Abstract
Nanomaterials are being vigorously investigated for their use in anticancer drug delivery regimes or as biomarkers agents and are considered to be a candidate to provide a way to combat severe weaknesses of anticancer drug pharmacokinetics, such as their nonspecificity. Because of this weakness, a bigger proportion of the drug-loaded nanomaterials flow toward healthy tissues and result in undesirable side effects. It is very important to evaluate drug loading and release efficiency of various nanomaterials to find out true pharmacokinetics of these drugs.
This observational study aims to evaluate various surface functionalized and naked nanomaterials for their drug loading capability and consequently strengthens the Reporting of Observational Studies in Epidemiology (STROBE). We analyzed naked and coated nanoparticles of transition metal oxides for their further loading with doxorubicin, a representative water-soluble anticancer drug.
Various uncoated and polyethylene glycol-coated metal oxide nanoparticles were synthesized and loaded with anticancer drug using simple stirring of the nanoparticles in a saturated aqueous solution of the drug. Results showed that surface-coated nanoparticles have higher drug-loading capabilities; however, certain naked metal oxide nanoparticles, such as cobalt oxide nanoparticles, can load a sufficient amount of drug.
INTRODUCTION
Chemotherapeutic anticancer drugs are commonly administered intravenously leading to general systemic distribution with poor targeted tumor specificity. This nonspecificity of the anticancer drug causes higher than expected drug uptake by the healthy tissues and results in undesirable side effects.1–3 Nanomaterials are being widely investigated for their use in diagnosis and therapy of various abnormalities including cancer due to their unique character and functioning at the cellular and molecular levels and scope of their usage is being broadened constantly.4–7 New improved drug delivery systems based on nanocarriers promise to enhance the specificity of these agents and are expected to reduced their side effects with better therapeutic efficacy due to improved pharmacokinetics and diffusion. However, there are few issues with nanomaterials-based drug delivery systems.8,9 First, drug-loaded nanomaterials are feared to be instantly recognized and cleared off the body by the reticuloendothelial system (RES) prior to reaching the target tumor. Second, various physiological barriers, for example, blood–brain barrier (BBB), hinder the carriage of these drug-loaded nanoparticles to and from different body organs or tissues. Surface modifications and morphological adjustments, such as size and shape of nanoparticles, are carried out to overcome both of these problems with an increase in their blood retention times for better therapy results.10 These adjustments directly influence uptake and clearance of these drug-loaded nanomaterials to and from different organs of the body.11,12
Organic and inorganic nanomaterials have extensively been investigated for targeted drug delivery through an attachment of the particles with some ligands for active transport.13–15 Organic nanomaterials are more related to the body chemicals; however, different inorganic nanomaterials with low toxicity and better biodegradability are being used because of their better storage and shelf life.16,17 For example, in some early preclinical trials, iron oxide nanoparticles in colloidal form have shown satisfactory acceptance by the patients in colloidal form. Various surface modifications may result in simultaneous reduction in drug dosage and undesirable side effects to the healthy tissues.11,12,18 Better physiochemical properties have been achieved by surface modification of magnetic nanoparticles using various polymers, silica, different surfactant, or various organic compounds. Sufficient amount of drug can be loaded onto the stable surfaced nanoparticles with magnetic core shell structure.15,19,20
Transition metal oxide nanoparticles have sufficient aqueous affinity with coating and can be synthesized with a size <100 nm and can avoid rapid clearance by the RES. Surface modifications have a key role in defining nanoparticles biotoxicity.15 Various polymers, such as polyethylene glycol (PEG), have been worked out as drug carriers that degrade in the body environment21,22 and can be used for tissue engineering.23,24 These polymers, which are easily degradable inside the body and are suitable for human intake, have reasonable chemical composition. Some nanoparticles surface-blended copolymers have been used to increase blood circulation time of these nanoparticles.25 Although, surface coatings may help in achieving better drug-loading profiles, certain inorganic nanomaterials are capable of loading reasonable drug quantities even without any surface modifications and help to reduce a step (surface coating) in the process of drug design.
