Abstract
To improve the outcome of allogeneic stem cell transplantation in refractory acute myeloid leukemia (AML), we conducted a single-arm phase II clinical trial to evaluate the efficacy and feasibility of conditioning regimen following cytoreduction chemotherapy with 7-day interval.
Adult patients with refractory AML were enrolled in the study and received fludarabine, cytarabine, and idarubicin (FLAG-IDA) as cytoreductive chemotherapy followed by busulfan and fludarabine (Flu-BU) conditioning regimen and transfusion of mobilized peripheral stem cells from human leukocyte antigen-matched sibling or unrelated donor. The primary endpoint of the study was 2-year leukemia-free survival (LFS) and secondary endpoints included complete-remission rate, 2-year overall survival (OS), nonrelapse mortality (NRM), and relapse rate.
A total of 16 patients were enrolled with median age of 36 (16–60), which included 9 primary induction failure, 2 early relapse, and 5 with relapse/refractory disease. The median cycles of previous chemotherapy were 4 (3–10) with a median of 55% (1%–90%) blasts in bone marrow. Six patients received transplantation from matched sibling and 10 from matched unrelated donors. After transplantation, 15 patients achieved bone marrow remission (11 complete remissions [CRs] and 4 bone marrow remissions without platelet recovery) at day +28. A total of 8 patients remained alive in CR with median LFS of 29.5 months (9.5–40.5 months). Four patients relapsed and 3 of them died of disease and another 4 patients died because of transplantation-related toxicity. The 2-year NRM and relapse rates were 25.0% ± 10.8% and 33.4% ± 13.8%, respectively with 2-year OS at 53.5% ± 13.1% and LFS at 50.0% ± 12.5%. Based on the Simon 2-stage design, 5 out of first eligible 14 patients remained leukemia-free for more than 2 years after allogeneic hematopoietic stem cell transplantation; thus, the null hypothesis of the study will be rejected and the study protocol is accepted as being warranted for further study.
Based on the above data, our phase II study demonstrated that the sequential FLAG-IDA cytoreduction chemotherapy followed by Flu-BU conditioning regimen given at the hematological nadir was feasible and has sufficient activity to warrant further investigation prospectively with a larger patient sample (clinicaltrials.gov identifier: NCT01496547).
INTRODUCTION
Refractory acute myeloid leukemia (AML) remains as the most difficult clinical scenario. Though different therapeutic regimens were tested in clinical trials, the overall outcome remained poor.1–3 Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative treatment for refractory AML.4,5 However, allogeneic transplantation with active leukemia failed to improve significantly the long-term outcome. Standard myeloablative conditioning regimen in these patients was associated with significant regimen-related mortality, whereas reduced intensity conditioning had less treatment-related complications, but was associated with extremely high relapse rates.6–8 The Center for International Blood and Marrow Transplant Research (CIMBTR) reported 3-year overall survival (OS) rates of 19% for AML patients with relapse disease or primary induction failure receiving myeloablative regimen.9 To further improve the outcome of allo-HSCT in such high-risk and refractory patients, sequential schedule of cytoreduction therapy followed by nonmyeloablative conditioning has been developed.10–14 Schmid et al had introduced a sequential conditioning regimen consisting of chemotherapy FLASMA followed by reduced-intensity conditioning (RIC) and prophylactic donor lymphocytes infusion (DLI) for patients with refractory AML. In their primary report, the 2-year OS and leukemia-free survival (LFS) were 40% and 37%, respectively and patients with only 2 cycles of previous chemotherapy might particularly benefit from the procedure.10 In another study using 5-day clofarabine as cytoreduction followed by initiation of conditioning during the nadir 14 days later for relapsed/refractory AML, 1-year progression-free survival (PFS), and OS were 25% and 38% respectively. Bone marrow biopsy 12 days after clofarabine treatment with effective cytoreduction was correlated with improved PFS.11
To further improve the outcome of patients with refractory leukemia, we developed a transplantation strategy consisting of sequential cytoreductive chemotherapy with fludarabine, cytarabine, and idarubicin (FLAG-IDA) followed by a reduced toxicity conditioning with busulfan and fludarabine (Flu-Bu) at the hematological nadir of chemotherapy-induced aplasia in refractory AML. The rationale is based on the significant anti-leukemia efficacy of FLAG-IDA regimen, which has been demonstrated in relapse or refractory AML, whereas the Flu-Bu regimen have been reported to be effective and less toxic as standard conditioning regimen in AML and myelodysplasia syndrome (MDS).15,16 In this study, we tested the hypothesis that maximizes the leukemia burden reduction by adding intensive chemotherapy regimen FLAG-IDA followed by the Flu-Bu conditioning given at the aplasia phase after cytoreduction chemotherapy may translate into better disease control after allo-HSCT.
