Abstract
Mucinous adenocarcinoma (MAC) is a histological subtype of colorectal cancer. The oncologic behavior of MAC differs from nonmucinous adenocarcinoma (non-MAC). Our aim in this study was to characterize patients with colorectal MAC through evaluation of a large, institutional-based cohort with long-term follow-up.
A total of 6475 patients with stages I to III colorectal cancer who underwent radical surgery were enrolled from January 2000 to December 2010. Prognostic comparison between MAC (n = 274, 4.2%) and non-MAC was performed.
The median follow-up period was 48.0 months. Patients with MAC were younger than those without MAC (P = 0.012) and had larger tumor size (P < 0.001), higher preoperative carcinoembryonic antigen (P < 0.001), higher pathologic T stage (P < 0.001), more right-sided colon cancer (49.3%, P < 0.001), and more frequent high-frequency microsatellite instability (10.2%, P < 0.001). Five-year disease-free survival (DFS) was 76.5% in the MAC group and 83.2% in the non-MAC group (P = 0.008), and 5-year overall survival was 81.4% versus 87.4%, respectively (P = 0.005). Mucinous histology (MAC vs non-MAC) in the entire cohort was not an independent prognostic factor of DFS but had a statistical tendency (P = 0.071). In subgroup analysis of colon cancer without rectal cancer, mucinous histology was an independent prognostic factor (P = 0.026).
MAC was found at more advanced stage, located mainly at the right side and was an independent factor of survival in colon cancer. Because of the unique biological behavior of MAC, patients with MAC require special consideration during follow-up.
INTRODUCTION
Mucinous adenocarcinoma (MAC) is a histological subtype of colorectal cancer. Population-based studies have indicated that MACs accounts for 3.9% to 19% of colorectal cancer worldwide.1–5 According to the World Health Organization, MAC is “an adenocarcinoma in which a substantial amount of mucin (>50% of the tumor) is retained within the tumor.”6
The oncologic behavior of MAC differs from nonmucinous adenocarcinoma (non-MAC).7 In particular, MAC has a worse prognosis than the non-MAC form. Several studies have reported the characteristics of MAC, but these reports have limitations. Because MAC is relatively rare among adenocarcinomas, few large-scale studies on it have been performed. Even for a large-scale study, the period of data collection would need to be as long as 30 years to compare the results with hereditary nonpolyposis colorectal cancer (HNPCC) and sporadic cancer. Limited information is provided in retrospective studies.2,5,8 Postoperative survival for patients with MAC by stage is unclear and factors related to the survival of patients with MAC have not been well studied. Therefore, our aim in this study was to characterize patients with colorectal MAC through evaluation of a large, institution-based cohort with long-term follow-up.
METHODS
Records of 10,413 consecutive patients who underwent surgery for colorectal cancer from January 2000 to December 2010 were analyzed. Patients with palliative resection, emergent surgery, familial adenomatous polyposis or HNPCC, nonproven adenocarcinoma, signet ring cell type, and stage IV and recurrent or metachronous cancer were excluded. Patients who underwent preoperative therapy such as concurrent chemoradiotherapy, radiotherapy, or chemotherapy were also excluded. A total of 6475 patients were enrolled. Demographic data including age, sex, preoperative carcinoembryonic antigen (CEA) level, clinical bowel obstruction, clinical bowel perforation, pathologic features (T stage, N stage, lymphovascular and perineural invasion, microsatellite instability [MSI] status), and adjuvant therapy were collected and analyzed. MAC was defined as a tumor containing more than half mucin by volume on histologic examination with pools of extracellular mucin containing malignant epithelium as acinar structure, strips of cells, or single cells.4 Colorectal cancer was staged according to the seventh American Joint Committee on Cancer tumor node metastasis staging system. All pathologic slides were examined by 2 experienced colorectal pathologists. High-frequency MSI (MSI-H) was defined as ≥2 of the 5 markers exhibiting instability, and low-frequency MSI (MSI-L) was defined as when only 1 of the 5 markers was unstable. Tumors with MSI-L belong to the category of microsatellite stability (MSS) tumors because they have a common molecular background.9 Tumors that exhibited MSI-H were classified as MSI and other tumors were classified as having MSS.10 All colorectal resection was performed with curative intent. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea.
