Abstract
Immunoglobulin G4-related disease (IgG4-RD) is a recently discovered systemic condition, in which various organ manifestations are linked by a similar histological appearance. Our knowledge of this condition is still fragmented, as most studies have examined only a few dozen patients or focused on a particular organ manifestation. This study was conducted to learn the demography and patient characteristics of IgG4-RD using a large cohort. A total of 235 consecutive patients with IgG4-RD, diagnosed in 8 general hospitals in the same medical district, were identified by searching the institutions’ radiology database. Inclusion criteria were histology-proven IgG4-RD according to the Pathology Consensus Statement and/or definitive type 1 autoimmune pancreatitis meeting the International Consensus Diagnostic Criteria. Clinical notes and images of selected patients were retrospectively reviewed. All patients were adults (M/F = 4/1). The median age was 67 years (range 35–86). Nine tenths were diagnosed in their 50s to 70s. Among 486 manifestations identified in total, the most common was pancreatitis diagnosed in 142 patients (60%), followed by sialadenitis (34%), tubulointerstitial nephritis (23%), dacryoadenitis (23%), and periaortitis (20%). The majority of patients (95%) had at least 1 of the 5 most common manifestations. Male and female patients differed in their organ manifestations (periaortitis more common in males and sialodacryoadenitis more common in females). Serum IgG4 (normal ≤135 mg/dL) was elevated to >135 mg/dL in 208 patients (88%) and >270 mg/dL in 167 (71%). The IgG4 value was significantly higher in patients with multiorgan involvement than in those with a single manifestation (median 629 mg/dL vs 299 mg/dL, P < 0.01). Of 218 patients, for whom both IgG4 and IgG values were available, the IgG4/IgG ratio was raised to >10% in 194 (89%). Corticosteroids were effective, but the relapse rate was estimated to be 24% in the study period (median 37 months). During the follow-up, 15 malignant diseases were diagnosed in 13 patients (6%). This figure is similar to the incidence (12.9 cancers) expected from the Japanese nationwide study for cancer epidemiology (standardized incidence ratio 1.16). In conclusion, this reliable dataset could improve the characterization of IgG4-RD, particularly its unique demography and the frequency of each organ manifestation.
INTRODUCTION
Immunoglobulin G4-related disease (IgG4-RD) is an emerging systemic condition characterized by mass-forming sclerosing lesions, elevated serum IgG4 concentrations, and extensive tissue infiltration by IgG4+ plasma cells.1 The concept was coined from autoimmune pancreatitis (AIP), which is now called type 1 AIP or IgG4-related pancreatitis.2 Since 2001, when hyper-IgG4 was discovered in type 1 AIP, pathological studies have identified similar lesions in a variety of organs,3–5 eventually leading to the recognition of systemic IgG4-RD.1,6 “Idiopathic” sclerosing lesions, called by different names such as Küttner tumor, Mikulicz disease, and Ormond disease for nearly a century, appeared to be a part of this condition.7–11
Several hundred papers on IgG4-RD have been published in the last decade. However, because most studies examined only dozens of patients or focused on a particular organ manifestation, our knowledge of this condition is still fragmented.8–11 For example, we do not have the answer to even simple questions such as what the most common organ manifestation of IgG4-RD is. The fact that patients are seen in various specialities makes it difficult to analyze all organ manifestations in a single study.
To tackle this problem, we reviewed 235 patients with IgG4-RD consecutively diagnosed in various departments. This is the largest cohort covering all organ manifestations and should give a reliable dataset for IgG4-RD patients.
PATIENTS AND METHODS
Enrolled Hospitals and Patients
This study was approved by the ethics committee of Kanazawa University Hospital in Japan (reference no. 1354). Eight hospitals participated: Kanazawa University Hospital, Toyama Prefectural Central Hospital, Fukui Saiseikai Hospital, Toyama City Hospital, Kouseiren Takaoka Hospital, Tonami General Hospital, Noto General Hospital, and Fukui Prefectural Hospital, all general hospitals with 260 to 800 beds located in the Hokuriku region, population approximately 3 million. These hospitals are affiliated with Kanazawa University Graduate School of Medical Science.
We managed to identify all patients with IgG4-RD diagnosed in these hospitals from January 2005 to December 2013. Although IgG4-RD has become widely recognized only in the last few years, we have studied this condition since 2003 and prospectively diagnosed it.4,8 We searched our institutions’ radiology database using the terms “IgG4,” “autoimmune pancreatitis,” “mass-forming pancreatitis,” “Küttner's tumour,” “Mikulicz's disease,” “sclerosing sialadenitis,” “sclerosing cholangitis,” “sclerosing dacryoadenitis,” “inflammatory pseudotumor,” “periaortitis,” “periarteritis,” “retroperitoneal fibrosis,” “interstitial nephritis,” “inflammatory abdominal aneurysm,” and “multifocal fibrosclerosis” for all fields of radiology reports, including clinical summary, imaging findings, diagnosis, and comments. Clinical notes, images, and pathology data of selected patients were reviewed to identify cases that meet the inclusion criteria described below.
One reason why we used a radiology database is that IgG4-RD patients are supposed to undergo imaging at least once during either the diagnostic process or the follow-up period. Another reason is that radiology databases are superior to pathology archives for that purpose, as pathological examination is not necessary for diagnosis, particularly of type 1 AIP.12 Even if a diagnosis of IgG4-RD was raised by pathological examination first, radiological procedures were most likely carried out subsequently to check other organs.
