Abstract
Contrast-enhanced computed tomography (CECT) and positron emission tomography with 18-FDG (FDG-PET/CT) are used to identify malignant solitary pulmonary nodules. The aim of the study was to evaluate the accuracy of CECT and FDG-PET/CT in diagnosing the etiology of solitary pulmonary nodule (SPN).
Eighty patients with newly diagnosed SPN >8 mm were enrolled. The patients were scheduled for either or both, CECT and FDG-PET/CT. The nature of SPN (malignant or benign) was determined either by its pathological examination or radiological criteria.
In 71 patients, the etiology of SPN was established and these patients were included in the final analysis. The median SPN diameter in these patients was 13 mm (range 8–30 mm). Twenty-two nodules (31%) were malignant, whereas 49 nodules were benign.
FDG-PET/CT was performed in 40 patients, and CECT in 39 subjects. Diagnostic accuracy of CECT was 0.58 (95% confidence interval [CI] 0.41–0.74). The optimal cutoff level discriminating between malignant and benign SPN was an enhancement value of 19 Hounsfield units, for which the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CECT were 100%, 37%, 32%, and 100%, respectively. Diagnostic accuracy of FDG-PET/CT reached 0.9 (95% CI 0.76–0.9). The optimal cutoff level for FDG-PET/CT was maximal standardized uptake value (SUV max) 2.1. At this point, the sensitivity, specificity, PPV, and NPV were 77%, 92%, 83%, and 89%, respectively.
The diagnostic accuracy of FDG-PET/CT is higher than that of CECT. The advantage of CECT is its high sensitivity and negative predictive value.
INTRODUCTION
Solitary pulmonary nodules (SPNs) are commonly identified by lung imaging studies. They are found in 0.2% to 2% of chest radiographs and in 10% to 40% of computed tomography (CT) scans.1–4 The prevalence of malignancies among SPNs diagnosed in the frame of lung cancer screening programs is about 0.5% to 3.5%. The probability of malignancy is related to both patients’ characteristics and radiological features of the nodule.4 The patient-related risk factors of malignant nature of the lesion are: age, current or past smoking, and previous history of malignancies. Radiological features associated with increased risk of malignancy include large nodule diameter and volume, spiculated margins, and upper lobe location.5,6 Unfortunately, these features are neither sensitive nor specific enough to predict SPN nature. In the context of the large number of patients with SPN detected by CT, there is an urgent need for a high performance diagnostic tool differentiating between malignant nodules, which should be removed without delay and benign lesions where surgery should be avoided. As sensitivity of various sampling techniques is limited and these methods are associated with the substantial risk of complications, novel imaging studies are perceived as a promising solution. In our earlier study, we showed that simplified method of dynamic contrast-enhanced computed tomography (CECT) can be used to predict the benign etiology of SPN.7 Other methods used to differentiate between malignant and benign SPN include nuclear magnetic resonance, single-photon emission CT (SPECT) and positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET).8 Recently updated American College of Chest Physicians’ (ACCP) guidelines strongly point out FDG-PET as the most sensitive and specific imaging technique differentiating between malignant and benign SPN.6 Although FDG-PET is the most accurate test in diagnosing SPN, its costs are substantial and availability is limited. The aim of our study was to evaluate the diagnostic accuracy of CECT and FDG-PET/CT in predicting malignant versus benign SPN etiology.
MATERIALS AND METHODS
The study was approved by the institutional review board of the Medical University of Warsaw. Eighty adult consecutive patients with newly diagnosed SPN referred to the Department of Internal Medicine, Pneumonology and Allergology between 2007 and 2011 were initially enrolled into the prospective study. The inclusion criteria were: age over 18 years and the largest nodule diameter measured in CT scan ≥8 mm. Patients with smaller nodules and those with pulmonary nodules with features strongly suggesting benign etiology (central dense nidus, diffuse solid, or laminal calcification) were excluded. The pre-test probability of malignant SPN etiology was calculated according to Mayo Clinic calculator proposed by Swensen et al.9 In patients with recent history of malignancy, a different model as described by Gould et al10 was used. The diagnostic approach included medical history, CECT, FDG-PET/CT, bronchoscopy, and video-assisted thoracoscopic surgery with resection of the nodule. However, the ultimate management was individualized according to the probability of malignancy, attending physician recommendations and patient preferences.