Postoperative adjuvant treatment depended on the patient's general condition and compliance and physician preference. Postoperative 5-fluorouracil-based chemotherapy was considered for all patients with T3, T4, or node-positive disease. Local recurrence, distant metastasis, 5-year disease-free survival (DFS), and 5-year overall survival (OS) were assessed. Patients were followed at 3-month intervals for 2 years, at 6-month intervals for the next 3 years, and annually thereafter. On a semiannual basis or on suspicion of recurrence, follow-up examinations, including clinical history, physical examination, serum CEA assay, chest x-ray, abdominopelvic computed tomography (CT) or magnetic resonance imaging, colonoscopy, or positron emission tomography (PET) scanning, were performed. Recurrence was determined by clinical and radiologic examination or histologic confirmation. The main pattern of recurrence was recorded as the first site of detectable failure during the follow-up period.
Statistical analyses were carried out using the Statistical Package for the Social Sciences for Windows, version 18.0 (SPSS Inc, Chicago, IL). The significance of differences between the groups was evaluated using a χ2 test or analysis of variance, as appropriate. Survival rates were calculated using the Kaplan–Meier method and prognostic factors and survival curves were compared using log-rank test factors. Factors that were significant (P ≤ 0.05) on univariate analysis were entered into multivariate analysis using the Cox model. Variables potentially related to the risk of DFS or OS with P ≤ 0.100 on univariate analysis were included in multivariate analysis. A P value of ≤0.05 was considered statistically significant.
RESULTS
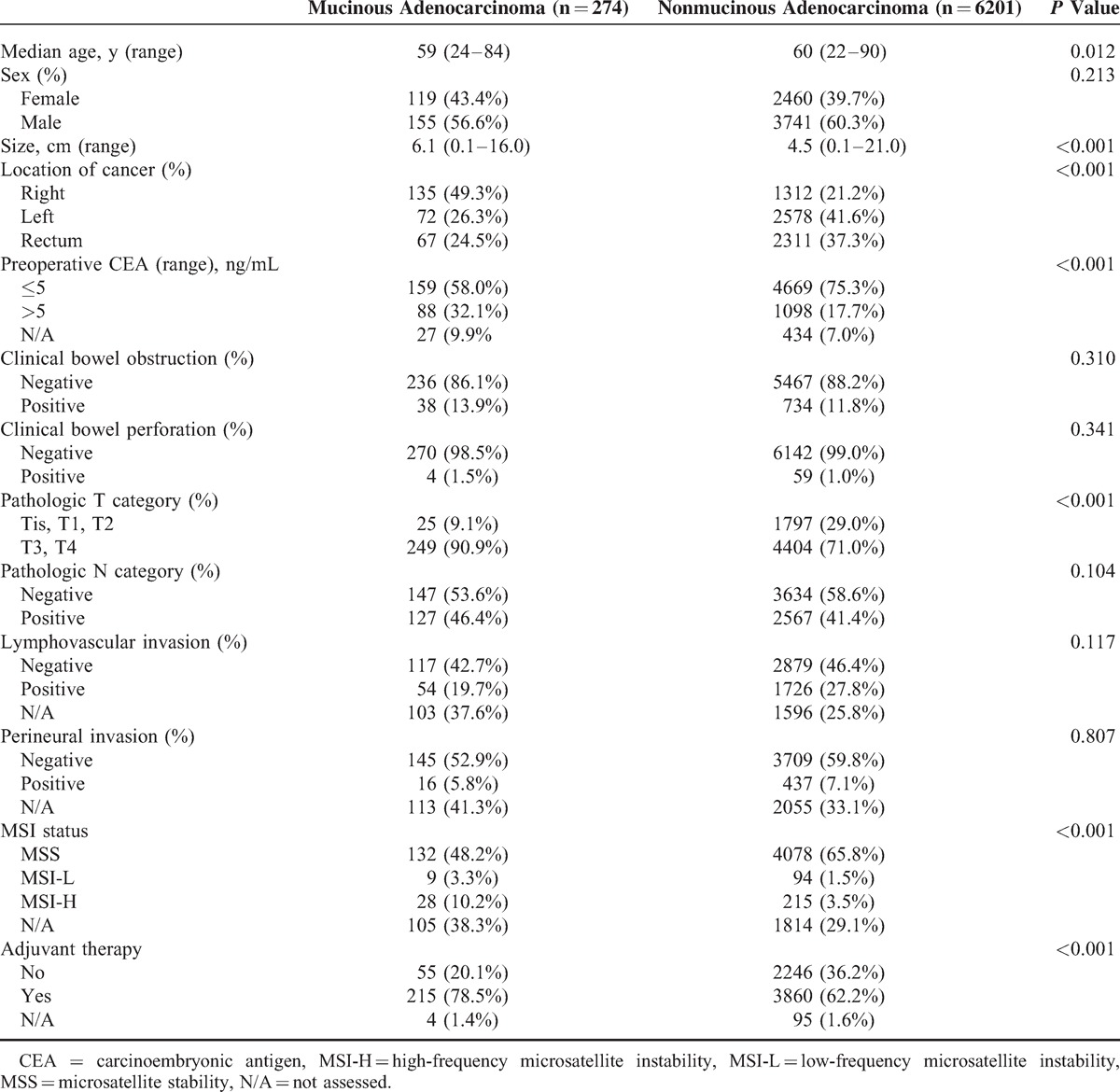