In passive drug delivery, drug uptake by the targeted tumor is generally relied on specificity and diffusion process. Nanocarriers may help in achieving reasonably good specificities of the particles loaded with anticancer drugs, whereas this parameter may be influenced by an additional surface coating. This form of passive drug delivery of antitumor agents, adsorbed on the surface of nanoparticles, is a promising alternative to conventional chemotherapy to increase drug-targeting probability and reduce undesirable side effects. A very critical parameter need to be known in this case is the amount of drug loaded on to the nanoparticles behaving as nanocarriers.26 This loaded drug is then needed to be released at the targeted tumor side to have its therapeutic effect. No such analysis is available that how the drug is loaded on what type of nanoparticles. It will also be beneficial for the sake of drug design to study this behavior for various inorganic nanomaterials. Keeping in view the above-mentioned points, this work aims to compare and analyze loading efficiency of a representative water-soluble anticancer drug (doxorubicin [DOX]) onto various transition metal oxide nanoparticles due to their low toxicities, reasonably good biocompatibility, and ease of preparation and drug-loading methods. Drug-loading capabilities of naked and PEG surface-functionalized metal oxide nanoparticles of iron oxide, nickel oxide (NiO), tin oxide (SnO), and cobalt oxide (CoO) were evaluated by ultraviolet (UV)–visual spectrometry. These results, we hope, will be helpful in the design of some targeted realm of water-soluble anticancer drug. We intend to evaluate biodistribution and specificities of these transition metal oxide nanomaterials in future.
METHODS
Following methods were used for nanoparticles synthesis and characterization. It is to be stated that our study does not include any human or animal participants and does not need an approval of the ethical committee. However, our study was approved by the review board of the Government College University (GCU), Lahore, Pakistan.
Nanoparticles Synthesis
Iron Oxide Nanoparticles
Ferrous chloride tetrahydrates (FeCl2·4H2O) and ferric chloride hexahydrate (FeCl3·6H2O) were used for synthesis of iron oxide nanoparticles. We used all chemicals of reagent grade and further purification was not carried out. Molar concentrations of FeCl3 and FeCl2, in their 2:1 ratio, respectively, were dissolved in 50-mL deionized water; 50 mL of sodium hydroxide was added to 50 mL mixture of iron salts under vigorous mechanical stirring at 1200 revolutions/min. This reaction was performed for 30 minutes at 20°C in air medium. Black precipitate of Fe3O4 nanoparticles appeared in the solution instantly with mixing of the alkali.18 Particles were washed with deionized water thrice and dried in oven at 60°C.
Nickel Oxide Nanoparticles
To prepare NiO nanoparticles, 0.25 M aqueous solution of nickel chloride (NiCl4) and 0.25 M aqueous solution of oxalic acid were separately prepared in distilled water in beakers. This solution containing oxalic acid was heated to a temperature of 55°C. NiCl4 solution was added drop wise (slowly for 1 hour) to this heated solution, under vigorous stirring. Nickel oxalate precipitate settled at the bottom and was separated and washed with deionized water 3 times.27 The particles were dried at 60°C in oven and further heated up to 450°C in tube furnace to get NiO nanoparticles.
Cobalt Oxide Nanoparticles
To prepare CoO nanoparticles, 0.25 M aqueous solution of cobalt chloride (CoCl2) and 0.25 M aqueous solution of oxalic acid were prepared in distilled water. Solution containing oxalic acid was heated to a temperature of 55°C and then CoCl2 solution was added drop wise (slowly for 1 hour) to this heated solution under high-speed stirring. Cobalt oxalate precipitate settled at the bottom and was separated and washed with deionized water thrice. The particles were dried at 60°C in oven and then heated up to 450°C in tube furnace to get CoO nanoparticles.28
Tin Oxide Nanoparticles
To prepare SnO nanoparticles, 0.25 M aqueous solution of tin chloride (SnCl2) and 0.25 M aqueous solution of oxalic acid were prepared in distilled water. The solution containing oxalic acid was heated at temperature of 55°C and SnCl2 solution was added drop wise (slowly for 1 hour) to this heated solution under high-speed stirring. Tin oxalate precipitate settled at the bottom and was separated and washed with deionized water thrice. The particles were dried at 60°C in oven and heated up to 450°C in tube furnace to get CoO nanoparticles.29
Characterization
For nanoparticles characterization, scanning electron microscope (SEM) and x-ray diffraction (XRD) were used. Scherrer formula was used for small nanocrystallite size, and for size distribution, SEM images were analyzed.