Inclusion Criteria
Two inclusion criteria were used in this study. The first was histology-proven IgG4-RD. A pathological diagnosis of IgG4-RD was made based on the IgG4-RD Pathology Consensus Statement13: morphological characteristics, an increased absolute number of IgG4+ plasma cells, and elevated IgG4+/IgG+ plasma cell ratio. Diffuse lymphoplasmacytic infiltration with irregular, typically “storiform,” fibrosis, and/or obliterative phlebitis is considered a morphological hallmark. Findings against the diagnosis of IgG4 include necrosis, discrete granuloma, and extensive neutrophilic infiltration. Cut off values of the required absolute number of IgG4+ plasma cells per high-power field were determined based on the organ examined according to a consensus.13 An IgG4+/IgG+ plasma cell ratio of >40% was another requisite, when IgG-stained sections were available.
The second inclusion criterion was having type 1 AIP diagnosed based on the International Consensus Diagnostic Criteria (the category of “definitive” type 1 AIP).12 The diagnosis of type 1 AIP does not always require histological confirmation, as imaging features of this organ manifestation are well established (Figure 1A). For instance, typical imaging findings and serum IgG4 elevation >135 mg/dL lead to the definitive diagnosis of type 1 AIP.
FIGURE 1.
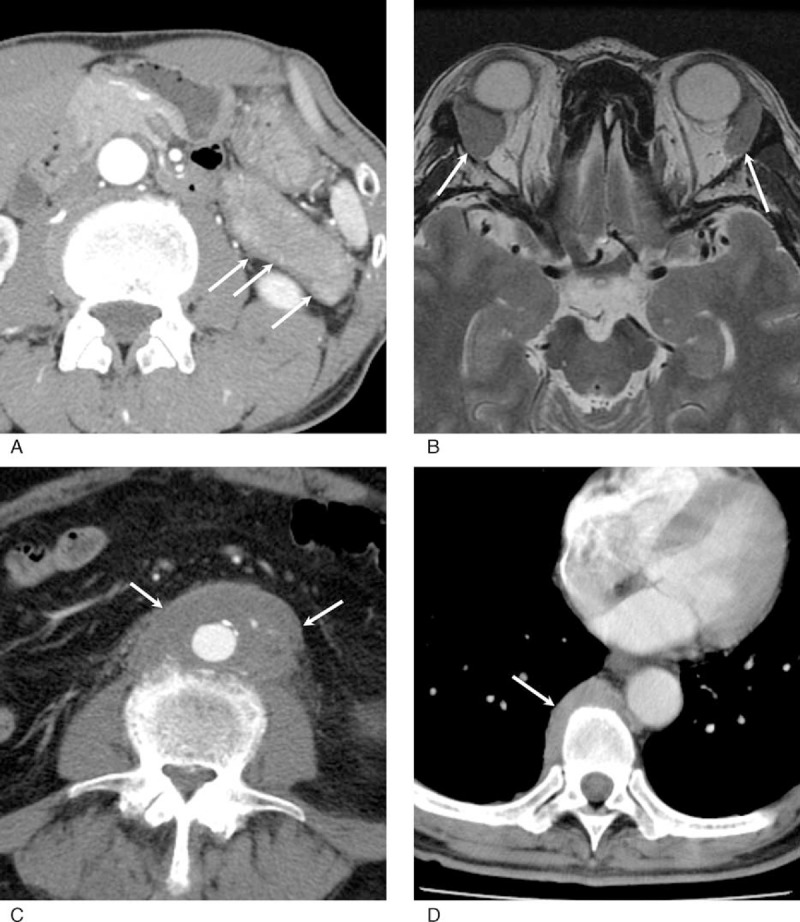
Radiological features of IgG4-RD. (A) Contrast-enhanced CT. The pancreatic tail is diffusely enlarged with a peripancreatic capsule-like rim (arrows). (B) T2-weighted MR image. Bilateral lacrimal glands are enlarged without a discrete mass (arrows). (C) Contrast-enhanced CT. The abdominal aorta has a large aorta-centered mass continuous to the adventitia (arrows). (D) Contrast-enhanced CT. Soft tissue mass is noted at the paravertebral site (arrow). CT = computed tomography, IgG4-RD = immunoglobulin G4-related disease, MR = magnetic resonance.
Clinical Features
Electronically stored clinical notes for all patients were retrospectively reviewed, particularly in terms of initial symptoms, details of treatment, and past medical history of diabetes and autoimmune or allergic disorders. As IgG4-RD is currently believed to yield radiologically detectable abnormalities at affected sites (Figure 1), all archived images were reviewed to identify organ manifestations with reference to imaging features of IgG4-RD described in previous studies.14–20 Organ manifestations identified at the time of diagnosis and manifestations that appeared during the follow-up period were separately recorded. Cross-sectional images (computed tomography [CT] or magnetic resonance imaging [MRI]) of the head and neck were available for 160 patients (68%) (Figure 1B), chest CT for 188 (80%), abdominal CT and/or MRI for 230 (98%), and F-18 fluorodeoxyglucose positron emission tomography for 85 (36%).