METHODS
Study Design
Between June 15, 2011 and Jan 15, 2014, a total of 16 patients with refractory AML received allogeneic stem cell transplantation from HLA-matched donors in Blood & Marrow Transplantation Center, Department of Hematology, Rui Jin Hospital. All these patients were enrolled in a prospective, single-arm phase II clinical trial to study the efficacy and feasibility of new transplantation protocol consisting of intensive cytoreductive chemotherapy followed by sequential conditioning regimen given with a 7-day interval. The study was performed in accordance with the Declaration of Helsinki and the ICH Guidelines for Good Clinical Practice. The protocol was approved by the Ethics Committee of Rui Jin Hospital and was registered at www.clinicaltrials.gov (clinicaltrials.gov identifier: NCT01496547).
Eligibility Criteria
Patients were included if they fulfilled the following criteria defining refractory AML10,17: primary induction failure (PIF) after 2 cycles of chemotherapy, first early relapse after a remission duration of <6 months, relapse disease refractory to salvage chemotherapy containing high-dose Ara-C. Further inclusion criteria were age between 16 and 60 years with available donor (HLA-matched family or unrelated stem cell donor with 8/8 or 10/10 HLA matching) and written informed consent. The exclusion criteria were creatinine clearance <50 mL/min, bilirubin or transaminases >3 times the upper limit of normal, cardiac shortening fraction <30% and pregnancy.
Treatment Protocol
Before the treatment, bone marrow aspiration was performed to determine the disease status in all patients before a cytoreductive chemotherapy consisting of fludarabine (30 mg/m2), AraC (2 g/m2), and G-CSF 5 μg/kg from day −20 ∼ −16 and idarubicin 12 mg/m2 from day −16 ∼ day −14 (FLAG-IDA). A bone marrow aspiration was performed then on day −7 to access the marrow cellularity and residual blasts. A reduced toxicity conditioning regimen was consisted of fludarabine (30 mg/m2) from day −6 ∼ day −2 and busulfan 3.2 mg/kg from day −5 ∼ day −3 (Flu-Bu). G-CSF-mobilized peripheral stem cells were infused on day 0 in all patients (Figure 1). The graft-versus-host disease (GVHD) prophylaxis regimen included cyclosporine (CsA) 3 mg/kg from day −1 to day+60 with short methotraxate (MTX; 15 mg/m2 on day +1 and 10 mg/m2 day +3 and +6) and mycophenolate mofetil (MMF; 1000 mg/day) from day 0 to day +30 for all patients. Rabbit antithymocyte globulin (ATG, Thymoglobulin®, Sanofi/France) was given with a total dose of 6 mg/kg for unrelated donor from day −4 ∼ −1. The tapering of CsA started at Day +61 if no acute (GVHD) was documented. The prophylactic DLI was not eligible in the study. DLI was allowed only in case of documented loss of donor chimerism by polymerase chain reaction analysis of short-tandem repeat markers, increased minimal residual disease via flowcytometry or hematological relapse during follow-up.
FIGURE 1.
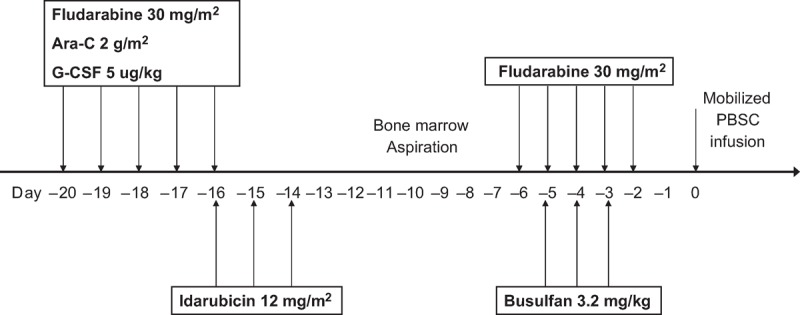
This figure illustrates the treatment scheme of the study protocol.