Drug-Loading Analysis
Five milligram of DOX was dissolved in 10 mL of distilled water and continuously shaken on an orbital shaker. Sixty milligrams of nanoparticles were added to this solution for drug-loading purposes. With continuous shaking, a small amount of this solution was taken out to evaluate its absorption/transmission using UV–visual spectroscopy.
RESULTS
Results using above methodology are presented in this section.
Nanoparticles Characterization
Particle morphology was assessed with SEM images and XRD patterns. The average particle size was found to be from 30 to 60 nm for all nanomaterials discussed. Size distribution was also found to be very fine around this range, as indicated by the SEM images in Figure 1. XRD patterns for various nanoparticles match with their standard profiles. Figure 2 presents XRD profiles for Fe3O4, NiO, CoO, and SnO. From XRD we see that material of the particles have very close pattern to the standard ones that confirmed the material and its purity level. The purity and material confirmation grade is >95% for all kinds of particles. The size of all particles was counterchecked by applying Scherrer formula by considering the peak broadness and full-width half maxima (FWHM) of all patterns. This confirmed that the size of all particles is in nanorange. Figure 3 shows results for iron oxide nanoparticles coated with monoethylene glycol (MEG), diethylene glycol (DEG), and PEG. These figures show that even after coating with PEG, iron oxide nanoparticles maintain their crystalline structure. However, different levels of peak broadening are evident (give FWHM of different curves). In Figure 3, broader peaks for iron oxide nanoparticles coated with different PEG can be observed. The peak broadening increases with the size of PEG molecule and for PEG peaks are wider as compared with the MEG. These broader peaks indicate thinner size of the particles due to coating material.
FIGURE 1.
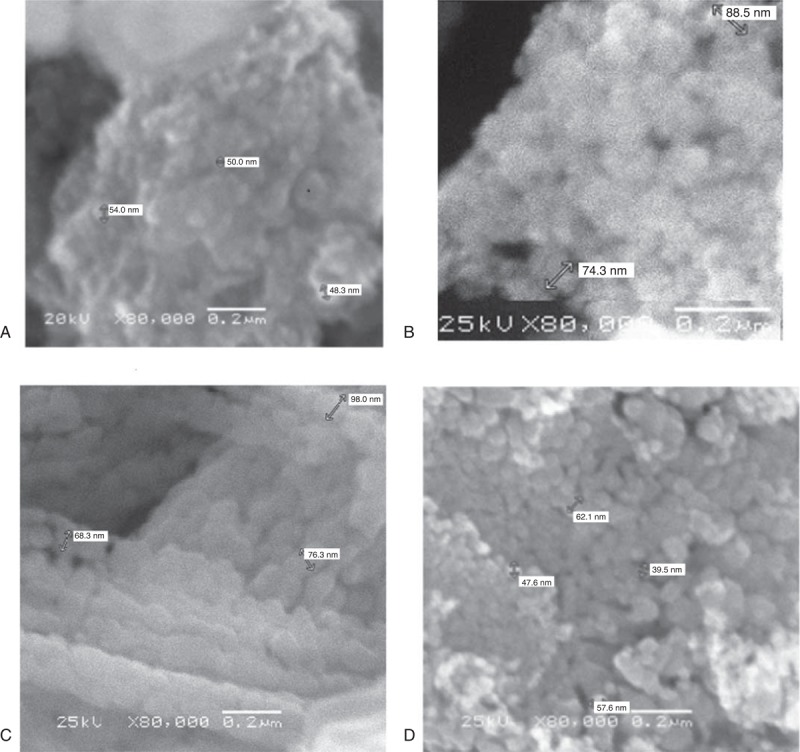
SEM images of various nanoparticles. Images show that nanoparticles are of the size of 30 to 60 nm and size distribution is almost uniform and thin. (A) Iron oxide. (B) Nickle oxide. (C) Cobalt oxide. (D) Stannous oxide. SEM = scanning electron microscope.