Sclerosing cholangitis was defined as hilar or intrahepatic bile duct involvement. Lower bile duct stricture was not considered as a biliary manifestation as it is usually related to pancreatitis, and isolated intrapancreatic cholangitis is unusual in the context of IgG4-RD. Orbital manifestations involving soft tissue, muscles, or nerves were recorded separately from dacryoadenitis.21 Localized retroperitoneal lesions centered on the aorta were diagnosed as IgG4-related periaortitis (Figure 1C), whereas lesions broadly affecting the retroperitoneum or mainly involving other anatomical structures such as the ureter were called retroperitoneal fibrosis. The involvement of iliac arteries was determined as a part of IgG4-related periaortitis, as it is generally continuous to the periaortic mass. Other separate arterial lesions were described as arterial manifestations. Lymphadenopathy (>1.5 cm in size) was recorded but not counted as a manifestation of IgG4-RD because discrimination between IgG4-related and nonspecific lymphadenitis requires tissue diagnosis.
Follow-up data were also reviewed in terms of history of relapses and malignant diseases. The median follow-up period was 37 months (range 2–105). Remission was defined as the disappearance of organ enlargement, nodular lesions, or wall thickening of ductal organs on imaging along with loss of related symptoms. Relapse was defined as symptomatic or radiologically detectable disease recurrence. We also had an interest in the prevalence of cerebral aneurysms in IgG4-RD patients, as growing evidence suggests that arteries are one of the targets of IgG4-RD, but the association with cerebral aneurysms was not examined.18,22 Images of magnetic resonance (MR) angiography of the cerebrovascular system were reviewed, if available.
Serological Aspects
In addition to IgG4 levels, IgG concentrations and titers of antinuclear antibody (ANA) at the time of diagnosis were recorded. The IgG4 to IgG ratio (normal range ≤5%) was calculated for each case.
Statistical Analysis
Statistical analysis was performed using the χ2 test or Mann–Whitney U test to analyze variables. A probability of P < 0.05 was considered to be statistically significant.
RESULTS
Patients
Review of clinical notes, imaging, and histology for the patients selected by database search eventually identified 235 cases of IgG4-RD that met the inclusion criteria. In the same period, an additional 43 patients were suspected of having IgG4-RD, but they were not included in this study because of the lack of either histological confirmation or classic type 1 AIP. Out of the 235 patients, 150 (64%) had histology-proven IgG4-RD, whereas 143 (61%) were diagnosed to have definitive type 1 AIP based on the International Diagnostic Criteria. Fifty-eight patients had both. All patients were adults (189 males, 46 females; M/F = 4/1). The median age was 67 years (range 35–86). Two hundred twelve patients (90%) were diagnosed in their 50s to 70s (Figure 2).
FIGURE 2.
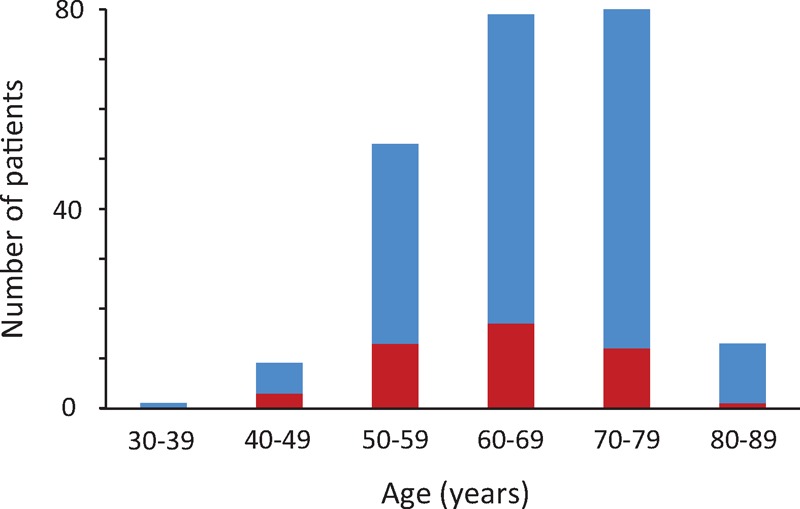
Age distribution of patients with IgG4-RD. Two hundred patients (91%) were 50 to 80 years of age. Blue and red bars represent male and female patients, respectively. IgG4-RD = immunoglobulin G4-related disease.
Seventy-one patients (30%) were incidentally found to have IgG4-RD during routine medical checkups or surveillance for other diseases, whereas others (70%) presented symptomatically. The symptoms of 96 patients (41%) were attributed to a mass effect of affected organs (ie, obstructive jaundice due to pancreatic enlargement, exophthalmos caused by dacryoadenitis). Forty-three (18%) had nonspecific abdominal symptoms such abdominal pain or epigastralgia. The others presented with renal (n = 8, 4%) or pulmonary (n = 7, 3%) symptoms, and general malaise (n = 10, 4%). Patients were seen by doctors of various specialities including rheumatology, gastroenterology, surgery, nephrology, pulmonary medicine, otolaryngology, ophthalmology, and hematology.