The demographic features of patients with MAC and non-MAC are in Table 1. The 2 groups showed no differences in sex, pathologic N category, bowel obstruction, bowel perforation, and lymphovascular or perineural invasion. Patients with MAC were younger than those with nonmucinous carcinoma (P = 0.012) and had larger tumor sizes (P < 0.001), higher preoperative CEA (P < 0.001) and higher pathologic T stage (P < 0.001). MAC was mainly located in the right colon (49.3% MAC vs 21.2% non-MAC, P < 0.001). Patients with MSI-H were more often in the mucinous group (Table 1). Adjuvant therapy such as concurrent chemoradiotherapy, chemotherapy, or radiotherapy was administered to 215 patients (78.5%) in the mucinous group and 3860 (62.2%) in the nonmucinous group. The median follow-up period was 48.0 months (range: 0–165 months).
TABLE 1.
Clinicopathological Features of Patients With Colorectal Nonmucinous and Mucinous Adenocarcinoma
We analyzed 5-year DFS and 5-year OS between the 2 groups and found that 5-year DFS was 76.5% in the mucinous group and 83.2% in the nonmucinous group (P = 0.008). The 5-year OS results were significantly different at 81.3% for the mucinous group versus 87.4% for the nonmucinous group (P = 0.005) (Figure 1). In subgroup analysis for stage 3 colorectal cancer, there was difference in the 5-year DFS, 75.7% in the mucinous group and 68.0% in the nonmucinous group (P = 0.018). Five-year OS of stage 3 was also different (88.5% vs 71.0%, respectively) (P < 0.001). But, 5-year DFS and OS in stages 0, 1, and 2 revealed that there were no significant differences between the 2 groups.
FIGURE 1.
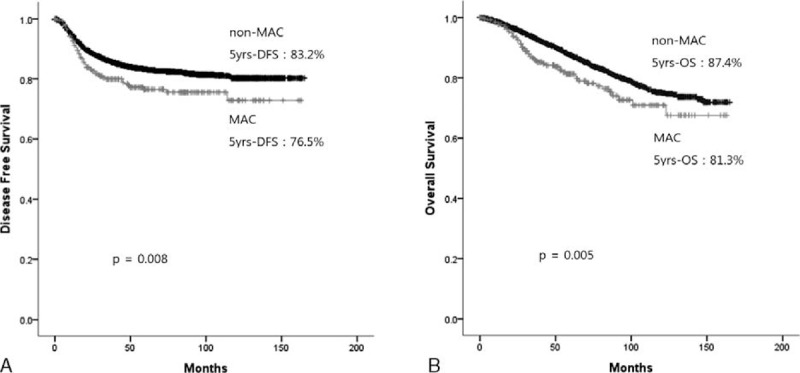
Disease-free survival rate and overall survival rate for the mucinous carcinoma and nonmucinous carcinoma groups.