Toxicity Assessment
The electronic medical record was reviewed to grade toxicities according to NCI CTC Toxicity scale Version 2.0. aGVHD and chronic GVHD (cGVHD) were diagnosed and graded accordingly.18,19
Sample Size and Statistical Consideration
The primary endpoint of the study was 2-year LFS, whereas LFS was defined as being alive without relapse of leukemia. A 2-year LFS of 15% with the study protocol will be of no interest clinically because it is not superior to the conventional or standard transplantation protocol according to our own historical control data and available literature, whereas a 2-year LFS reaching 45% will be of interest for further study.9–11 All patients enrolled in the study must be followed-up to the occurrence of event (relapse of leukemia or death from any cause) or at least 2 years after allo-HSCT without any event defined above.
The sample size of 14 patients was determined based on Simon 2-stage minimax design with type one error rate α at 0.05 and power at 0.8.20 If no patient among the first 5 enrolled in the study maintained leukemia-free at 2 years after allo-HSCT, the trial will be stopped early for futility. At the end, if only ≤4 patients out of 14 enrolled patients in the study maintained leukemia-free at the landmark of 2 years after allo-HSCT, no further investigation of the study protocol is warranted. Otherwise, the FLAG-IDA/Flu-Bu protocol will be considered as being warranted for larger-scale clinical trial.
The secondary endpoints of study included complete remission rate (CR) on day 28 after allo-HSCT, 2-year nonrelapse mortality (NRM), 2-year relapse rate, and 2-year OS (the time from enrollment to death from any cause). The distribution of time-to-event endpoints such as LFS and OS was estimated using the Kaplan–Meier method. Probability of NRM and relapse rate was calculated using reciprocal cumulative incidence estimates. Additionally, we compared the overall outcome of the study with our historical control series in terms of OS, LFS, NRM, and relapse rate. Log-rank test was used for the analysis with P values reported as 2-sided and <0.05 as statistical significance.21 SPSS (SPSS Inc, Chicago, IL) software packages were used for data analysis.
RESULTS
Patients’ Characteristics
Between June 15, 2011 and Jan 15, 2014, a total of consecutive 20 patients with refractory AML were referred to Blood & Marrow Transplantation Center and received allo-HSCT as salvage therapy. Among them, 4 patients without HLA-matched donor sibling (MSD) and unrelated donor (MUD, not available in 3 and refusal from donor in 1) received allo-HSCT from related haploidentical donors were excluded from the analysis. The remaining 16 patients with MSD (n = 6) or MUD (n = 10) were enrolled in the study. For those patients enrolled for MSD transplantation, no salvage chemotherapy was given, whereas for those patients for MUD transplantation, salvage chemotherapy was allowed during the work-up phase to MUD transplantation based on the decision of their primary hematologists.
Initially, 14 patients enrolled received cytoreductive chemotherapy and conditioning regimen on schedule except for 2 patients (UPN4 and 8) who experienced severe neutropenic fever with sepsis after FLAG-IDA chemotherapy, which lead to a 3-day delay of Flu-Bu conditioning delivery after successful antibiotics treatment and the day-7 bone marrow evaluation was not performed. Thus, 2 more patients were enrolled who received the FLAG-IDA chemotherapy, day-7 bone marrow assessment, and Flu-Bu conditioning on schedule. All these 16 patients completed the cytoreduction chemotherapy, conditioning regimen, and mobilized peripheral stem cells infusion sequentially. The data of first 14 patients enrolled were eligible for determination of either rejection or acceptance of the study null hypothesis in the statistical consideration, whereas data of all 16 patients were included for the analysis of overall transplantation outcome, toxicity, and comparison study to the historical control.