Past Medical History
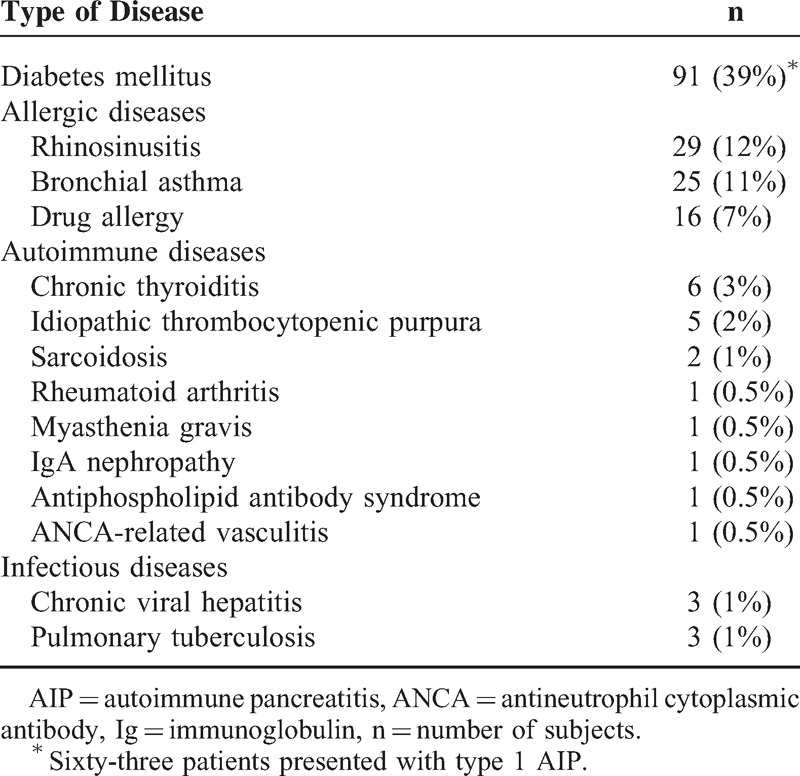
Previous history is summarized in Table 1. Patients frequently suffered from allergic diseases and type 2 diabetes mellitus. Four patients had a history of sialadenitis, which was originally diagnosed as Sjögren syndrome or sialadenitis of unknown etiology, but a retrospective review of histological slides with IgG4 immunostaining confirmed the diagnosis of IgG4-related sialadenitis. Another patient had a history of a surgical procedure for inflammatory abdominal aortic aneurysm, which was retrospectively proved to be IgG4-related periaortitis.
TABLE 1.
Past Medical History
Organ Manifestations
In total, 486 manifestations were identified in 235 patients at the time of diagnosis. The most common manifestation was pancreatitis, diagnosed in 142 patients (60%). The next most common was sialadenitis in 81 patients (34%), followed by tubulointerstitial nephritis in 54 (23%), dacryoadenitis in 53 (23%), and periaortitis in 28 (20%) (Figure 3). Two hundred twenty-three patients (95%) had at least 1 of the 5 most common manifestations. The other organ lesions were seen in <15% of patients each. IgG4-related paravertebral plaques, which have not been reported so far, were identified in 11 patients (5%) (Figure 1D). Lymph node enlargement was seen at various sites (ie, mediastinum, neck, para-aorta, or systemic) in 34 patients (14%).
FIGURE 3.
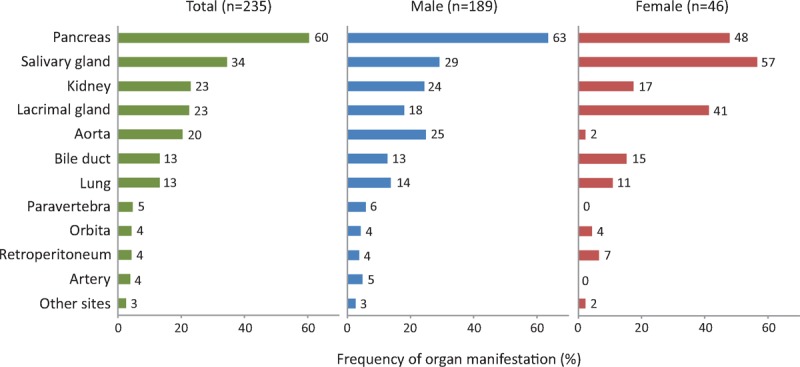
Frequency of each organ manifestation at the time of diagnosis in patients with IgG4-RD. Other sites include the prostate, peripheral nerve, pituitary gland, skin, and pericardium. IgG4-RD = immunoglobulin G4-related disease.
Male and female patients differed in terms of their organ manifestations. Periaortitis was significantly more common in males than in females (25% vs 2%, P = 0.001). Lesions that more commonly developed in females were sialadenitis and dacryoadenitis (57% vs 29%, P < 0.001 and 41% vs 18%, P < 0.001, respectively). Sialadenitis was the leading manifestation in females (Figure 3).
One hundred thirty-seven patients (58%) had multiple organ involvement. Patients with sclerosing cholangitis, tubulointerstitial nephritis, and periarteritis most often had other organ manifestations. In contrast, 98 patients (42%) presented with an isolated lesion. This presentation was most common for pancreatitis patients, with 38% lacking extrapancreatic lesions (Figure 4).
FIGURE 4.