The definite nature of the nodule was determined on the basis of cyto-histopathological findings and/or radiological follow-up. The criteria of nodule benignity were as follows: absence of malignant cells/tissue in the resected nodule or stable nodule dimensions in CT scan followed-up for at least 24 months from the initial diagnosis. Time intervals between subsequent CT scans were consistent with ACCP recommendations.5
Thorax CT scans were performed with 16-row CT scanner (LightSpeed 16 General Electric Medical Systems, Milwaukee, WI) using 1.25-mm collimation, pitch 1.375, 120–140 kV (peak), 250 mA current, matrix size 512 × 512 and 0.5 second scanning time. In general, the CECT procedure was based on the protocol proposed by Swensen et al,11 but the number of post-contrast measurements was reduced to 2, as described elsewhere.7 Thus, 3 CT scans were performed in each patient: before contrast administration, 30 sec and 4 min after intravenous contrast injection (Iomeron, Bracco; 2 mL/sec; 300 mg/mL). Insignificant nodule enhancement (≤15 Hounsfield units, HU) after contrast injection has been suggested to be strongly predictive of its benignity.11 The measurements were performed by an experienced chest radiologist with 20 years of experience in chest radiology, who was unaware of the final diagnosis of the nodule.
FDG-PET/CT was done according to the protocol proposed by Gould et al.12 Half-body PET/CT examinations from vertex to upper thighs were performed on a Biograph 64 TruePoint PET/CT scanner (Siemens Medical Solutions, Knoxville, TN) using 3-dimensional mode. Patients were injected 300 to 370 MBq 18F-FDG and imaged after a 60-min uptake period. CT was acquired continuously in spiral mode using a pitch of 0.8, 120 kV (peak), 170 mA current, and 2-mm postprocessing slice thickness. PET study was acquired covering an area identical to that covered by CT at 2 min per bed position (6–7 bed positions depending on the size of the patient). Emission data were reconstructed on a 168 × 168 matrix using ordered subsets expectation maximization algorithm (2 iterations, 14 subsets) and corrected for attenuation using CT. The PET/CT images were transferred to a Multi-Modality Work Station, Syngo (TrueD) (Siemens Medical Solutions) for analysis. The malignant etiology of the nodule was suspected if the nodule was visible in PET examination and maximal standardized uptake value (SUV max) was >2.5.13,14 The results of FDG-PET/CT were analyzed by 1 radiologist and 1 nuclear medicine specialist with 10 years of experience in nuclear medicine, both of them were unaware of the final diagnosis of the nodule.
Statistical analysis was performed using STATISTICA 10.0 (StatSoft, Inc. Tulsa, OK) and MedCalc 9.5.2.0 (MedCalc Software bvba, Ostend, Belgium) software packages. Quantitative variables are presented as median, interquartile range (IQR), and/or ranges, whereas qualitative variables are presented as number and percentage. Nonparametric Mann–Whitney U test or Chi-square test was used to assess the difference between variables in different groups. Receiver-operating characteristic (ROC) curve was analyzed to evaluate the diagnostic accuracy of CECT and FDG-PET/CT. Diagnostic performance of an earlier proposed algorithm that included CECT as the first diagnostic step (if CECT is negative no further tests are performed, if positive, then FDG-PET/CT is conclusive) and FDG- PET/CT as the second,15,16 was also analyzed. Diagnostic accuracy of imaging tests was expressed in 2 ways, as area under the ROC curve (AUC-ROC) and as the proportion of true-positive and true-negative results to all results. The Spearman rank correlation coefficient was applied to test correlations between quantitative variables. A P value <0.05 was regarded as significant.
RESULTS
Of 80 patients enrolled into the study, 9 were lost to follow-up and had to be excluded from analysis. Thus, the final analysis included 71 patients (45 females, 26 males), median age 69 years (range 45–88 years). Six patients had a history of malignancy in the last 10 years preceding the diagnosis of SPN. The median nodule diameter was 13 mm, range 8–30 mm. There were 43 and 28 patients with SPN located in the right and the left lung, respectively. The distribution of nodule size was as follows: 8–10 mm in 23 patients; 11–20 mm in 34 patients, and 21–30 mm in 14 patients. In 61 patients, CT showed a solid nodule, 6 patients were diagnosed with ground glass opacity, and 4 had a subsolid nodule (both solid and ground glass areas).
In 49 of 71 (69%) patients, benign SPN was diagnosed. Of these, 9 patients underwent nodule resection with pathological examination which revealed: hamartoma (n = 4), nonspecific inflammation (n = 2), tuberculoma (n = 2), and neurinoma (n = 1). All resections were performed within 4 to 14 weeks from CECT and FDG-PET/CT imaging. In 10 patients, a significant decrease of nodule size or even complete resolution was documented in follow-up CT scans. In the remaining 30 patients, the benign nature of SPN was defined based on stable nodule dimensions in CT scan performed at least 24 months after the initial diagnosis.