FIGURE 2.
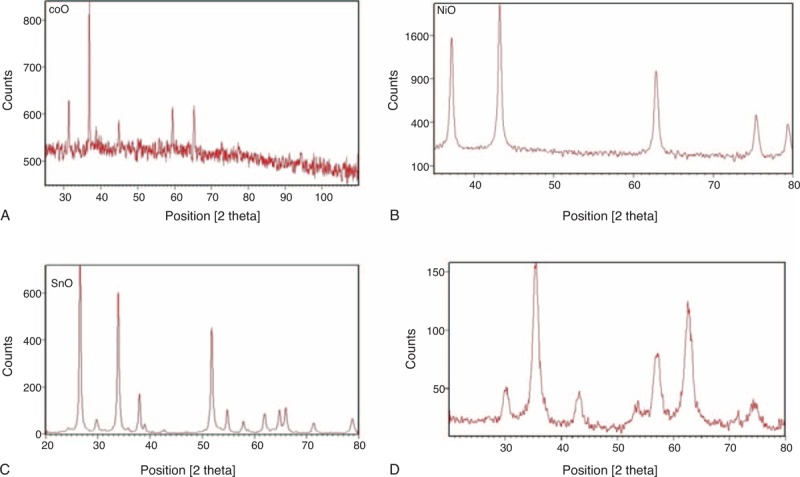
XRD profiles for noncoated nanoparticles of different metal oxides. These profiles indicate the formation of crystalline particles of a particular material. (A) XRD profile for cobalt oxide, (B) nickel oxide, (C) stannous oxide, and (D) iron oxide. XRD = x-ray diffraction.
FIGURE 3.
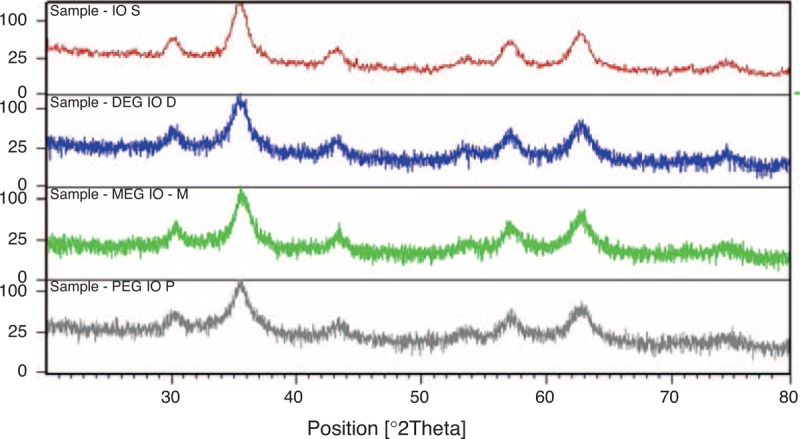
XRD patterns for surface-coated iron oxide nanomaterials. Peaks get broadened and a little noisy with coated iron oxide nanoparticles whereas simple iron oxide pattern is less noisy. XRD = x-ray diffraction.