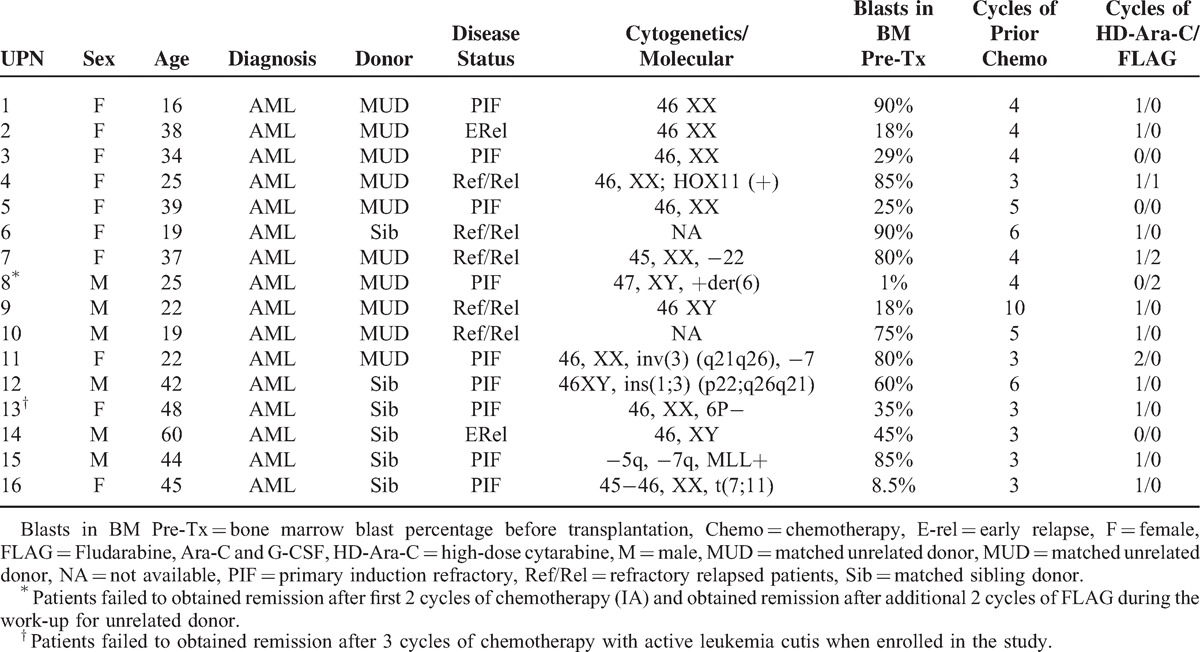
The patients’ characteristics of all 16 patients are summarized in Table 1. The median age of patients was 36 (16–60). Among 16 patients enrolled, 9 had PIF, 2 early relapse and 5 had relapse/refractory disease. All these patients received at least 3 cycles of chemotherapy (median 4, range 3–10). Fifteen patients had active disease and only 1 patient (UPN 8) who failed to obtained CR after first 2 cycles of induction chemotherapy (idarabicin + cytarabine) with a 9/10 matched MUD donor identified was enrolled in the study. During the work-up period for MUD transplantation with delay for almost 2 months, the very patient obtained CR with 2 additional cycles of salvage chemotherapy (Fludarabine + cytarabine + G-CSF, FLAG). Since the patient insisted to undergo the study protocol, the allo-HSCT was performed accordingly. Overall, the median percentage of blasts in bone marrow before the FLAG-IDA chemotherapy was 55% (1%–90%). The graft contained a median of 7.15 × 108/kg mononuclear cells (MNC, range 3.8–12.7) and 6.45 × 106 CD34+/kg (range 2.26–22.0).
TABLE 1.
Patients’ Characteristics
Transplantation Outcome
As to the cytoreduction, the bone marrow analysis on day -7 was evaluable in a total of 14 patients (except for UPN 4 and 8) and all demonstrated an extremely hypocellularity without any blasts (n = 12) or only few blasts (<5 in per bone marrow smear, n = 2).
As to the engraftment, 1 patient (UPN 4) died before engraftment, whereas the remaining 15 patients achieved neutrophil engraftment (>0.5 × 109 cells/L) with median of 15 days (range 9–24). The engraftment of platelet (>20 × 109 cells /L) occurred in 12 patients with a median of 19.5 days (range 10–30), whereas remaining 3 patients had poor platelet recovery dependent on platelet transfusions for at least 3 months after transplantation (Figure 2).
FIGURE 2.
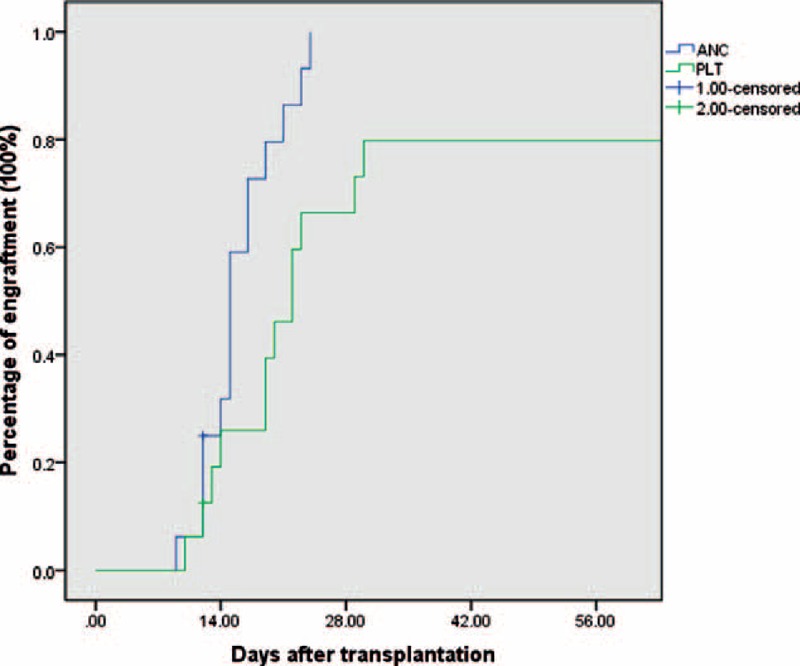
The probability of neutrophils and platelet engraftment after transplantation in 16 patients.