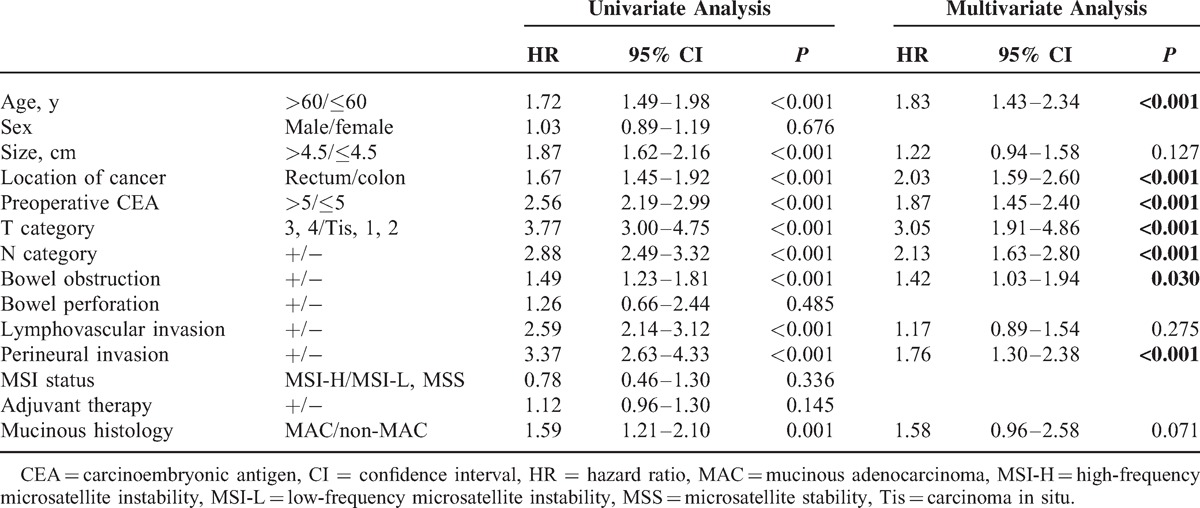
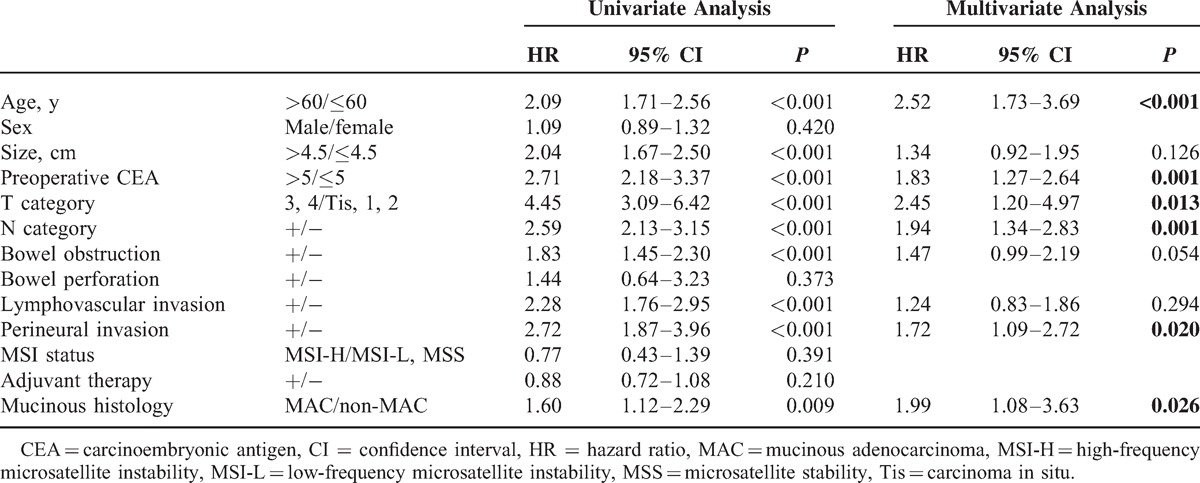
Univariate analyses demonstrated that age (P < 0.001), tumor size (P < 0.001), cancer location (P < 0.001), preoperative CEA (>5 ng/mL) (P < 0.001), pathologic T category (P < 0.001), pathologic N category (P < 0.001), bowel obstruction (P < 0.001), lymphovascular invasion (P < 0.001), perineural invasion (P < 0.001), and mucinous histology (P = 0.001) were associated with DFS. Multivariate analysis confirmed that age (P < 0.001), location of cancer (P < 0.001), preoperative CEA (>5 ng/mL) (P < 0.001), pathologic T category (P < 0.001), pathologic N category (P < 0.001), bowel obstruction (P = 0.030), and perineural invasion (P < 0.001) were independent prognostic factors for DFS. However, mucinous histology (MAC or non-MAC) was not an independent factor for DFS (P = 0.071) (Table 2).