Drug Loading
Results of drug loading on different nanoparticles are presented in Figures 4 and 5. Figure 4 compares drug loading of various nanomaterials without any surface coating, whereas Figure 5 presents comparative drug loading for coated iron oxide nanoparticles. For noncoated CoO, nanoparticles seem to have a sufficient drug-loading values, whereas other nanomaterials do not have any drug loaded or entrapped into the particle structure. Similarly, iron oxide nanoparticles accept almost half of the drug as compared with the CoO nanoparticles. However, SnO and NiO seem to accept least level of drug loading. Figure 5 presents DOX loading onto PEG-coated iron oxide nanoparticles. These profiles suggest that we get maximum loading with MEG whereas DOX loading reduces with DEG and PEG. With PEG, there is some loading that is gradual for the initial 12 hours whereas for DEG and MEG, loading is abrupt in the initial couple of hours and get almost sustained afterward.
FIGURE 4.
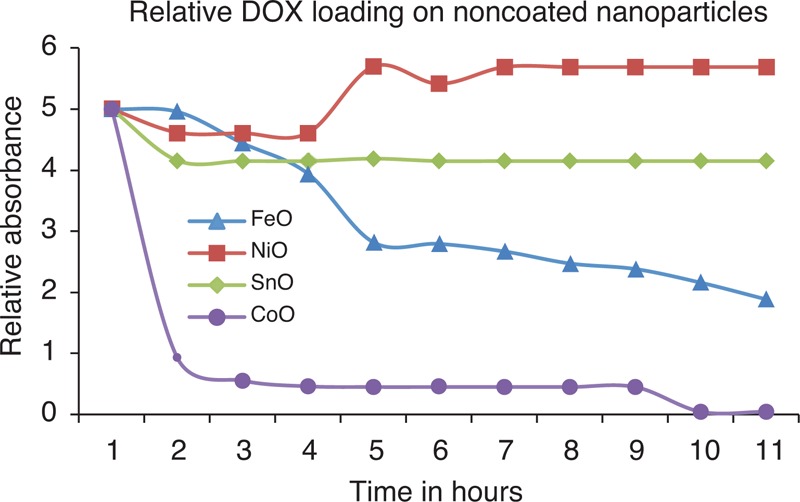
Results for DOX drug loading onto the nanoparticles. These results are for uncoated nanoparticles and it is obvious that cobalt oxide nanoparticles can load a sufficient amount of drug onto them even without any surface coating. DOX = doxorubicin, XRD = x-ray diffraction.
FIGURE 5.
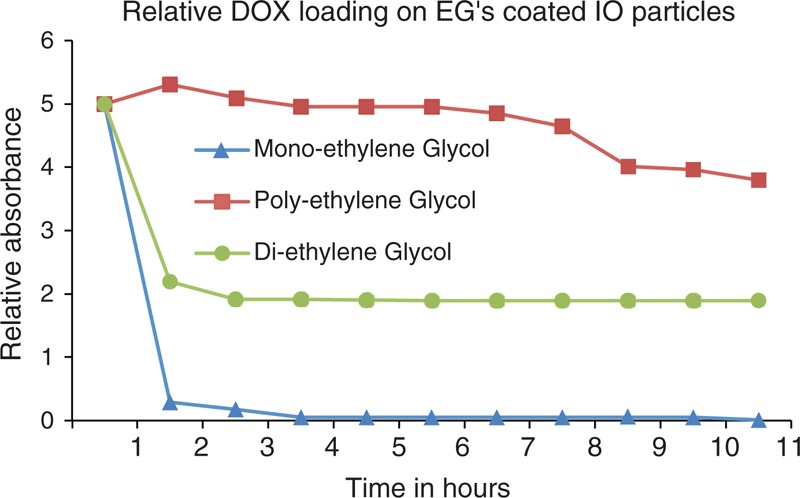
Results for DOX loading onto the coated iron oxide nanoparticles with different coated molecules. DOX = doxorubicin.