There were 22 of 71 (31%) patients with malignant pulmonary nodules. The most common tumor was primary lung adenocarcinoma (n = 12), followed by squamous cell carcinoma (n = 5), typical carcinoid (n = 1), and small cell lung carcinoma (n = 1). In 3 patients, metastases from extrapulmonary tumors were diagnosed (colon adenocarcinoma in 2 cases and 1 pheochromocytoma). The median time between CECT and FDG-PET/CT scanning and tumor resection was 5 weeks (range–12 weeks).
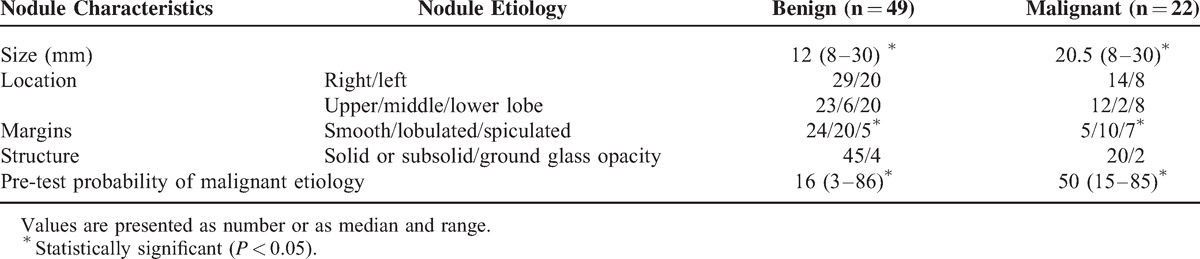
Comparison of selected demographic data and nodule characteristics in patients with benign versus malignant nodules is presented in Table 1 and Table 2. There were no significant differences between these 2 groups in terms of age and sex. Although the proportion of active smokers, ex-, and never smokers was not different in benign and malignant SPN groups, the total exposure to tobacco smoke was significantly higher in patients with malignant as compared with patients with benign nodules (P = 0.00005) (Table 1). Benign nodules were significantly smaller (P = 0.0004) and more frequently had smooth margins (P = 0.03) (Table 2). The pre-test probability of malignancy was significantly higher for SPNs, which occurred to be malignant (P = 0.0000).
TABLE 1.
Characteristics of the Patients
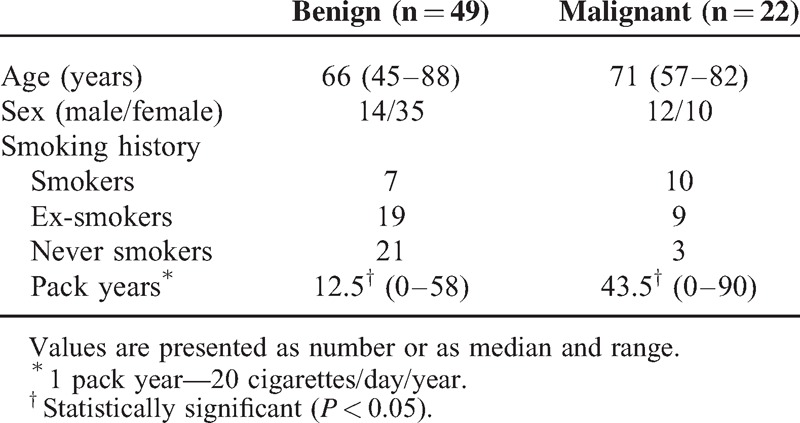
TABLE 2.
Characteristics of the Nodules
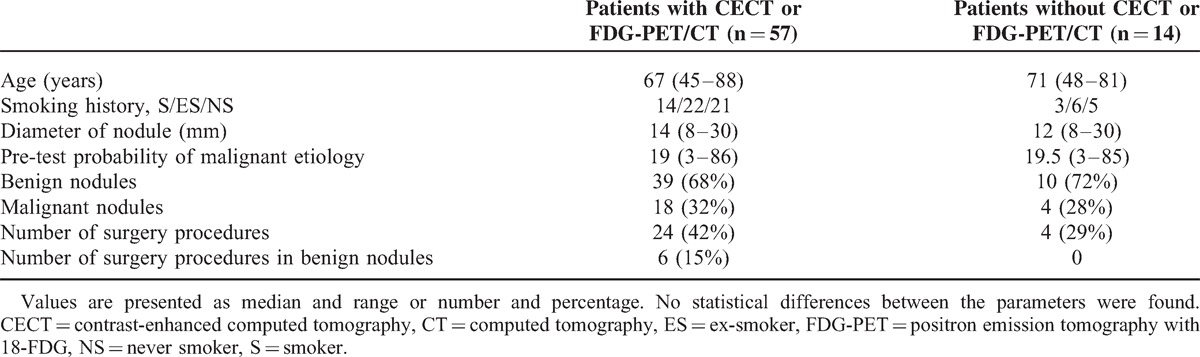
CECT was performed in 39 patients and FDG-PET/CT in 40 patients. Fifty seven patients (57/71, 80%) underwent at least one of these diagnostic procedures. In 14 of 71 patients with SPNs, neither CECT nor FDG-PET/CT was performed due to very high probability of malignancy (>60%, n = 4), patient's noncompliance with the study protocol (n = 7), or contraindications to PET and CECT (n = 3). The differences between patients’ and nodules’ characteristics who/that were managed with or without functional imaging techniques are shown in Table 3.