Among the 15 patients with evaluable chimerism data at day 28, all had complete donor chimersim (>99%) in unfractionated bone marrow nucleated cell compartments in which 11 patients achieved CR and 4 with bone marrow remission without platelet recovery (CRp, Table 2).
TABLE 2.
Clinical Outcome
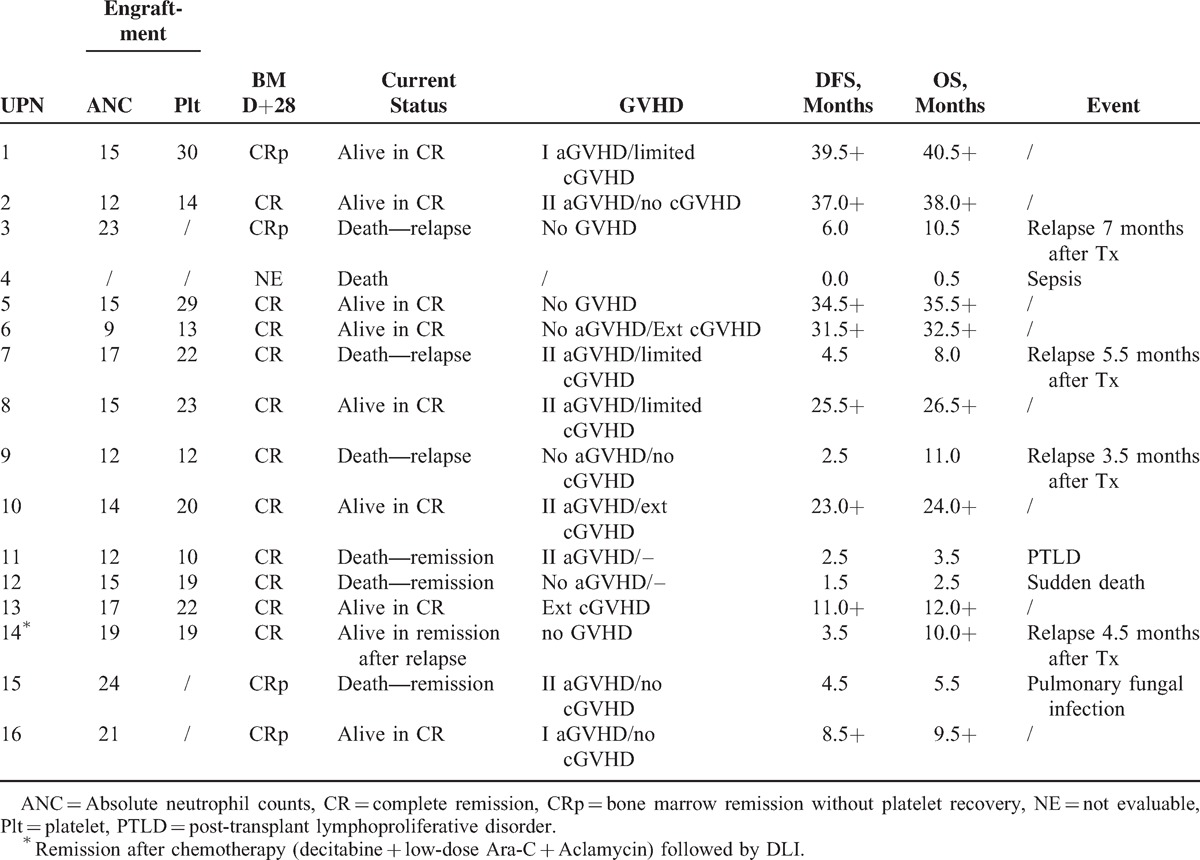
Up to the last follow-up at Nov 30, 2014, with a median follow-up of 10.5 months (0.5–40.5) for all patients, 4 patients died because of transplantation-related toxicity including infection before engraftment, post-transplant lymphoproliferative disorder (PTLD), sudden death, and pulmonary fungal infection respectively (as shown in Table 2). Accumulated 100-day NRM and 2-year NRM were 18.7% ± 9.8% and 25.0% ± 10.8%, respectively.
