.2 months.
SLE-Associated Clinical Manifestations
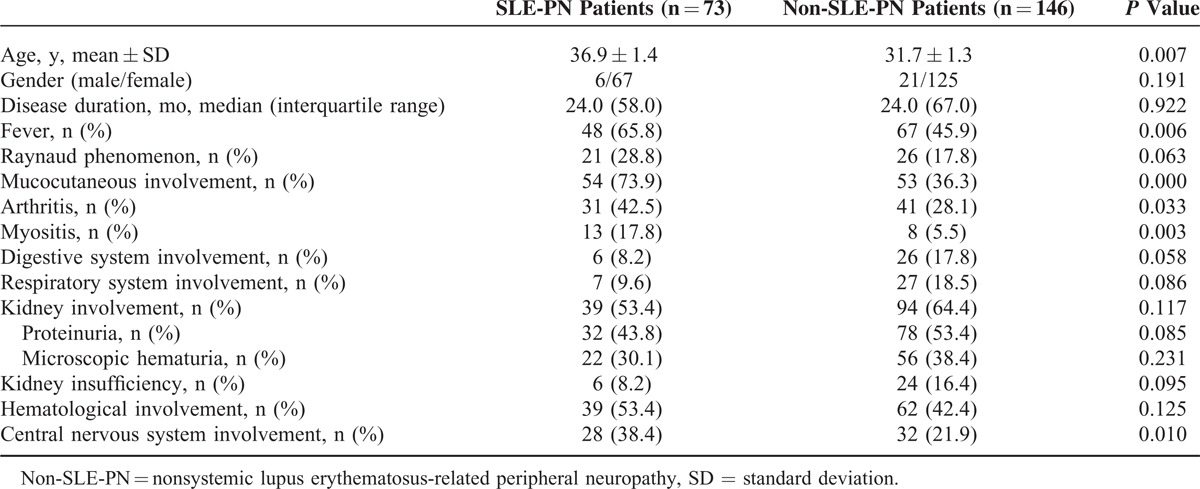
Table 2 shows a comparison of demographic information and clinical manifestations between SLE-PN and non-SLE-PN patients. No statistical difference was found in SLE duration in SLE-PN patients, and these patients were older than patients with non-SLE-PN at the onset of the disease (P = 0.007). Compared with non-SLE-PN patients, SLE-PN patients were prone to develop fever (P = 0.006), skin and mucous membrane conditions (P = 0.000), arthritis (P = 0.033), myositis (P = 0.003), and central nervous system conditions (P = 0.010). In addition, 5 patients had neuromyelitis optica.
TABLE 2.
Demographic Information and Clinical Manifestations in SLE-Related PN and Non-SLE-Related PN Patients
Laboratory Tests, Treatment, and Mortality of SLE
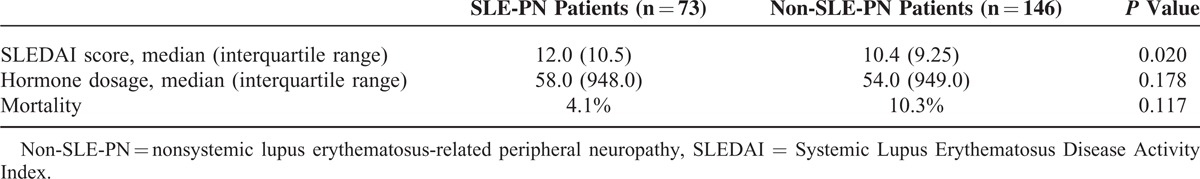
Serological markers for SLE-PN and non-SLE-PN patients are shown in Table 3. Anti-Sm antibody (anti-Sm) positivity and complement 3 hypocomplementemia occurred more frequently in the patients with SLE-PN compared with non-SLE-PN patients (P < 0.05). In addition, remarkable elevated levels of serum immunoglobulin G (IgG) in SLE-PN patients were observed compared with non-SLE-PN patients (P < 0.05). The Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score, treatment, and mortality are shown in Table 4, which revealed a significantly higher SLEDAI score in SLE-PN patients compared with non-SLE-PN patients (P < 0.05). No significant difference was found in the mortality of non-SLE-PN patients compared with SLE-PN patients (P = 0.117).
TABLE 3.