TABLE 2.
Predictive Factors for Disease-Free Survival by Univariate and Multivariate Analyses of the Cohort (n = 6475)
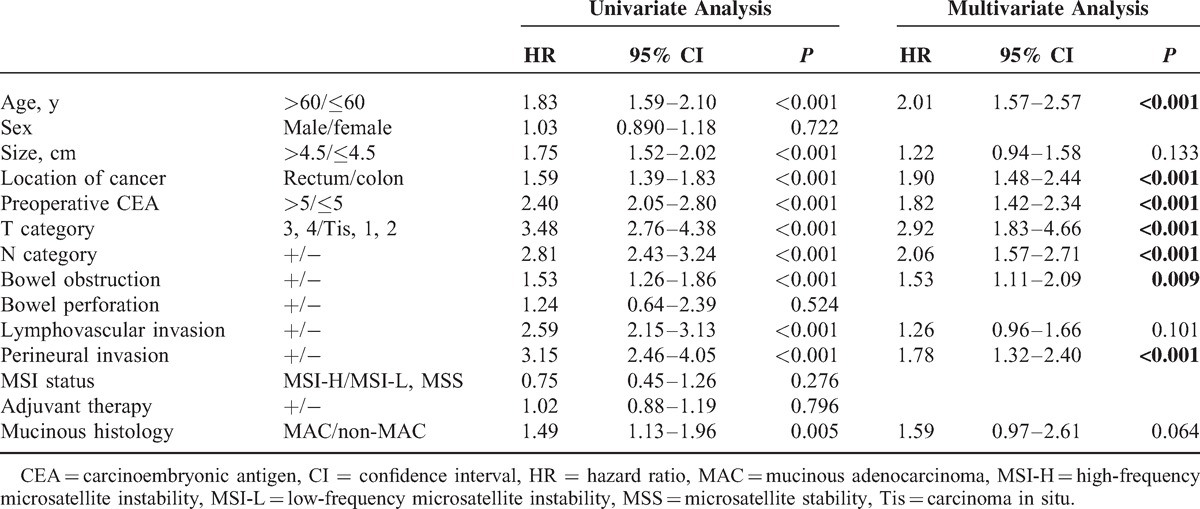
The results of univariate analyses of the relationships between tested factors and OS were not different from the results for DFS. Multivariate analysis confirmed that age (P < 0.001), cancer location (P < 0.001), preoperative CEA (>5 ng/mL) (P < 0.001), pathologic T category (P < 0.001), pathologic N category (P < 0.001), bowel obstruction (P = 0.009), and perineural invasion (P < 0.001) were independent prognostic factors for OS (Table 3).
TABLE 3.
Predictive Factors for Overall Survival by Univariate and Multivariate Analyses of the Cohort (n = 6475)
Multivariate subgroup analysis for colon location without rectal cancer revealed that age (P < 0.001), preoperative CEA (>5 ng/mL) (P < 0.001), pathologic T category (P = 0.013), pathologic N category (P = 0.001), perineural invasion (P = 0.020), and mucinous histology (P = 0.026) were independent factors for DFS (Table 4). However, in multivariate analysis, mucinous histology for rectal location was not an independent prognostic factor (P = 0.854). In addition, we performed subgroup analysis according to cancer stage. Multivariate analysis for stage III colorectal cancer revealed that mucinous histology had no significance for DFS (P = 0.057).
TABLE 4.
