Abstract
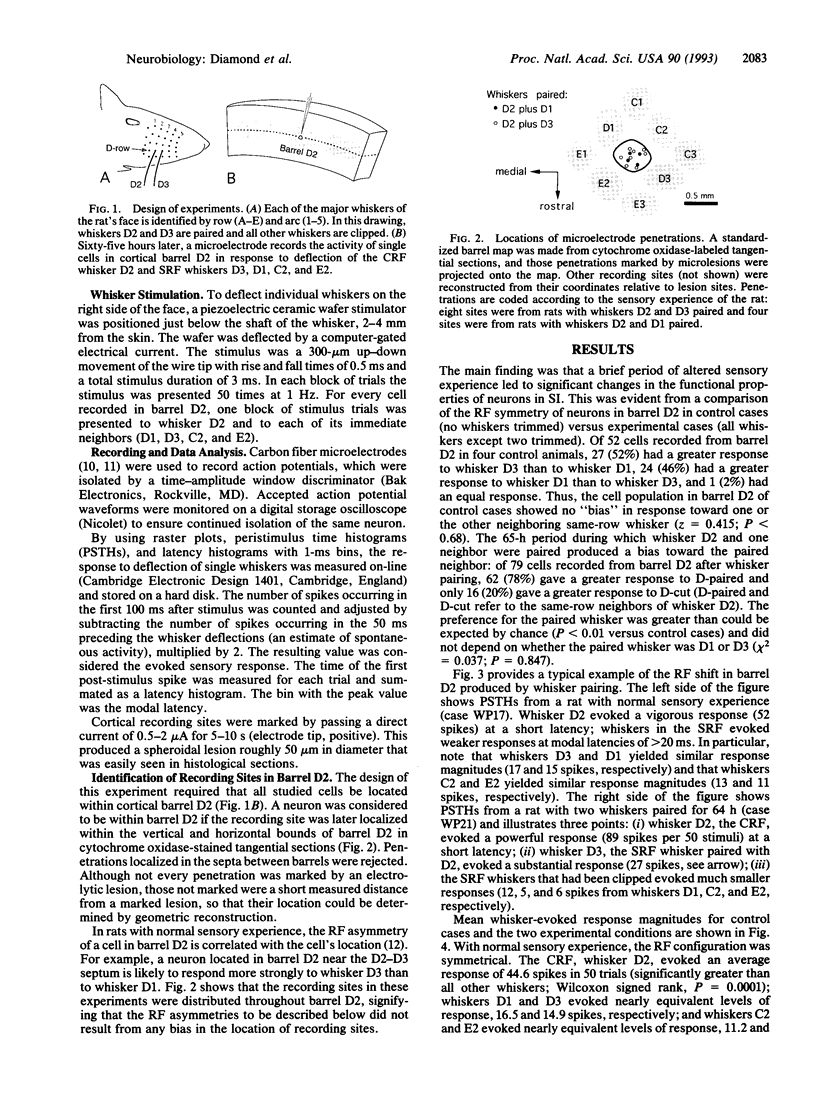
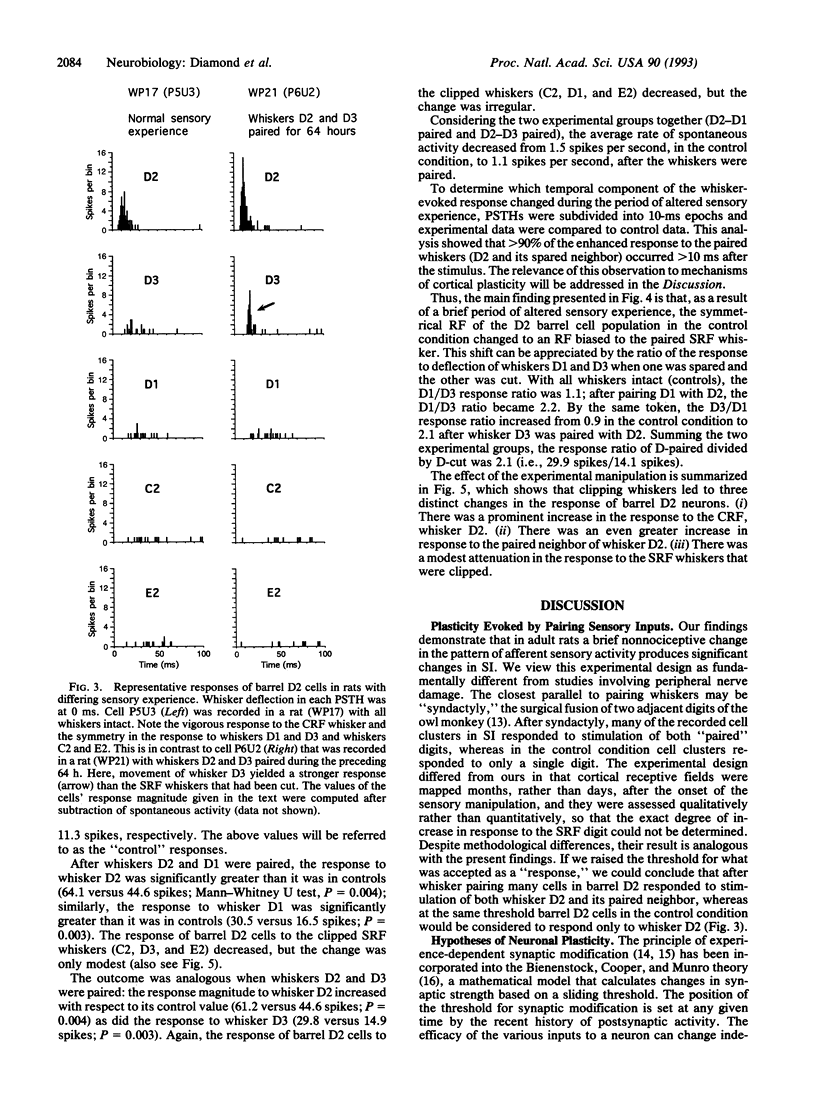
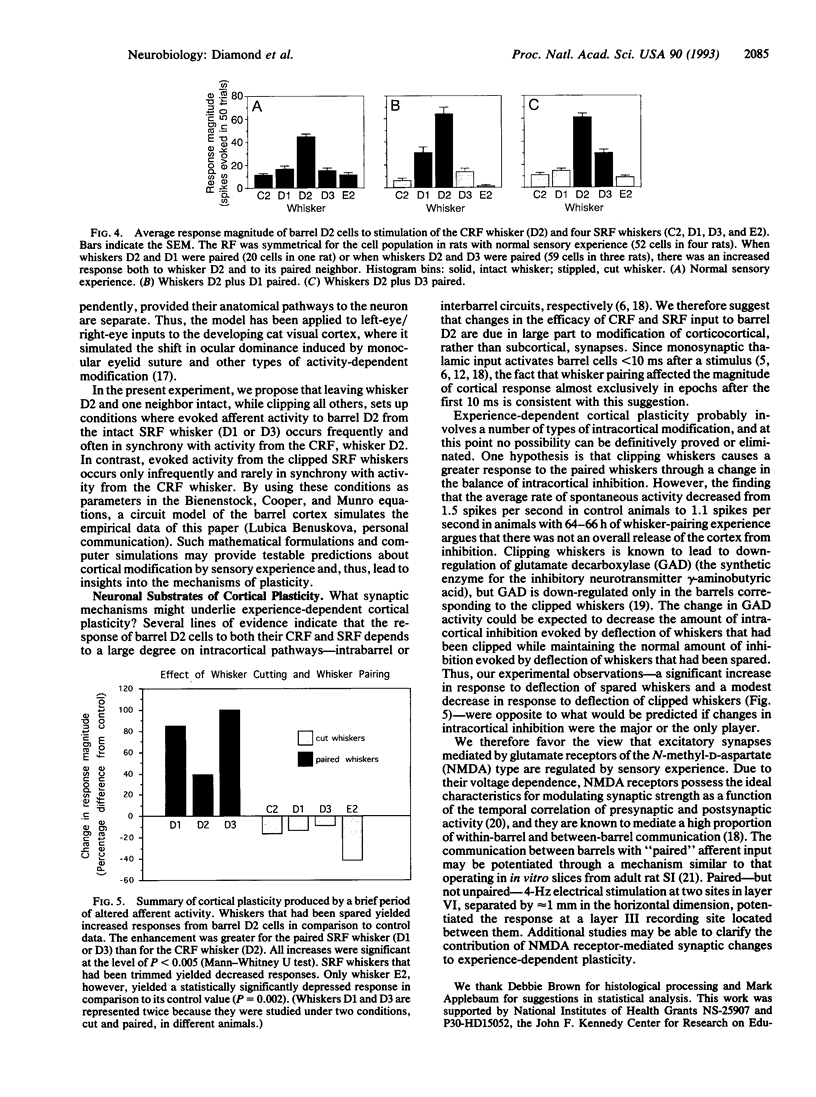
This study tested the hypothesis that the receptive fields (RFs) of neurons in the adult sensory cortex are shaped by the recent history of sensory experience. Sensory experience was altered by a brief period of "whisker pairing": whiskers D2 and either D1 or D3 were left intact, while all other whiskers on the right side of the face were trimmed close to the fur. The animals were anesthetized 64-66 h later and the responses of single neurons in contralateral cortical barrel D2 to stimulation of whisker D2 (the center RF) and the four neighboring whiskers (D1, D3, C2, and E2; the excitatory surround RF) were measured. Data from 79 cells in four rats with whiskers paired were compared to data from 52 cells in four rats with untrimmed whiskers (control cases). During the period of whisker pairing, the RFs of cells in barrel D2 changed in three ways: (i) the response to the center RF, whisker D2, increased by 39%, (ii) the response to the paired surround RF whisker increased by 85-100%, and (iii) the response to all clipped (unpaired) surround RF whiskers decreased by 9-42%. In the control condition, the response of barrel D2 cells to the two neighboring whiskers, D1 and D3, was equal. After whisker pairing, the response to the paired neighbor of D2 was more than twice as large as the response to the cut neighbor of D2. These findings indicate that a brief change in the pattern of sensory activity can alter the configuration of cortical RFs, even in adult animals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar N. D., Land P. W. Activity-dependent regulation of glutamic acid decarboxylase in the rat barrel cortex: effects of neonatal versus adult sensory deprivation. J Comp Neurol. 