TABLE 3.
Characteristics of Patients and Nodules Diagnosed With and Without CECT or FDG-PET/CT
Diagnostic accuracy of CECT (measured as AUC-ROC) was 0.58 (95% confidence interval [CI] 0.41–0.74). ROC analysis revealed that the optimal cutoff level to discriminate between malignant and benign SPN was enhancement value of 19 HU. Diagnostic accuracy of FDG-PET/CT (measured as AUC-ROC) was higher than that of CECT and was calculated as 0.9 (95% CI 0.76–0.9). The optimal discriminating cutoff level for FDG-PET/CT was SUV max 2.1. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and likelihood ratio and diagnostic accuracy of CECT and FDG-PET/CT at different decision thresholds (as indicated by proportion of true positive and true negative results to all results) are presented in Table 4 and Table 5.
TABLE 4.
Diagnostic Performance of Simplified Dynamic CECT in Evaluation of Pulmonary Nodules
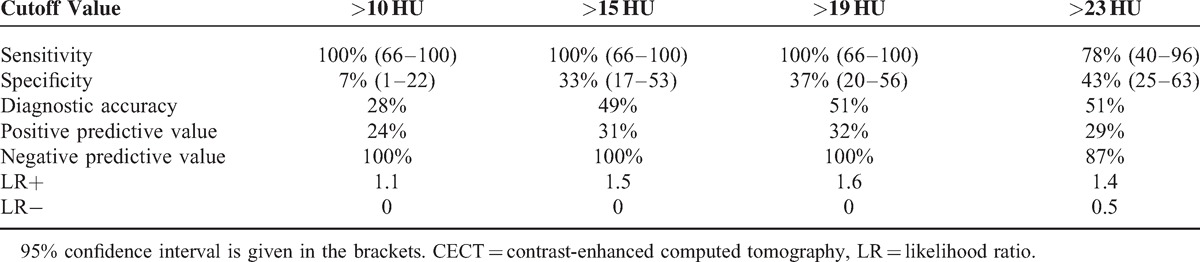
TABLE 5.
Diagnostic Performance of FDG-PET/CT in the Evaluation of Pulmonary Nodules (Cutoff Levels Indicated by ROC)

Both imaging tests were carried out in 22 cases. We found no correlation between enhancement value and SUV (r = −0.1, P = 0.6). In 10 patients, both functional imaging techniques gave consistent results, whereas in the remaining 12 patients, the results were discordant. Comparison of CECT and FDG-PET/CT results in individual patients is presented in Table 6.
TABLE 6.
Comparison of Results of CECT and FDG-PET/CT
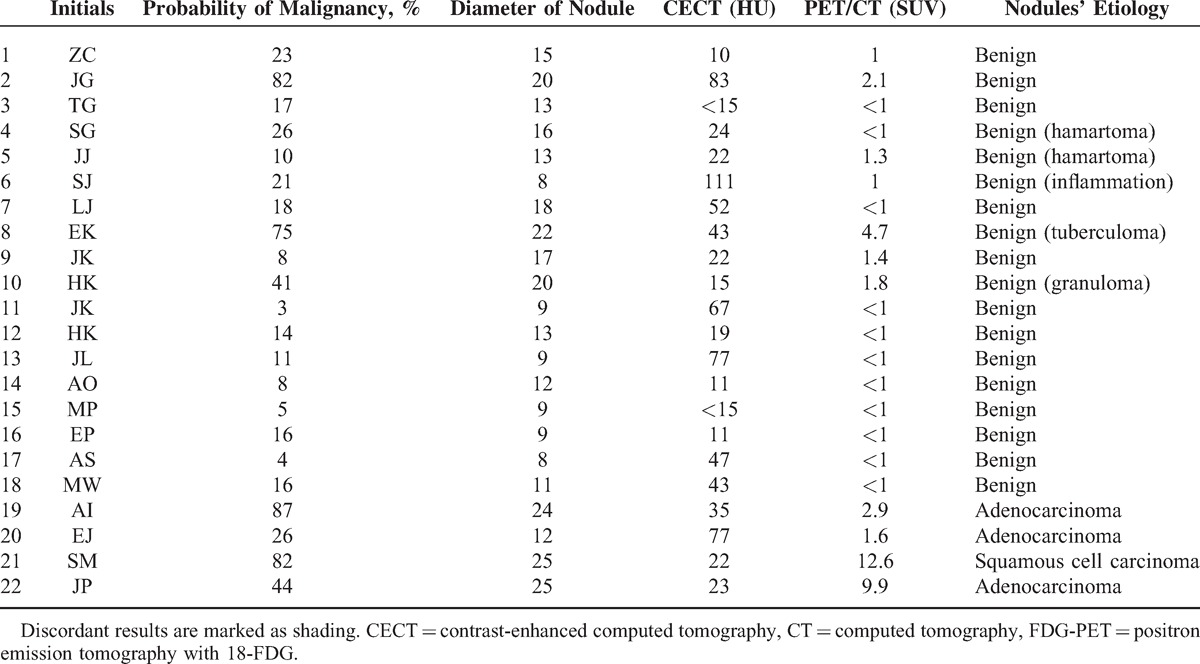
Assuming CECT cutoff value 19 HU and FDG-PET/CT cutoff value SUV >2.1, the sensitivity, specificity, PPV and NPV, likelihood ratio for positive and negative result, and accuracy for the diagnostic algorithm including CECT (first), and FDG-PET/CT (second) were calculated as 75%, 94%, 75%, 94%, 12.5, 0.26, 91%, respectively.