DISCUSSION
This work describes synthesis and characterization of various transition metal oxide nanomaterials with a water-soluble anticancer drug-loading efficiency for a very simple loading method. Different metal oxide nanoparticles were synthesized by wet chemical method of coprecipitation because of its simplicity and popularity and well-defined analysis of the results available in the literature.6,20 Results have shown that sufficiently small nanoparticles of sizes from 30 to 60 nm can be synthesized with fine size distribution using the above-mentioned methods. Particles <4 μm are eliminated by cells of the RES, mainly in the liver (60%–90%) and the spleen (3%–10%). Particles >200 nm are usually filtered by the spleen,18 the cutoff point of which extends up to 250 nm. Particles up to 100 nm are mainly phagocytosed via liver cells. In general, it is observed that the larger the particles, the shorter their plasma half-life. Another limiting factor is due to the physiological barriers present in the human body, such as BBB and low blood retention times. Morphological characteristics of the particles, such as particles size, shape, distribution of size and surface charges available, vitally effect the pharmaco-kinectics of these particles in-vivo.30,31 These properties directly influence the uptake and clearance of these particles to and from various organs of the body.
Nanomaterials are being widely used for drug delivery purposes in cancer treatment to alleviate the problems of undesirable side effects due to unwarranted accumulation of anticancer drug in the healthy tissues. Our comparison results show that nanomaterials, in functionalized form, are able to load sufficient quantities of the target drug onto their nanocarriers. This study describes an analysis of various transition metal oxide nanoparticles for their drug-loading behavior, with or without being functionalized, because of their better shelf life and biotoxicity. We observed that CoO nanoparticles are able to load DOX without any surface coating. This is not a case with other transition metal oxide nanoparticles, for example, NiO and SnO, whereas Fe3O4 nanoparticles seem to load relatively more quantity of the anticancer drug. This is in contrast to the most published results where surface-modified nanoparticles are used to facilitate relatively more anticancer drug onto the nanoparticles. It is well known that certain characteristics of nanocarriers, such as morphology including shape and size, surface charges, electrostatic charges, or availability of binding sites, count toward the drug quantity loaded onto the nanoparticles. Surface functionalization affects these parameters and ultimately accounts for the amount of the loaded drug. Our sample characterization showed all favorable results for the sake of drug-loading purposes.
Instead of synthesis analysis, our main analysis in this work was on drug-loading efficiency and behavior. We see that surface-coated particles of reasonable size (10–30 nm) are useful for biomedical applications as well as surface coating seems to have pronounced effect on drug loading of different nanoparticles. Controlled drug loading and release is very important for water-insoluble anticancer drugs or to reduce undesirable side effects caused by drug delivery to the healthy tissue. Understanding of drug-loading phenomena onto the nanoparticles is really challenging, although it is very simple to carry out actual loading. Drugs may be loaded during the synthesis or postsynthesis process. Our view is that the ultimate same amount of drug should be loaded onto the nanocarriers if particle morphology, loading methods, and environmental parameters are kept same. We stirred nanoparticles in a saturated aqueous drug solution for the drug to be absorbed or adsorbed onto the particles. Different procedures are considered to be responsible for entrapment of the drug onto the nanoparticles such as diffusion, surface charges, or encapsulation of the drug into the space between coating and the particle. Interpretation of drug loading are generally considered to be chemical-covalent/noncovalent/physical bonding including all other bindings such as hydrophobic, electrostatic, or hydrogen bonding, which may result in surface attachments or encapsulation of the drug into the nanocarriers. Both of these phenomena should facilitate optimal loading sites and minimal hindrance to diffusion process of the drug on to the nanoparticles surface, which certainly depends on the particle itself and its neighboring environment. Noncovalent bonding is believed to be less firmly attached drug to the particle and therefore it is considered to be more sensitive to the particle and its environment.