Predictive Factors for Disease-Free Survival by Univariate and Multivariate Analyses for Cancers of the Colon (n = 4097)
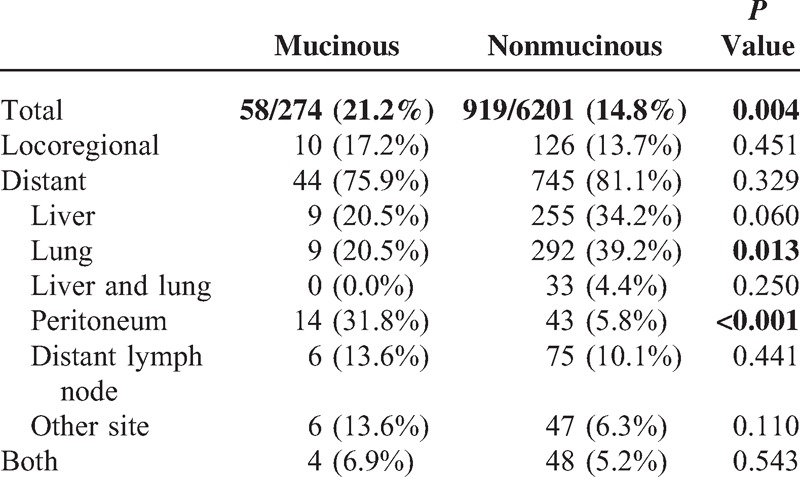
Recurrence occurred in 58 of 274 patients (21.2%) in the MAC group and in 919 of 6201 (14.8%) in the non-MAC group (P = 0.004). Locoregional recurrence was 17.2% (10/58) in the MAC group and 13.7% (126/919) in the non-MAC group (P = 0.451). Distant recurrence was 75.9% (44/58) and 81.1% (745/919), respectively (P = 0.329). In distant recurrence, lung metastasis occurred more often in the nonmucinous group (20.5% vs 39.2%, P = 0.013) but peritoneal metastasis was found more often in the mucinous group (31.8% vs 5.8%, P < 0.001) (Table 5).
TABLE 5.
Recurrence Patterns
DISCUSSION
MAC is an uncommon and rare histopathological type of colorectal cancer. In this study, patients with MAC were younger, had larger tumor sizes, a majority of right-sided colon cancer, and more advanced status and MSI-H compared with patients with non-MAC. These results were in accordance with other several studies.1–5 The proportion of MAC was 4.2% in this study, in close agreement with other studies, which reported results of 3.9% to 19%.1–5 Although several similar studies have been reported, they had a small sample size; our report was a large-scale, long-term study.
Even though poorer DFS and OS were seen for the MAC group compared with the non-MAC group, MAC histology was not a significant prognostic factor for DFS or OS. These results were in agreement with the previous studies.3,5,11 In subgroup analysis according to primary cancer location, mucinous histology was not a prognostic risk factor in subgroup analysis of cancers of the rectum but was an independent prognostic factor for cancers of the colon. The reason might be that MAC tended to be found more often in the colon. This was also a characteristic of MAC in other reports.3,7,11 In addition, in subgroup analysis according to stage, mucinous histology had marginal significance for stage III (P = 0.057). This result corresponded to other results on stage III patients.12 Although distinct clinical results were not shown, the worse prognosis with MAC might be because metastatic regional lymph nodes were involved. The patients with MAC underwent more adjuvant therapy (78.5%) than patients with non-MAC (62.2%) (P < 0.001). It presumed that the patients with MAC had more advanced cases than non-MAC. Although the patients with stage III were not different (46.4% vs 41.4%, P = 0.104), the patients with stage II in MAC (48.2% vs 34.9%, P < 0.001) were more than non-MAC. Pathologic T category in MAC was higher than non-MAC (90.0% vs 71.0%, P < 0.001). This result is in close agreement with other study that the patients with MAC had more advanced tumor stage and could profit from closer follow-up or even intensified adjuvant therapy.11
More local or distant recurrences after radical surgery occurred in the mucinous group. Peritoneal metastasis was common in the mucinous group, whereas lung metastasis occurred more often in the nonmucinous group. Liver metastasis had marginal significance in the nonmucinous group. Several studies suggest that a high rate of peritoneal metastases (36%–48.2%) is observed in patients with MAC.13–15 The reason for the peritoneal metastasis is not clear, but 1 study hypothesized that the production of mucus under pressure allows cancers to separate tissue planes in the bowel wall and gain access to the peritoneal cavity. In addition, the fluid produced by these tumors is taken up by the lymphatic system, which helps to push the tumor into regional lymph nodes.16 Peritoneal metastases are associated with poor prognosis. Survival is even worse if metastases to other organs are present.17 Therefore, in patients with MAC, imaging techniques such as PET-CT should be employed carefully during follow-up for early detection of peritoneal metastases.