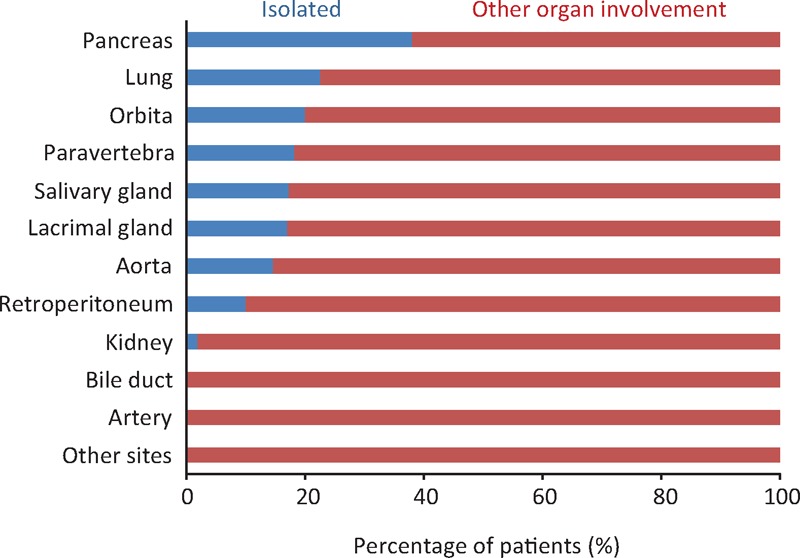
Frequency of organ manifestation presented with an isolated lesion or other organ involvement at disease onset.
Serological Features
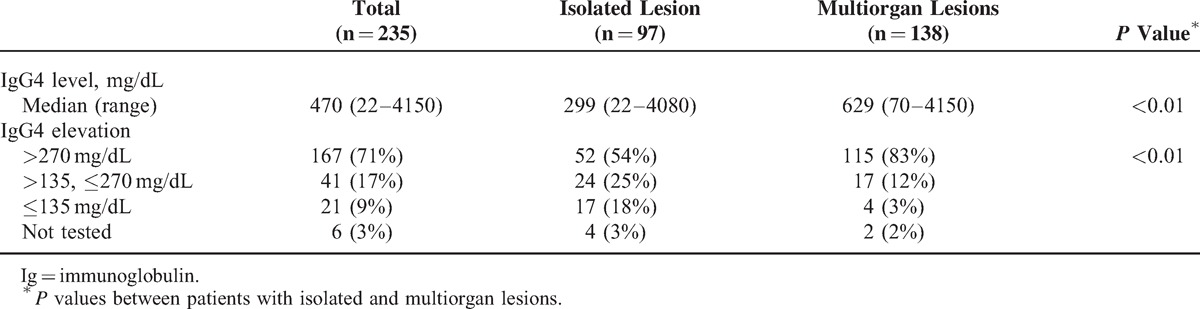
Serum IgG4 was elevated in 208 patients (88%), within the normal range in 21 (9%), and not tested in 6 (3%). The median concentration was 470 mg/dL (range 22–4150). Serum IgG4 was raised to >270 mg/dL, twice the upper limit of normal, in 167 patients (71%). The IgG4 value was significantly higher in the patients with multiple organ manifestations (Table 2).
TABLE 2.
Serum IgG4 Concentrations at the Time of Diagnosis in Patients With Isolated and Multiorgan Lesions
Total IgG concentrations were also raised to >1600 mg/dL in 155 of the 224 patients tested (69%). Of 218 patients, for whom both IgG4 and IgG values were available, the IgG4/IgG ratio was raised to >10% in 194 (89%), >20% in 133 (61%), and even >30% in 80 (37%) (Figure 5). ANA, which was tested in 197, was positive with ≥40 titers in 98 (50%) and ≥80 in 45 (23%).
FIGURE 5.
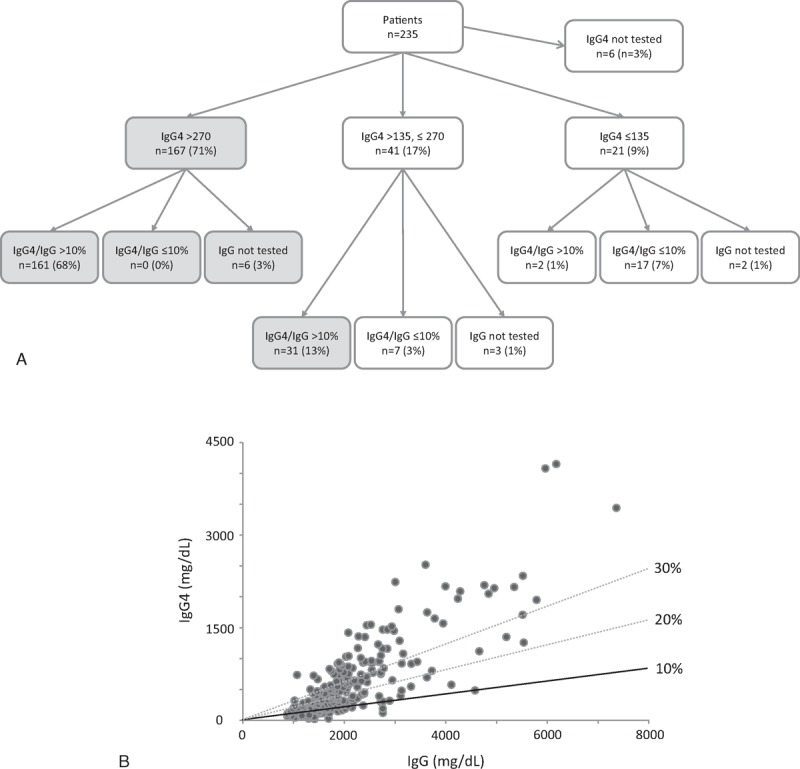
Serum IgG4 in patients with IgG4-RD. (A) IgG4 concentrations and the IgG4/IgG ratio at the time of diagnosis are summarized. Gray circles indicate 198 patients (84%) having either IgG4 >270 mg/dL or IgG4 135–270 mg/dL and IgG4/IgG >10%. (B) Correlation between IgG4 and IgG concentrations in IgG4-RD patients. The black line indicates the 10% IgG4/IgG ratio. Dotted lines are for 20% and 30%. Ig = immunoglobulin, IgG4-RD = IgG4-related disease.