DISCUSSION
The results of our study showed that albeit both functional imaging techniques, CECT and FDG-PET/CT, may be applied to differentiate between benign and malignant pulmonary nodules, the diagnostic performance of the latter is significantly higher. We believe this finding is particularly important in the context of considerable experience of our team with the use of CECT as a diagnostic tool.11 Nevertheless, we are convinced there might still be a role for CECT in patients with SPN.
Pulmonary nodules are increasingly identified in routine chest radiographs and in low-dose CT scanning used as lung cancer screening.5,6 The proportion of benign and malignant pulmonary nodules found in different studies may vary. We previously demonstrated that 21% of SPNs identified in chest radiographs of 5726 patients were malignant.2 In the present study, the percentage of malignant nodules was higher (31%). This difference might be easily explained by the differences between demographic and clinical features of the study populations and by the major inclusion criterion of >8 mm for nodule diameter in this study. In general, our findings are concordant with the results presented by Swensen et al,9 who analyzed a cohort of 419 patients with SPN detected on chest radiographs. In that article, the prevalence of malignancy in SPN was 23%. Significantly higher as well as significantly lower proportions of malignant nodules were also reported. The prevalence of malignancy in 375 veterans with pulmonary nodules followed-up by Gould et al was as high as 54%. It must be emphasized that such a high prevalence was found in a highly selected group of males, current or ex- smokers, with SPN diameter >6 mm.10 Lower proportions of patients with malignant SPN can be expected on the basis of the results of some lung cancer screening programs involving low-dose CT. Since SPN had been identified in about 20% to 50% of screened patients4 and depending on the inclusion criteria, malignant nodules had been diagnosed in about 0.5% to 3.5% of all screened patients, it might be calculated that approximately 1% to 15% of all SPNs may be malignant in nature. Given the fact that lung cancer screening programs usually involve patients over 50 years of age with relevant smoking history,17 these numbers seem to be relatively low. The prevalence of malignancy in the population with pulmonary nodule may be of significance when assessing the predictive role of different diagnostic methods used to differentiate between benign and malignant lesions.18,19 The positive result of the test with known high PPV in a population with a relatively high prevalence of malignant nodules identifies nodules with a very high probability of malignancy that require surgical resection. However, diagnostic methods characterized by high NPV may be more useful in populations with low prevalence of malignancy. Under these circumstances, negative test result makes malignant nodule etiology highly unlikely and thus justifies a “watchful waiting” strategy.
The reliability of various functional imaging techniques in differentiating between malignant and benign pulmonary nodules is still a matter of discussion. Both CECT and FDG-PET have been used for >25 years, but FDG-PET/CT became more available in the last 15 years. Previous ACCP guidelines recommended both FDG-PET/CT and CECT in the evaluation of SPN in patients with low to moderate (5–65%) pre-test probability of malignancy.5 Recent guidelines also mention both methods, but highlight higher specificity and accuracy of FDG-PET/CT.6 The superiority of FDG-PET/CT over CECT in terms of diagnostic accuracy was clearly shown in our present study (AUC-ROC for FDG-PET/CT was 0.9 as compared with 0.58 for CECT). Similar results were earlier reported by Christensen et al,15 who compared the utility of CECT and FDG-PET in diagnosing the etiology of SPN in 42 patients with pulmonary nodules >7 mm. In that study, the sensitivity and specificity for CECT were 100% and 29% and for FDG-PET 88% and 76%, respectively. Surprisingly enough, a meta-analysis of 4 imaging modalities (CECT, FDG-PET, SPECT, and magnetic resonance imaging) did not reveal significant differences in the ability to discriminate benign and malignant SPNs.7 Relatively low—comparable with other methods—diagnostic value of FDG-PET might have been related to the fact that the meta-analysis included mainly studies reporting results of FDG-PET only and not FDG-PET/CT what seems to be the standard procedure at the moment.19 The higher accuracy of integrated FDG-PET/CT as compared with FDG-PET alone has been documented.20,21 However, the diagnostic accuracy of FDG-PET/CT is lower in small nodules (diameter <10 mm) and in ground glass opacities or subsolid nodules.6,22 As only 10 ground glass and subsolid nodules were evaluated in our study, the diagnostic accuracy of CECT or FDG-PET/CT in these specific types of SPNs could not have been reliably calculated.