From the drug-loading results for coated nanoparticles, we found that the amount of anticancer drug load reduces with increasing size of the coating molecule. This analysis is found notably interesting because it is very important to decide for the coating material for drug delivery purposes. However, our important result is that in case of CoO nanoparticles, drug may be loaded without any surface coating of the material. Drug loading on cobalt nanoparticles may be similar to the loading of anticancer drug onto the PEG coated or surface functionalized nanoparticles, which depends on the solubility of the drug to the recipient matrix, drug polymer interactions, molecular weights, and end-functional groups both in drug and the polymer. Two parameters that are binding site availability and drug diffusion mainly affect the drug-loading efficiency. Stability may be evaluated in ideal conditions or pure solvent and a better understanding requires studying behavior in other complex environment. Following may be the forces that keep the conjugate stable (or loading): van der Waals attractive forces, electrostatic stabilization, and static repulsion stabilization. Drug release is considered to be dependent on surrounding environment, such as pH or temperature, and similarly loading will also be influenced by these parameters. Ratio of loaded to free drug in a particular environment may give an indication of drug loading, release, or stability and may be studied, for example, by reducing fluorescence of DOX molecules while being loaded onto gold nanoparticles.
CONCLUSIONS
We compared DOX drug-loading efficiency of various transition metal oxide nanoparticles with or without surface coating in terms of drug diffusion onto the particles using UV–visual results. It was observed that surface functionalization of the nanomaterials helps obtaining more drug load onto the nanoparticles. A keen observation was that nanoparticles coated with lighter molecules in terms of molecular weight carry more drugs as compared with the particles coated with heavier coating material. CoO nanoparticles, however, can exceptionally load enough anticancer drugs even without any surface coating. This behavior requires further detailed insight into the loading physics and chemistry.
Footnotes
Abbreviations: BBB = blood–brain barrier, DEG = diethylene glycol, DOX = doxorubicin, FWHM = full-width half maxima, MEG = monoethylene glycol, PEG = polyethylene glycol, RES = reticuloendothelial system, SEM = scanning electron microscope, XRD = x-ray diffraction.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Floyd JD, Nguyen DT, Lobins RL, et al. Cardiotoxicity of cancer therapy. J Clin Oncol 2005; 23:7685–7696. [DOI] [PubMed] [Google Scholar]
- 2.Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 1989; 49:4373–4384. [PubMed] [Google Scholar]
- 3.Teicher BA. Molecular targets and cancer therapeutics: discovery, development and clinical validation. Drug Resist Updat 2000; 3:67–73. [DOI] [PubMed] [Google Scholar]
- 4.Akbarzadeh A, Samiei M, Joo SW, et al. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J Nanobiotechnol 2012; 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Davaran S, Rashidi MR, Pourabbas B, et al. Adriamycin release from poly(lactide-coglycolide)-polyethylene glycol nanoparticles: synthesis, and in vitro characterization. Int J Nanomed 2006; 1:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gref R, Minamitake Y, Peracchia MT, et al. Biodegradable long-circulating polymeric nanospheres. Science 1994; 263:1600–1603. [DOI] [PubMed] [Google Scholar]
- 7.Lehman SE, Larsen SC. Zeolite and mesoporous silica nanomaterials: greener syntheses, environmental applications and biological toxicity. Environ Sci: Nano 2014; 1:200–213. [Google Scholar]
- 8.Lim EK, Jang E, Lee K, et al. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics 2013; 5:294–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 2008; 3:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shikata F, Tokumitsu H, Ichikawa H, et al. In vitro cellular accumulation of gadolinium incorporated into chitosan nanoparticles designed for neutron-capture therapy of cancer. Eur J Pharm Biopharm 2002; 53:57–63. [DOI] [PubMed] [Google Scholar]