Treatment and Relapse
One hundred eighty-three patients (78%) were treated. They had corticosteroid therapy (n = 162, 69%), surgical resection (n = 16, 7%), or both (n = 5, 2%). All went into clinical remission. One patient showed a rapid enlargement of IgG4-related aneurysmal abdominal periaortitis, which required emergency surgery, 32 months after commencing corticosteroid therapy. The initial doses of prednisolone were 20 to 60 mg/d, with the majority of patients given 30 or 40 mg daily. They were gradually tapered by 5 mg every 1 to 2 weeks over several months, based on clinical and serological improvements. Following remission, most patients had maintenance therapy (2.5 or 5.0 mg/d) for 1 to 3 years. IgG4-RD relapsed in 44 of the 183 treated patients (24%). The median period from remission to relapse was 29 months (range 2–83). Thirty-five patients had 1 episode of relapse, whereas 9 patients had 2 or more. Recurrent disease was found at the same site as the first manifestation (n = 14, 32%), another site (n = 18, 41%), or both (n = 12, 27%). All recurrent diseases responded well to additional therapy with prednisolone. Steroid-sparing immunomodulators such as azathioprine or rituximab were not used.
Fifty-two patients (22%) were not treated because of no symptoms (n = 28), diabetes mellitus (n = 15), history of malignant disease (n = 5), spontaneous regression (n = 2), and refusal to undergo treatment (n = 2). Only 5 of them (10%) had either local disease progression or additional organ involvement during the study period. In the remaining cases, the disease was almost stable or slightly regressed in radiological appearance even without treatment.
Association With Malignancy and Cerebral Aneurysm
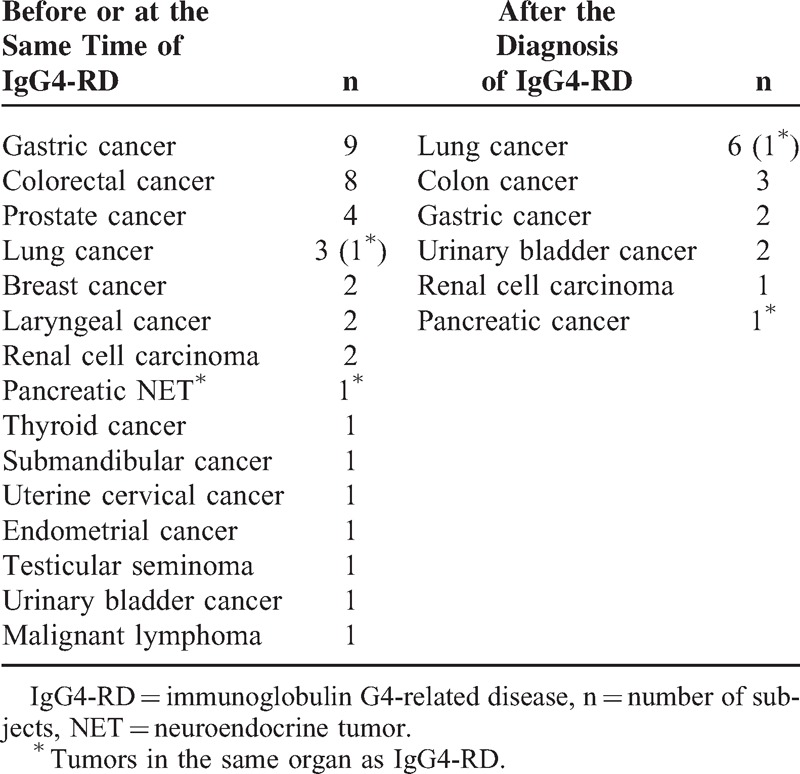
Thirty-eight malignant diseases had been diagnosed in 34 patients (15%) before or at the presentation of IgG4-RD (Table 3). The median period between the malignant disease and IgG4-RD was 4 years (range 0–22). Apart from a small pancreatic neuroendocrine tumor and lung cancer, malignant diseases and IgG4-RD differed in terms of the organ of origin.
TABLE 3.
Malignant Diseases in Patients With IgG4-RD
Fifteen malignant diseases were diagnosed in 13 patients (6%) during the follow-up for IgG4-RD. This figure is almost similar to the incidence (12.9 cancers) expected from the Japanese nationwide study for cancer epidemiology (standardized incidence ratio 1.16).23 Only 2 cases developed in an organ that had been involved in IgG4-RD (Table 3). The median period to the complication was 2 years (range 1 month to 8 years).