A total of 4 patients relapsed 3.5, 4.5, 5.5, and 7 months, respectively after transplantation with 2-year accumulated relapse rate of 33.4% ± 13.8%. One patient (UPN3) declined any further treatment and 2 patients (UPN 7 and 9) failed to respond to rescue chemotherapy and all died of disease. Only 1 patient (UPN 14) achieved remission with salvage therapy with decitabine and low-dose chemotherapy followed by DLI and remained in remission up to last follow-up (as shown in Table 2).
Overall, 8 patients remained alive without relapse for a median of 29.5(9.5–40.5) months and 6 of them already maintained leukemia-free for >2 years after allo-HSCT. Based on a median follow-up of 26.5 months for all alive patients, the estimated 2-year OS and LFS were 53.5% ± 13.1% and 50.0% ± 12.5%, respectively.
Transplantation Toxicity
The toxicities of this transplantation protocol were mainly hematological toxicities and toxicity of gastric intestinal tract as shown in Tables 3 and 4. All patients developed grade IV neutropenia and thrombocytopenia. The median duration of neutropenia was 24 days (18–36). All these patients experienced at least 1 episode of neutropenic fever (14 grade III and 2 grade IV) and septic shock (grade IV) was documented in 2 patients after FLAG-IDA chemotherapy causing a 3-day delay of conditioning regimen given. The gastric intestine (GI) toxicity such as anorexia, mucositis, and diarrhea occurred almost in all patients and total parenteral nutrition (TPN) support had to be given in 12 of 16 patients. Grade III GI bleeding was documented in 1 patient after conditioning regimen and recovered well after intensive supportive care. The liver toxicity was mild to moderate and only 1 veno-occlusion disease was documented (as shown in Tables 3 and 4).
TABLE 3.
Major Toxicities
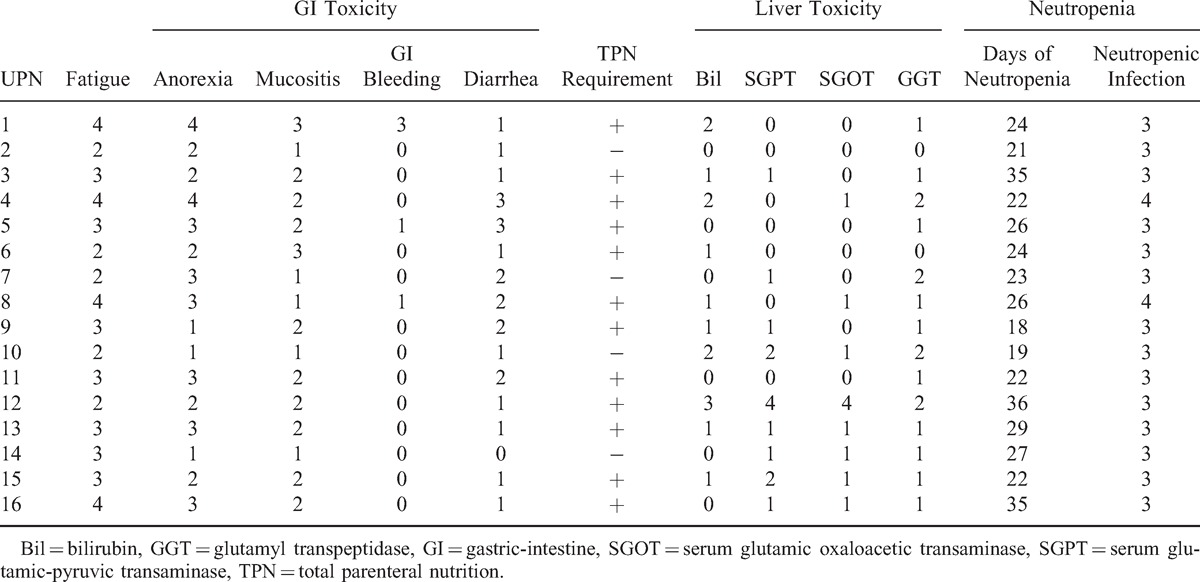
TABLE 4.