More MSI-H is found in MAC than non-MAC patients.12,18 MSI status was not a significant prognostic factor in our results, even in subgroup analysis according to stage (data not shown). This result was in close agreement with a previous study.12 CRC patients with MSI-H have a significantly better prognosis compared to patients with intact mismatch repair.19,20 Well-planned studies are required to resolve this issue.
The limitations of this study were that it was from a single institution and the retrospective study design might result in biases. The 2 groups had different clinicopathologic characteristics. However, our report did not intend to compare and analyze equivalent groups, but to determine the characteristics of MAC and non-MAC.
In conclusion, MAC was found at more advanced stage and was mainly located at the right side of the colon. MAC indicated a worse prognosis for colon cancer. Our data suggested that patients with MAC could benefit from closer follow-up because their high rates of peritoneal metastasis are a known factor for poor prognosis. The biological behavior of MAC differs from non-MAC, so patients with MAC require special awareness during follow-up.
Footnotes
Abbreviations: CEA = carcinoembryonic antigen, CT = computed tomography, DFS = disease-free survival, HNPCC = hereditary nonpolyposis colorectal cancer, MAC = mucinous adenocarcinoma, MSI = microsatellite instability, MSS = microsatellite stability, OS = overall survival, PET = positron emission tomography.
JSP contributed to data collection, analysis, and drafting of the manuscript. JWH developed the study design and proposal, and performed data analysis and final revision of the manuscript. JWH and WYL are responsible for fielding correspondence. Patients were enrolled by YAP, YBC, SHY, HCK, and HKC. All authors approved the final version of the manuscript.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (grant number 2012R1A1A1004888).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stewart SL, Wike JM, Kato I, et al. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer 2006; 107:1128–1141. [DOI] [PubMed] [Google Scholar]
- 2.Du W, Mah JT, Lee J, et al. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum 2004; 47:78–85. [DOI] [PubMed] [Google Scholar]
- 3.Chew MH, Yeo SA, Ng ZP, et al. Critical analysis of mucin and signet ring cell as prognostic factors in an Asian population of 2,764 sporadic colorectal cancers. Int J Colorectal Dis 2010; 25:1221–1229. [DOI] [PubMed] [Google Scholar]
- 4.Lee WS, Chun HK, Lee WY, et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg 2007; 194:294–298. [DOI] [PubMed] [Google Scholar]
- 5.Kang H, O’Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005; 48:1161–1168. [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT. WHO Classification of Tumors of the Digestive System. 4th ed.Lyon: IARC; 2010. [Google Scholar]
- 7.Verhulst J, Ferdinande L, Demetter P, et al. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012; 65:381–388. [DOI] [PubMed] [Google Scholar]
- 8.You JF, Hsieh LL, Changchien CR, et al. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res 2006; 12:4244–4250. [DOI] [PubMed] [Google Scholar]
- 9.Laiho P, Launonen V, Lahermo P, et al. Low-level microsatellite instability in most colorectal carcinomas. Cancer Res 2002; 62:1166–1170. [PubMed] [Google Scholar]
- 10.Sung CO, Seo JW, Kim KM, et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol 2008; 21:1533–1541. [DOI] [PubMed] [Google Scholar]
- 11.Nitsche U, Zimmermann A, Spath C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013; 258:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Shin SJ, Lee KY, et al. Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: a retrospective cohort study. Ann Surg Oncol 2013; 20:3407–3413. [DOI] [PubMed] [Google Scholar]
- 13.Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014; 25:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catalano V, Loupakis F, Graziano F, et al. Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 2009; 100:881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negri FV, Wotherspoon A, Cunningham D, et al. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 2005; 16:1305–1310. [DOI] [PubMed] [Google Scholar]
- 16.Sugarbaker PH. Mucinous colorectal carcinoma. J Surg Oncol 2001; 77:282–283. [DOI] [PubMed] [Google Scholar]
- 17.Lemmens VE, Klaver YL, Verwaal VJ, et al. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011; 128:2717–2725. [DOI] [PubMed] [Google Scholar]
- 18.Ward R, Meagher A, Tomlinson I, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 2001; 48:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005; 23:609–618. [DOI] [PubMed] [Google Scholar]
- 20.Merok MA, Ahlquist T, Royrvik EC, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol 2013; 24:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]