Of 65 patients who had MR angiography of the brain for reasons including dizziness, double vision, and to exclude pituitary involvement of IgG4-RD, 7 (11%) were discovered to have intracranial aneurysms. One patient presented with subarachnoid hemorrhage and another with an unruptured aneurysm underwent surgical clipping. The remaining 5 cases did not have interventional therapy because of the small size of the aneurysm (3–6 mm).
During the study period, 10 patients died of malignancies (n = 4), myocardial infarction (n = 1), brain hemorrhage (n = 1), infection (n = 1), accident (n = 1), and unknown causes (n = 2). This figure was not higher than the estimated number of deaths (14.9 of 235 people) of age- and sex-matched general population in Japan.
DISCUSSION
When this study was designed, 3 aspects were considered. First, we attempted to find as many patients as possible. Second, the cohort should include all patients consecutively diagnosed in various departments. The use of a radiology database was chosen for this purpose. Third, we asked multiple hospitals located in our medical district to contribute to the study, considering the risk that a single-center study might lead to biased results. For example, if the hospital has a large gastroenterology department, the incidence of pancreatic manifestations would be overestimated.
One of the main purposes of this study was to learn the demography of IgG4-RD and the frequency of each organ manifestation. It is now clear that IgG4-RD is an adult disease with >90% of patients being aged >50 years. It is interesting that male and female patients differed in their organ manifestations. IgG4-RD is less common in females, but sialadenitis and dacryoadenitis frequently develop when females get the disease. Although IgG4-RD can involve various organs/sites, 60% of patients have pancreatitis, suggesting that pancreatitis is a prototypic manifestation of IgG4-RD, the same as lung lesions for sarcoidosis, another systemic condition. A caveat is that this figure may represent the fact that only the pancreatic manifestation can be diagnosed without histology. However, even if an additional 42 patients who were suspected of having IgG4-RD but no tissue diagnosis are included, pancreatitis was the leading manifestation, seen in 52% of patients. Another possibility is that pancreatitis most commonly causes serious symptoms (ie, obstructive jaundice), which may increase the chance of getting diagnosed.
There is now a need to develop a useful diagnostic scheme for IgG4-RD. Among the sets of diagnostic criteria proposed so far, only the one for type 1 AIP is universally accepted.12 It is worth emphasizing here that 95% of patients had at least 1 of pancreatitis, sialadenitis, dacryoadenitis, periaortitis, and tubulointerstitial nephritis. A diagnostic algorithm that can cover the 5 manifestations would be applicable to most patients with IgG4-RD. In our experience, IgG4-RD is usually suspected based on radiological examination first, followed by additional serological testing for IgG4 and tissue diagnosis. Type 1 AIP is the single organ manifestation in which the definitive diagnosis can be established without tissue examination. The main reason for this is that imaging features highly specific to type 1 AIP are known. The more radiological data are available for extrapancreatic manifestations, the fewer patients may require tissue diagnosis.
Serum IgG4 is certainly the most useful noninvasive test for the diagnosis of IgG4-RD. Here, it is worth emphasizing 2 points. First, IgG4 elevations more than twice the upper limit of the normal range are highly specific for IgG4-RD. Ghazale et al24 examined serum IgG4 levels in 510 patients with various pancreatic diseases and found the specificity of IgG4 elevations >280 mg/dL (normal range <140) for the diagnosis of type 1 AIP to be 99%. Second, mild IgG4 elevations can be seen in a variety of diseases including both inflammatory and neoplastic conditions.25,26 In the above study, the specificity of IgG4 elevations >140 mg/dL for the diagnosis of type 1 AIP was 76%.24 This figure was confirmed by another study, in which 132 patients with IgG4-RD were compared with 48 patients with other disorders.27 A potential approach to increase diagnostic specificity in this setting is to calculate the IgG4/IgG ratio. An IgG4/IgG ratio >10% is known to be highly suggestive of IgG4-RD (specificity 91%).27 Meeting either IgG4 >270 mg/dL or IgG4 135–270 mg/dL and IgG4/IgG >10% may be a more valuable criterion than the currently used cut off point (IgG4 >135 mg/dL). This stringent criterion covered 84% of patients in this study, suggesting this to be not only specific but also sensitive enough for the diagnosis of IgG4-RD (Figure 3). Although some previous studies suggested that serum IgG4 monitoring is useful to predict relapse, it has not been validated universally.28 It may be interesting to see how useful monitoring the IgG4/IgG ratio for disease management is.
Whether IgG4-RD can increase the risk of malignancy remains controversial.29,30 Only a marginal increase in the incidence of cancer was identified in this study, but the short follow-up period (median 3 years) does not allow us to draw a definitive conclusion. From a pathological point of view, the epithelium is known to be intact despite the severe inflammatory process,5 which may suggest that IgG4-RD is less likely to increase the risk of cancers. Another interesting point is that 10% of patients who underwent MR angiography of the brain had cerebral aneurysms. This figure appears higher than that for “healthy” subjects (2–5%). If the intracranial aneurysm is a true manifestation of IgG4-RD, we need to consider the risk that corticosteroid therapy may lead to aneurysmal rupture by diminishing the aneurysmal wall, as seen in one of our patients with aneurysmal periaortitis.