Overall Toxicity Grading
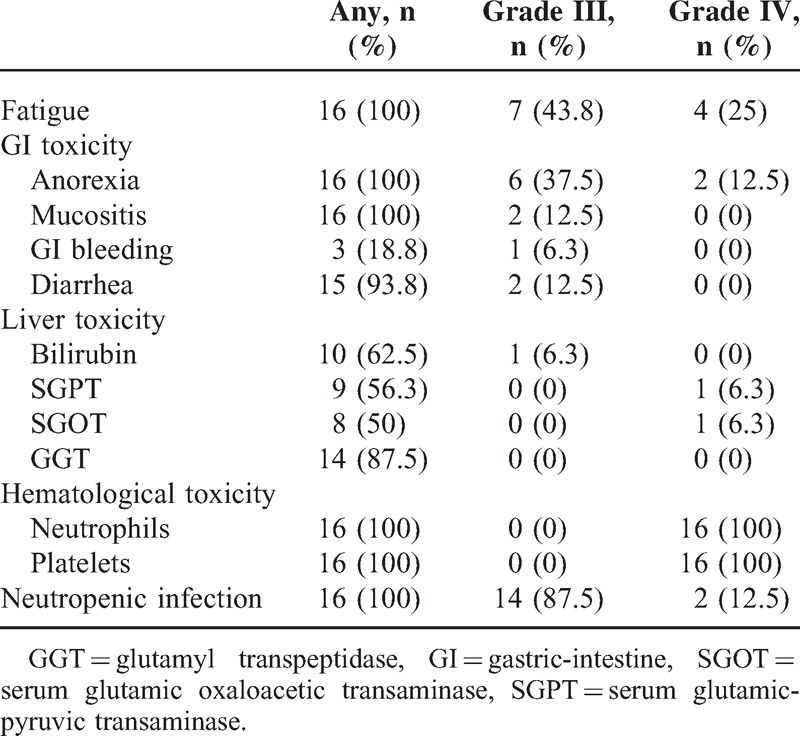
As to the aGVHD, in 15 evaluable patients, 8 developed aGVHD in which grade II in 6 and no grade III-IV aGVHD occurred. For 12 evaluable patients for cGVHD, 3 patients had limited cGVHD and another 3 had experienced extensive cGVHD (as shown in Table 2).
Comparison with Historical Control
In the next analysis, we compared the transplantation outcome of patients enrolled in the clinical trial with our historical control series of 26 patients with refractory AML received allogeneic transplantation from Jan 1st, 2000 to May 30, 2011 in our Blood and Marrow Transplantation Center (patient's characteristics as show in Table 5). When compared to the study group, the historical control group had significant lower bone marrow blasts before transplantation and all other clinical features were not significantly different between 2 groups. As to the transplantation outcome, most patients in the historical control group relapsed or died of transplantation toxicity within 6 months with only 3 patients remained disease-free 6 months after transplantation and 2 remained alive in continuous remission for >3 years up to the last follow-up. When compared with the historical control group, the outcome of patients enrolled in the study group was significantly improved in relapse rate (vs 81.2% ± 9.1%, P = 0.002), LFS (vs 11.1% ± 6.0%, median 3.7 months, P = 0.01), and OS (vs. 11.19% ± 6.0%, median 4.5 months, P = 0.002), whereas the NRM was not significantly different (vs 41.9% ± 14.8%, P = 0.5) as shown in Figure 3.
TABLE 5.