Although FDG-PET/CT is considered the most precise imaging tool differentiating malignant and benign pulmonary nodules, the optimal cutoff value of SUV is still discussed. SUV max >2.5 was commonly used as a diagnostic threshold strongly suggesting malignancy.23,24 In our study, the value of SUV >2.1 was associated with the highest area under ROC curve and the highest diagnostic accuracy. However, recent studies indicate that a higher cutoff level (SUV max >4) may be more even accurate.25 Our results do not confirm this observation. Increasing the cutoff level of SUV to 2.9 resulted in only a modest increase in specificity and PPV at the cost of a significant decrease in sensitivity and NPV. The use of a low cutoff value <1.4 enables the most reliable selection of patients with benign nodules (the highest NPV). In CECT, 15 HU was proposed as the most accurate post-contrast enhancement threshold discriminating between malignant and benign nodules.11 Some authors suggested the treshold value of 20 HU or even higher.26,27 In our study, the cutoff level of post-contrast enhancement between 10 and 19 HU resulted in 100% NPV; its increase >19 HU gave a notable decrease in NPV.
The relationship between the enhancement value in CECT and SUV max measured in FDG-PET/CT is an interesting issue. We expected a significant correlation between these 2 indices; however, this was not the case in our study. Nonetheless, we are aware that such a correlation had earlier been reported.28 Tateishi et al29 demonstrated a significant correlation between CECT enhancement and SUV max in malignant pulmonary tumors (r = 0.665; P < 0.0001), but not in benign lesions. There was also a significant correlation between microvessel density in malignant tumors and the enhancement measured in CECT as well as SUV max found in FDG-PET/CT. In our study, we could not reliably evaluate this relation because only 4 patients with a malignant nodule underwent both CECT and FDG-PET/CT. The direct comparison of CECT and FDG-PET/CT results may carry a certain risk, as these functional imaging methods reflect slightly different aspects of SPN characteristics. The result of FDG-PET/CT depends on glucose metabolism, whereas the result of CECT is closely related to angiogenesis, blood vessel network, and blood flow.29,30 Interestingly, an increased glucose metabolism measured as SUV might have a prognostic value in terms of tumor progression, recurrence, and hazard of death.30–32 However, Cappabianca et al33 reported no correlation between SUV and grading of malignant SPN.
Although FDG-PET/CT seems to be a more accurate method in evaluating the nature of SPN, it is not flawless. The availability of FDG-PET/CT, although increasing, is still limited; the procedure is expensive, and is associated with quite high effective dose of radiation. In contrast, CECT is widely available and its costs are significantly lower. When an appropriate cutoff level is applied, CECT can reliably select SPNs with very low probability of malignancy (high NPV). Thus, combination of both methods, with sequential use of CECT as the first diagnostic step and FDG-PET/CT as the second, may decrease the number of FDG-PET/CT procedures and reduce the costs of diagnostics. Such approach has already been proposed by Christensen et al.15 The authors assumed that with a negative result of CECT, a malignant SPN is highly unlikely and, if so, further diagnostics could be avoided. If CECT enhancement was >15 HU, the patient was referred to FDG-PET/CT. The authors concluded that their diagnostic algorithm allowed a more accurate characterization of indeterminate SPN. The cost-effectiveness of this approach had been documented in another study.16
It might be calculated that application of the above diagnostic algorithm in our study group would result in a 27% reduction of FDG-PET/CT procedures without a negative impact the diagnostic performance. In our hospital, the cost of CECT was calculated as 700 PLN (167 EUR) and of FDG-PET/CT as 4100 PLN (976 EUR). Therefore, implementing this diagnostic approach would save the sum of 267 EUR per patient. These numbers clearly show the advantages of the combined CECT and FDG-PET/CT diagnostic algorithm and justify the use of both methods in a patient with SPN.