Laboratory Results for SLE-Related PN and Non-SLE-Related PN Patients
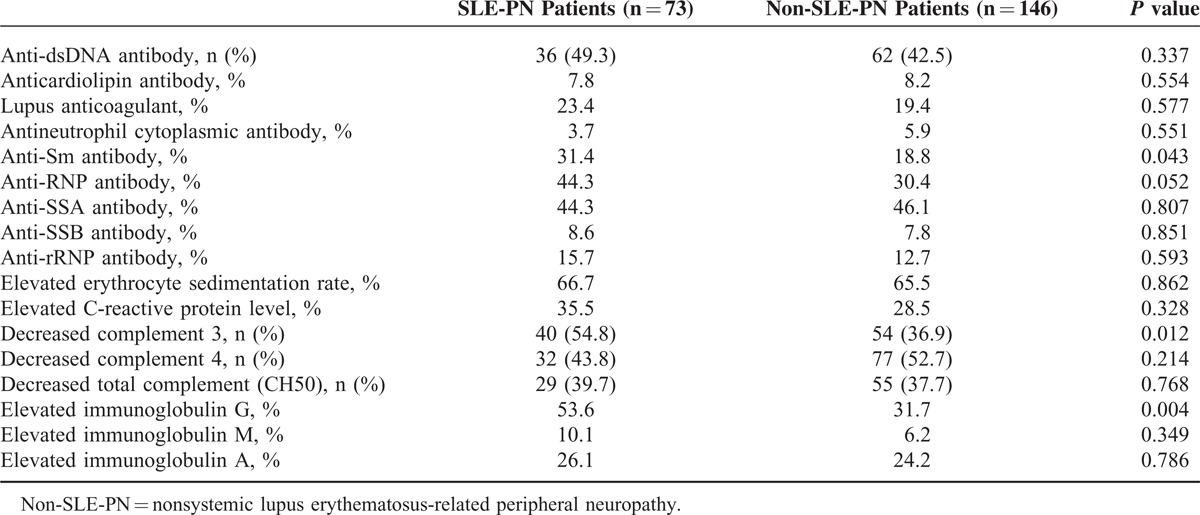
TABLE 4.
Treatment and Prognosis for SLE in SLE-Related PN and Non-SLE-Related PN Patients
Multivariate Logistic Regression
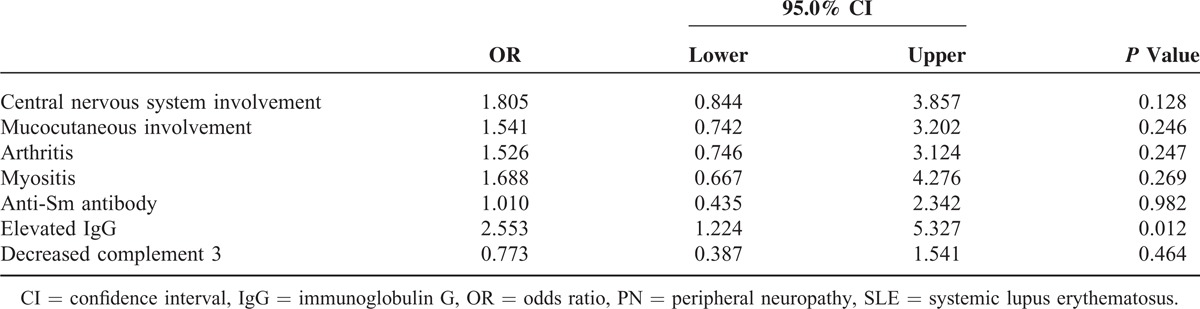
Seven covariates were further analyzed. The only characteristic associated with SLE-PN was elevated levels of serum IgG (odds ratio = 2.553, 1.224–5.327, P = 0.012) (Table 5).
TABLE 5.
Multivariate Logistic Regression in SLE-Related PN and Non-SLE-Related PN Patients
DISCUSSION
In this study, we present a retrospective longitudinal study with a large sample size and focused on the prevalence, clinical symptoms, and prognosis of patients with SLE-PN in China.
In our study, the prevalence of PN was 1.5%, which is lower than that found in the previous studies in which SLE-PN was reported to occur in 2.2% to 32% of the population.7–9 We hypothesize that multiple factors contributed to this discrepancy. First, there was no normative definition for PN before 1999. Since the ACR proposed a definition for SLE-NP in 1999, 7 subtypes of PN in SLE have been identified and the diagnosis of PN has been subsequently standardized. The prevalence of SLE-PN was reported to be 2.2% to 8.2% after 1999, which is lower than in older reports, although still higher than that found in our study. Second, many non-SLE-related factors could cause PN in SLE patients, for example, compression syndromes, drug toxicity, and diabetes mellitus,10 making it difficult for clinicians to differentiate between them. Third, the diagnosis criteria of SLE-PN vary in different studies. For example, in a study by Hanly et al,11 the patients presenting with PN-related manifestations, but without electrophysiological data, were excluded. Our study is retrospective and the electrophysiological examination rate was low, which may have resulted in missed diagnoses of some asymptomatic patients. In addition, up to now no specific data for Asian populations have been reported, and genetic characteristics may contribute to these observations.