Unlike classic consecutive cohort studies, in which patients are prospectively enrolled, our study is based on a retrospective search of the radiology database. One might argue that this approach has a risk of selection bias. However, given the fact that IgG4-RD patients usually undergo imaging during either the diagnostic process or the follow-up period, our cohort likely includes at least a great majority of IgG4-RD patients and supposedly represents the general IgG4-RD population.
In conclusion, this study gave us a reliable dataset for IgG4-RD, which could help us to achieve an overall picture of IgG4-RD and better understand this emerging condition.
ACKNOWLEDGMENTS
The authors thank all colleagues who contributed to the diagnosis and treatment of patients with IgG4-RD.
Footnotes
Abbreviations: AIP = autoimmune pancreatitis, ANA = antinuclear antibody, CT = computed tomography, IgG4-RD = immunoglobulinG4-related disease, MR = magnetic resonance, MRI = magnetic resonance imaging.
YZ contributed to the study design, analysis and interpretation of data, drafting the article, and final approval; DI was responsible for study design, data collection, analysis and interpretation of data, and final approval; KY, NY, KO, TM, KN, KO, FT, JT, and TM contributed to data collection and final approval; TG and OM contributed to supervising the study and final approval.
This work was supported in part by a Grant-in-Aid for Scientific Research (26860986) from the Ministry of Education, Culture, Sports, Science and Technology.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366:539–551. [DOI] [PubMed] [Google Scholar]
- 2.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344:732–738. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Funata N, Hayashi Y, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut 2003; 52:683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zen Y, Harada K, Sasaki M, et al. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 2004; 28:1193–1203. [DOI] [PubMed] [Google Scholar]
- 5.Zen Y, Nakanuma Y. IgG4-related disease: a cross-sectional study of 114 cases. Am J Surg Pathol 2010; 34:1812–1819. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, Zen Y, Pillai S, et al. IgG4-related disease. Lancet [Epub ahead of print]. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 7.Masaki Y, Dong L, Kurose N, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis 2009; 68:1310–1315. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa S, Zen Y, Harada K, et al. Abundant IgG4-positive plasma cell infiltration characterizes chronic sclerosing sialadenitis (Küttner's tumor). Am J Surg Pathol 2005; 29:783–791. [DOI] [PubMed] [Google Scholar]
- 9.Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol 2010; 34:202–210. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M, Harada S, Ohara M, et al. Clinical and pathological differences between Mikulicz's disease and Sjögren's syndrome. Rheumatology (Oxford) 2005; 44:227–234. [DOI] [PubMed] [Google Scholar]
- 11.Zen Y, Onodera M, Inoue D, et al. Retroperitoneal fibrosis: a clinicopathologic study with respect to immunoglobulin G4. Am J Surg Pathol 2009; 33:1833–1839. [DOI] [PubMed] [Google Scholar]
- 12.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the international association of pancreatology. Pancreas 2011; 40:352–358. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012; 25:1181–1192. [DOI] [PubMed] [Google Scholar]
- 14.Irie H, Honda H, Baba S, et al. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol 1998; 170:1323–1327. [DOI] [PubMed] [Google Scholar]
- 15.Sahani DV, Kalva SP, Farrell J, et al. Autoimmune pancreatitis: imaging features. Radiology 2004; 233:345–352. [DOI] [PubMed] [Google Scholar]
- 16.Inoue D, Zen Y, Abo H, et al. Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology 2009; 251:260–270. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi N, Kawashima A, Fletcher JG, et al. Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology 2007; 242:791–801. [DOI] [PubMed] [Google Scholar]
- 18.Inoue D, Zen Y, Abo H, et al. Immunoglobulin G4-related periaortitis and periarteritis: CT findings in 17 patients. Radiology 2011; 261:625–633. [DOI] [PubMed] [Google Scholar]
- 19.Fujinaga Y, Kadoya M, Kawa S, et al. Characteristic findings in images of extra-pancreatic lesions associated with autoimmune pancreatitis. Eur J Radiol 2009; 76:228–238. [DOI] [PubMed] [Google Scholar]
- 20.Fujita A, Sakai O, Chapman MN, et al. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics 2012; 32:1945–1958. [DOI] [PubMed] [Google Scholar]
- 21.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum 2012; 64:3061–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasashima S, Zen Y, Kawashima A, et al. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. Am J Surg Pathol 2008; 32:197–204. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda T, Marugame T, Kamo K, et al. Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2012; 42:139–147. [DOI] [PubMed] [Google Scholar]
- 24.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol 2007; 102:1646–1653. [DOI] [PubMed] [Google Scholar]
- 25.Oseini AM, Chaiteerakij R, Shire AM, et al. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology 2011; 54:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto M, Tabeya T, Naishiro Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 2012; 22:419–425. [DOI] [PubMed] [Google Scholar]
- 27.Masaki Y, Kurose N, Yamamoto M, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol 2012; 2012:580814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sah RP, Chari ST. Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol 2011; 23:108–113. [DOI] [PubMed] [Google Scholar]
- 29.Hart PA, Kamisawa T, Brugge WR, et al. Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 2013; 62:1771–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiokawa M, Kodama Y, Yoshimura K, et al. Risk of cancer in patients with autoimmune pancreatitis. Am J Gastroenterol 2013; 108:610–617. [DOI] [PubMed] [Google Scholar]