1991 May 8;307(2):200–213. doi: 10.1002/cne.903070204. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Callahan C. A., Friedman M. A. Thalamo-cortical processing of vibrissal information in the rat. I. Intracortical origins of surround but not centre-receptive fields of layer IV neurones in the rat S1 barrel field cortex. J Comp Neurol. 1991 Jan 8;303(2):193–210. doi: 10.1002/cne.903030203. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Callahan C. A. Thalamo-cortical processing of vibrissal information in the rat. II. spatiotemporal convergence in the thalamic ventroposterior medial nucleus (VPm) and its relevance to generation of receptive fields of S1 cortical "barrel" neurones. J Comp Neurol. 1991 Jan 8;303(2):211–224. doi: 10.1002/cne.903030204. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Evidence for a specific role for cortical NMDA receptors in slow-wave sleep. Brain Res. 1988 Jun 7;451(1-2):189–196. doi: 10.1016/0006-8993(88)90763-9. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K., Millar J. A method for etching the tips of carbon fibre microelectrodes. J Neurosci Methods. 1980 Aug;2(4):431–432. doi: 10.1016/0165-0270(80)90009-6. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K. Spatiotemporal convergence and divergence in the rat S1 "barrel" cortex. J Comp Neurol. 1987 Sep 8;263(2):265–281. doi: 10.1002/cne.902630209. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Millar J. Carbon fibre microelectrodes. J Neurosci Methods. 1979 Oct;1(3):279–287. doi: 10.1016/0165-0270(79)90039-6. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Cooper L. N., Ebner F. F. A physiological basis for a theory of synapse modification. Science. 1987 Jul 3;237(4810):42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. A., Allard T., Jenkins W. M., Merzenich M. M. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988 Mar 31;332(6163):444–445. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Clothiaux E. E., Bear M. F., Cooper L. N. Synaptic plasticity in visual cortex: comparison of theory with experiment. J Neurophysiol. 1991 Nov;66(5):1785–1804. doi: 10.1152/jn.1991.66.5.1785. [DOI] [PubMed] [Google Scholar]
- Dawson D. R., Killackey H. P. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. J Comp Neurol. 1987 Feb 8;256(2):246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Lee S. M., Weisskopf M. G., Ebner F. F. Horizontal long-term potentiation of responses in rat somatosensory cortex. Brain Res. 1991 Mar 29;544(2):303–310. doi: 10.1016/0006-8993(91)90069-8. [DOI] [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Wong-Riley M. T., Welt C. Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and adult mice. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2333–2337. doi: 10.1073/pnas.77.4.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey T. A., Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970 Jan 20;17(2):205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]