The most common type of PN was polyneuropathy with sensory involvement, followed by mononeuropathy, which is similar to previous findings by Brandusa et al.4 There have been rare cases reported of SLE patients with myasthenia gravis. In our study, 8 patients with myasthenia gravis were reported, and 3 young females (37.5%) were confirmed to have myasthenia gravis at 6 to 14 months prior to the development of SLE (19, 23, and 36 years old, respectively). Therefore, proper follow-up should be carried out for young female patients with myasthenia gravis.
In accordance with the study by Brandusa et al,4 a remarkable increase was noted in the SLEDAI score in SLE-PN patients. In addition, SLE-PN patients often had complications with central nervous system involvement, especially in patients with optic nerve involvement, which was also more likely to be complicated by myelitis. All of these findings suggest active SLE disease in SLE-PN patients, which is also consistent with the high frequency of other manifestations of active disease, including fever, mucocutaneous involvement, arthritis, myositis, and the decrease of complement in those patients.12 However, a recent study reported lower disease activity and a decreased frequency of mononeuritis multiplex (7%)9; differences in disease activity are thought to account for differences in the frequency and spectrum of PN.
A higher rate of anti-Sm antibody-positive results has been noted in PN patients.13 Moreover, the presence of anti-cardiolipin antibody has been confirmed previously in SLE-PN children.14 Our study showed that the prevalence of PN was higher in patients with positive anti-Sm antibody; however, the association between anti-Sm and the pathogenesis of PN remains controversial.4,15
Currently, the pathogenesis of SLE-PN is still not well defined. Previous studies have revealed several factors associated with the onset of SLE-PN, including disorders of small vessel, vasculitis, deposits of immune complexes, or injury due to production of antibodies. Roald et al16 showed that small nerve fiber involvement was partially responsible for the onset of SLE. Small fiber neuropathy is a frequently occurring PN.9 In addition, Mawrin et al17 reported that the upregulation of matrix metalloproteinase-3 and matrix metalloproteinase-9 may be related to vessel wall injury, resulting in a chronic combined axonal and demyelinating type of SLE-PN.17 Our findings indicate that IgG elevation is an important factor of SLE-PN. IgG elevation is the result of immune dysfunction and reflects B lymphocyte hyperfunction, resulting in production of autoantibodies and cytokines, activation of T lymphocytes, and the onset of PN. Thus, we propose that the immunological reactions to nerve tissue may play an important role in the pathogenesis of SLE-PN.
Manifestations were comparatively absent in some PN patients, especially at the early stage, but the electrophysiological tests (neural conduction test and the electromyogram) were aberrant. In a study of 38 SLE patients, decreased nerve conductive velocity (NCV) was noted in 9 patients (23.6%), and approximately 50% of them showed no nervous system manifestations,10 indicating that electrophysiological investigations are of prime importance for the diagnosis of PN at early stage. In our study, electrophysiological investigations were carried out in 80% of the SLE-PN patients. NCV could be used as a suitable method for the diagnosis of PN. However, a recent study showed that some SLE patients present with patchy, asymmetric, and proximal neuropathic pain, which is markedly different from a stocking glove distribution. In these patients, abnormal skin biopsy findings, but normal findings from electrodiagnostic studies were noted.9
To date, there have not been any large-size or case–control studies on the treatment of PN. In 2010, the European League Against Rheumatism recommended that permanent treatment with glucocorticoids and immunosuppressants should be considered for SLE-PN patients with severe disease.18 A controlled clinical trial in SLE patients with severe PN manifestations showed that intravenous (IV) cyclophosphamide treatment was more effective than IV methylprednisolone.19 In addition, a previous retrospective study indicated that cyclophosphamide could prevent the relapse of vasculitic PN.20
It is hard to draw conclusions on the outcomes of the SLE-PN based on our retrospective study. In the future, large randomized controlled trials are needed to evaluate the most efficient treatment strategy for treating PN in SLE patients in order to improve the life quality and outcomes of these patients.