There are some limitations of our study. First, its results refer exclusively to patients with SPN ≥8 mm in diameter. Patients with smaller nodules could not have been included, as the diagnostic accuracy of CECT and FDG-PET/CT in nodules <8 mm is not satisfactory.5,6 Nonetheless, the criterion of the nodule diameter ≥8 mm used in our study seems to be consistent with recent suggestion that only nodules larger than 7 to 8 mm should be considered as positive results in lung cancer screening programs.34 This approach is intended to reduce the large number of false-positive results.35 Second, the number of patients in whom both CECT and FDG-PET/CT were performed is relatively small. Therefore, direct comparison of the results of both methods was possible in only one-third of our study group. Finally, our study included a small subgroup of patients (n = 14) in whom neither CECT nor FDG-PET/CT was performed. The diagnostic performance of advanced functional imaging techniques could not have been assessed in these patients. However, it seems striking that in this group of patients, a high accuracy of referral to surgery had been achieved. This group included 4 patients with a malignant nodule and all these patients underwent resection without delay (Table 3). Moreover, none of the 10 patients with a benign nodule was referred for unnecessary surgery. Thus, although no functional imaging had been used in this group, the prediction of the nature of the lesion was highly effective. This may call into question the usefulness of the advanced functional imaging of SPN, especially since 4 unnecessary surgical resections were performed in the CECT/FDG-PET/CT group (Table 3). Thus, in terms of proper patient selection for surgical resection, the results in non-CECT, non-FDG-PET/CT group were superior to those found in CECT/FDG-PET/CT group. We believe this might be an incidental finding resulting from the small number of patients and related to a selection bias. The small group of 14 patients included 4 patients with very high probability of malignancy and 7 patients with low probability of malignancy who a priori gave no consent to advanced imaging procedures and surgical treatment and in whom the benign character of SPN was proved in radiological follow-up. Thus, in our opinion, no reliable conclusion can be drawn from this observation. To study the above phenomenon, the patients should have been randomly assigned to 2 study arms with different diagnostic algorithms—including versus not including functional imaging techniques.
CONCLUSIONS
The diagnostic accuracy of FDG-PET/CT is higher than that of CECT. Nevertheless, both functional imaging methods may be useful in differentiating between a malignant and benign pulmonary nodule. As the advantage of CECT is very high sensitivity and NPV, the method might be preferred in populations with low prevalence of pulmonary malignancies with its major clinical application to exclude the malignant nature of the nodule. Conversely, the high specificity and high PPV of FDG-PET/CT may be effectively applied to confirm malignant nodule in patients with high prevalence of malignant pulmonary lesions. Diagnostic algorithm that includes CECT as the first diagnostic step may significantly decrease the number of patients that require FDG-PET/CT imaging and reduce the cost of the diagnostic work-up.
Footnotes
Abbreviations: 18-FDG-PET = positron emission tomography with 18-fluorodeoxyglucose, ACCP = American College of Chest Physicians, CECT = contrast-enhanced computed tomography, CT = computed tomography, ROC = receiver-operating characteristic curve, SPN = solitary pulmonary nodule, SUV max = maximal standardized uptake value.
KR is currently receiving 2 research grants from the National Science Center in Poland, which are not related to this study. KJ has received fee for consultation for Bayer HealthCare Polska. For the remaining authors, no conflicts of interest were declared.
REFERENCES
- 1.Ost DE, Gould MK. Decision making in patients with pulmonary nodule. Am J Respir Crit Care Med 2012; 185:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dąbrowska M, Kolasa A, Żukowska M, et al. Analysis of solitary pulmonary nodules found in chest radiograms. Pneum Alergol Pol 2009; 77:37–42. [PubMed] [Google Scholar]
- 3.Aberle D, DeMello S, Berg CD, et al. Results of the two incidence screenings in the National Lung Screening Trial. N Engl J Med 2013; 369:920–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midthun DE. Screening for lung cancer. Clin Chest Med 2011; 32:659–668. [DOI] [PubMed] [Google Scholar]
- 5.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest 2007; 132:108S–130S. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Chest 2013; 143:93S–120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dąbrowska M, Zukowska M, Krenke R, et al. Simplified method of contrast enhanced computed tomography in the evaluation of the indeterminate pulmonary nodules. Respiration 2010; 79:91–96. [DOI] [PubMed] [Google Scholar]
- 8.Cronin P, Dwamena BA, Kelly AM, et al. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology 2008; 246:772–782. [DOI] [PubMed] [Google Scholar]