- 11.Mitra S, Gaur U, Ghosh PC, et al. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release 2001; 74:317–323. [DOI] [PubMed] [Google Scholar]
- 12.Na K, Lee ES, Bae YH. Adriamycin loaded pullulan acetate/sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J Control Release 2003; 87:3–13. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Pei Y, Zhang X, et al. PEGylated PLGA nanoparticles as protein carriers: synthesis, preparation and biodistribution in rats. J Control Release 2001; 71:203–211. [DOI] [PubMed] [Google Scholar]
- 14.Jeong YI, Nah JW, Lee HC, et al. Adriamycin release from flower-type polymeric micelle based on star-block copolymer composed of poly(gamma-benzyl L-glutamate) as the hydrophobic part and poly(ethylene oxide) as the hydrophilic part. Int J Pharm 1999; 188:49–58. [DOI] [PubMed] [Google Scholar]
- 15.Kwon GS, Naito M, Yokoyama M, et al. Physical entrapment of adriamycin in AB block copolymer micelles. Pharm Res 1995; 12:192–195. [DOI] [PubMed] [Google Scholar]
- 16.Grimsdale AC, Mullen K. The chemistry of organic nanomaterials. Angew Chem Int Ed Engl 2005; 44:5592–5629. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Hu W. Organic Nanomaterials. In: Vajtai R, ed. Springer Handbook of Nanomaterials. Berlin/Heidelberg: Springer; 2013. 905–940. [Google Scholar]
- 18.Ahmd M, Rashid K, Nadeem M, et al. A simple method to prepare aqueous dispersion of iron oxide nanoparticles and their biodistribution study [abstract]. J Colloid Sci Biotechnol 2012; 1:201–209. [Google Scholar]
- 19.Nance EA, Woodworth GF, Sailor KA, et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci Transl Med 2012; 4:149ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GarcÃ-a-Jimeno S, Estelrich J. Ferrofluid based on polyethylene glycol-coated iron oxide nanoparticles: characterization and properties. Colloids Surf A Physicochem Eng Aspects 2013; 420:74–81. [Google Scholar]
- 21.Duncan R. Drug-polymer conjugates: potential for improved chemotherapy. Anticancer Drugs 1992; 3:175–210. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama M, Kwon GS, Okano T, et al. Preparation of micelle-forming polymer-drug conjugates. Bioconjug Chem 1992; 3:295–301. [DOI] [PubMed] [Google Scholar]
- 23.Colin d V, Dubernet C, Nemati F, et al. Uptake of doxorubicin from loaded nanoparticles in multidrug-resistant leukemic murine cells. Cancer Chemother Pharmacol 1994; 33:504–508. [DOI] [PubMed] [Google Scholar]
- 24.Minko T, Kopeckova P, Pozharov V, et al. HPMA copolymer bound adriamycin overcomes MDR1 gene encoded resistance in a human ovarian carcinoma cell line. J Control Release 1998; 54:223–233. [DOI] [PubMed] [Google Scholar]
- 25.Jeong JH, Lim DW, Han DK, et al. Synthesis, characterization and protein adsorption behaviors of PLGA/PEG di-block co-polymer blend films. Colloids Surf B Biointerfaces 2000; 18:371–379. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Li S, Pu Y, et al. Nanoparticles generated by PEG-Chrysin conjugates for efficient anticancer drug delivery. Eur J Pharm Biopharm 2014; 87:454–460. [DOI] [PubMed] [Google Scholar]
- 27.Salvadori MR, Nascimento CA, Correa B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci Rep 2014; 4:6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishore PN, Jeevanandam P. Synthesis of cobalt oxide nanoparticles via homogeneous precipitation using different synthetic conditions. J Nanosci Nanotechnol 2013; 13:2908–2916. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee A, Ahmaruzzaman M, Sinha T. A novel approach for the synthesis of SnO2 nanoparticles and its application as a catalyst in the reduction and photodegradation of organic compounds. Spectrochim Acta A Mol Biomol Spectrosc 2015; 136 (Pt B):751–760. [DOI] [PubMed] [Google Scholar]
- 30.Hoff D, Sheikh L, Bhattacharya S, et al. Comparison study of ferrofluid and powder iron oxide nanoparticle permeability across the blood-brain barrier. Int J Nanomed 2013; 8:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muhammad N, Munir A, Saeed MA, et al. Uptake and clearance analysis of Technetium99m labelled iron oxide nanoparticles in a rabbit brain. IET Nanobiotechnol 2015; E-First Article (14), 6 pp. [DOI] [PubMed] [Google Scholar]