Footnotes
Abbreviations: ACR = American College of Rheumatology, IgG = immunoglobulin G, NCV = nerve conductive velocity, PN = peripheral neuropathy, SLE = systemic lupus erythematosus, SLE-PN = SLE-associated PN.
WX and WM equally contributed to this study.
This project was supported by the National Major Scientific and Technological Special Project (2012ZX09303006-002), the Research Special Fund for Public Welfare Industry of Health (201202004), and the National High Technology Research and Development Project of China (2012AA02A513).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Ainiala H, Hietaharju A, Loukkola J, et al. Validity of the new American College of Rheumatology criteria for neuropsychiatric lupus syndromes: a population-based evaluation. Arthritis Rheum 2001; 45:419–423. [DOI] [PubMed] [Google Scholar]
- 2.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology 2002; 58:1214–1220. [DOI] [PubMed] [Google Scholar]
- 3.Hanly JG, McCurdy G, Fougere L, et al. Neuropsychiatric events in systemic lupus erythematosus: attribution and clinical significance. J Rheumatol 2004; 31:2156–2162. [PubMed] [Google Scholar]
- 4.Brandusa F, Ellie A, Jiandong SU, et al. Peripheral neuropathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum 2011; 41:203–211. [DOI] [PubMed] [Google Scholar]
- 5.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 6.ACR Ad Hoc Committee on Neuropsychiatric lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 1999; 42:599–608. [DOI] [PubMed] [Google Scholar]
- 7.Omdal R, Henriksen OA, Mellgren SI, et al. Peripheral neuropathy in systemic lupus erythematosus. Neurology 1991; 41:808–811. [DOI] [PubMed] [Google Scholar]
- 8.Feinglass EJ, Amett FC, Dorch CA, et al. Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine 1976; 55:323–339. [DOI] [PubMed] [Google Scholar]
- 9.Oomatia A, Fang H, Petri M, et al. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five-year study period. Arthritis Rheum 2014; 66:1000–1009. [DOI] [PubMed] [Google Scholar]
- 10.Luyendijk J, Steens SC, Ouwendijk WJ, et al. Neuropsychiatric systemic lupus erythematosus: lessons learned from magnetic resonance imaging. Arthritis Rheum 2011; 63:722–732. [DOI] [PubMed] [Google Scholar]
- 11.Hanly JG, Urowitz MB, Su L, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2010; 69:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huynh C, Ho SL, Fong KY, et al. Peripheral neuropathy in systemic lupus erythematosus. J Clin Neurophysiol 1999; 16:164–168. [DOI] [PubMed] [Google Scholar]
- 13.Mahler M, Stinton LM, Fritzler MJ. Improved serological differentiation between systemic lupus erythematosus and mixed connective tissue disease by use of an SmD3 peptide-based immunoassay. Clin Diagn Lab Immunol 2005; 12:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liora H, Masha M, Riva B, et al. Peripheral neuropathy in pediatric systemic lupus erythematosus. Pediatr Neurol 2002; 27:53–56. [DOI] [PubMed] [Google Scholar]
- 15.Omdal R, Loseth S, Torbergsen T, et al. Peripheral neuropathy in systemic lupus erythematosus: a longitudinal study. Acta Neurol Scand 2001; 103:386–391. [DOI] [PubMed] [Google Scholar]
- 16.Roald O, Svien IM, Lasse G, et al. Small nerve fiber involvement in systemic lupus erythematosus. Arthritis Rheum 2002; 46:1228–1232. [DOI] [PubMed] [Google Scholar]
- 17.Mawrin C, Brunn A, Röcken C, et al. Peripheral neuropathy in systemic lupus erythematosus: pathomorphological features and distribution pattern of matrix metalloproteinases. Acta Neuropathol 2003; 105:365–372. [DOI] [PubMed] [Google Scholar]
- 18.Bertsias GK, Ioannidis JPA, Aringer M, et al. Report of a task force of EULAR standing committee for clinical affairs. Ann Rheum Dis 2010; 69:2074–2082. [DOI] [PubMed] [Google Scholar]
- 19.Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, et al. Controlled clinical trial of IV cyclophosphamide versus IV methyl-predisonlone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis 2005; 64:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew L, Talbot K, Love S, et al. Treatment of vasculitic peripheral neuropathy: a retrospective analysis of outcome. QJ Med 2007; 100:41–51. [DOI] [PubMed] [Google Scholar]