- 9.Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997; 157:849–855. [PubMed] [Google Scholar]
- 10.Gould MK, Ananth L, Barnett PG. Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007; 131:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swensen SJ, Vigglano RW, Midthun DE, et al. Lung nodule enhancement at CT: multicenter study. Radiology 2000; 214:73–80. [DOI] [PubMed] [Google Scholar]
- 12.Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions. JAMA 2001; 285:914–924. [DOI] [PubMed] [Google Scholar]
- 13.Lowe VJ, Gobar L, Lawson M, et al. Prospective investigation of positron emission tomography in lung nodules. J CLin Oncol 1998; 16:1075–1084. [DOI] [PubMed] [Google Scholar]
- 14.Al-Sugair A, Coleman RE. Applications of PET in lung cancer. Semin Nucl Med 1998; 28:308–319. [DOI] [PubMed] [Google Scholar]
- 15.Christensen JA, Nathan MA, Mullan BP, et al. Characterization of the solitary pulmonary nodule: 18F-FDG PET versus nodule-enhancement CT. AJR Am J Roentgenol 2006; 187:1361–1367. [DOI] [PubMed] [Google Scholar]
- 16.Comber LA, Keith CJ, Griffiths M, et al. Solitary pulmonary nodules: impact of quantitative contrast-enhanced CT on the cost-effectiveness of FDG-PET. Clin Radiol 2003; 58:706–711. [DOI] [PubMed] [Google Scholar]
- 17.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013; 369:910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurney JW, Lyddon DM, McKAy JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II Application. Radiology 1993; 157:849–855. [DOI] [PubMed] [Google Scholar]
- 19.Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules. Chest 2013; 143:825–839. [DOI] [PubMed] [Google Scholar]
- 20.Kim SK, Auerbach BD, Goldin J, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med 2007; 48:214–220. [PubMed] [Google Scholar]
- 21.Chang CY, Tzao C, Lee SC, et al. Incremental value of integrated FDG-PET/CT in evaluating indeterminate solitary pulmonary nodule for malignancy. Mol Imaging Biol 2010; 12:204–209. [DOI] [PubMed] [Google Scholar]
- 22.Naidich DP, Bankler AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: A statement from the Fleischner Society. Radiology 2012; 266:304–331. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto Y, Tsuijkawa T, Kondo Ch, et al. Accuracy of PET for diagnosis of solid pulmonary lesions with 18FDG uptake below the standardized uptake value of 2.5. J Nucl Med 2006; 47:426–431. [PubMed] [Google Scholar]
- 24.Sim YT, Goh YG, Dempsey MF, et al. PET-CT evaluation of solitary pulmonary nodules: correlation with maximum standardized uptake value and pathology. Lung 2013; 191:625–632. [DOI] [PubMed] [Google Scholar]
- 25.Grgic A, Yuksel Y, Groschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using 18 F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Mol Imaging 2010; 37:1087–1094. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Kim H-J, Lee KH, et al. Solitary pulmonary nodules. A comparative study evaluated with contrast-enhanced dynamic MR imaging and CT. J Comp Assist Tomogr 2004; 28:766–775. [DOI] [PubMed] [Google Scholar]
- 27.Yi CA, Lee KS, Kim EA, et al. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology 2004; 233:191–199. [DOI] [PubMed] [Google Scholar]
- 28.Tateishi U, Nishihara H, Tsukamoto E, et al. Lung tumors evaluated with FDG-PET and dynamic CT: the relationship between vascular density and glucose metabolism. J Comput Assist Tomogr 2002; 26:185–190. [DOI] [PubMed] [Google Scholar]
- 29.Tateishi U, Nishihara H, Watanabe S, et al. Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic –pathologic correlation. J Comput Assist Tomogr 2001; 25:23–27. [DOI] [PubMed] [Google Scholar]
- 30.Berghmans T, Dusart M, Paesmams M, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUV max) measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: a systematic review and meta-analysis by European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008; 3:6–12. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal M, Brahmanday G, Bajaj SK, et al. Revisiting the prognostic value of preoperative 18F-fluoro2-deoxyglucose positron emission tomography in early stage (I&II) non-small cell lung cancers (NSCLC). Eur J Nucl Med Mol Imaging 2010; 37:691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higashi K, Ueda Y, Arisaka Y, et al. 18F-FDG uptake as a biologic factor for recurrence in patients with surgically resected non-small cell lung cancer. J Nucl Med 2002; 43:39–45. [PubMed] [Google Scholar]
- 33.Cappabianca S, Porto A, Petrillo M, et al. Preliminary study on the correlation between grading and histology of solitary pulmonary nodules and contrast enhancement and [18F] fluorodeoxyglucose standardize uptake value after evaluation by dynamic multiphase CT and PET/CT. J Clin Pathol 2011; 64:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013; 158:246–252. [DOI] [PubMed] [Google Scholar]
- 35.Richards TB, White MC, Caraballo RS. Lung cancer screening with low dose computed tomography for primary care providers. Prim Care 2014; 41:307–330. [DOI] [PMC free article] [PubMed] [Google Scholar]