Patients’ Characteristics for Patients in Historical Control Group Compared With Study Group
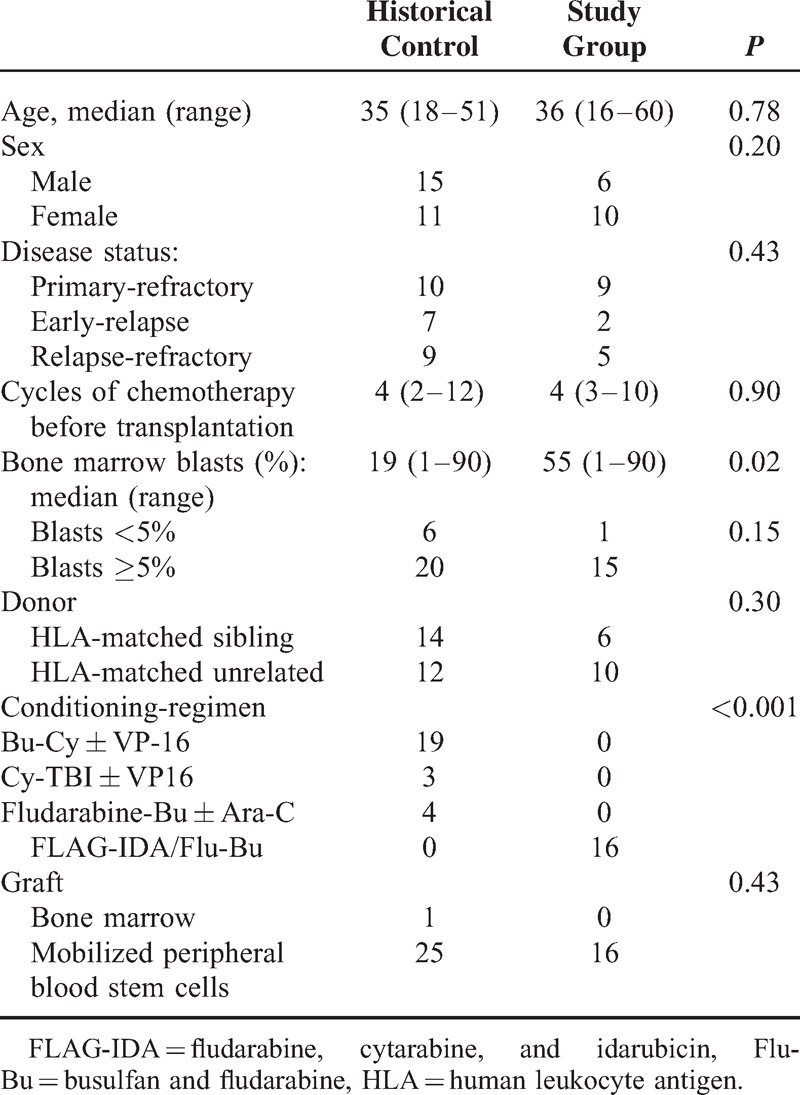
FIGURE 3.
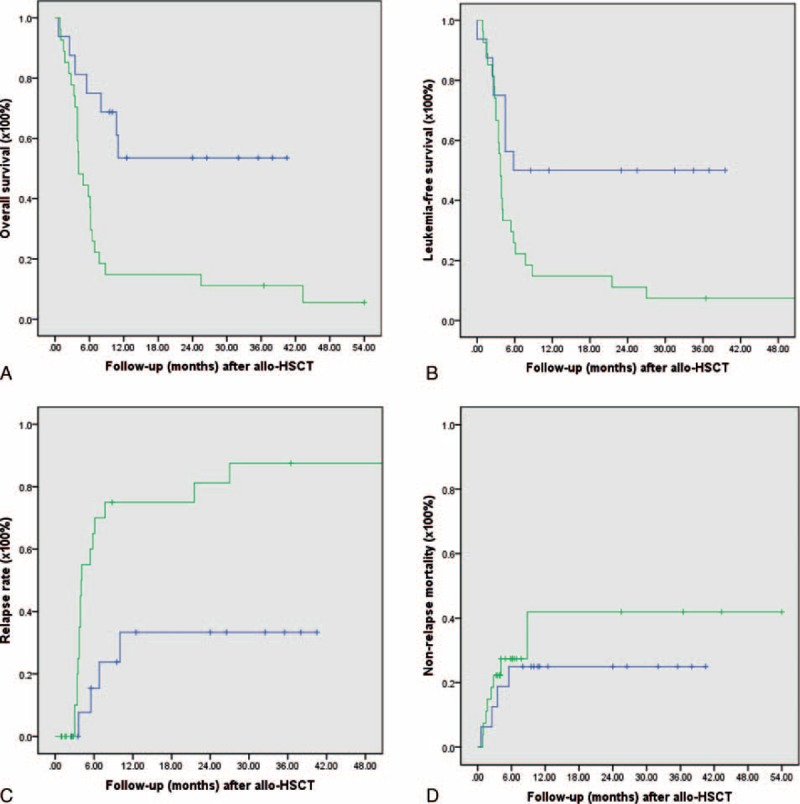
(A) The 2-year overall survival of patients in study group (53.5% ± 13.1%) compared with historical control (11.1% ± 6.0%, P = 0.002). (B) The 2-year leukemia-free survival of patients in study group (50.0% ± 12.5%) compared with historical control (11.1% ± 6.0%, P = 0.01). (C) The 2-year relapse rate of patients in study group (33.4% ± 13.8%) compared with historical control (81.2% ± 9.1%, P = 0.002). (D) The 2-year nonrelapse mortality in study group (25.0% ± 10.8%) compared with historical control (40.9% ± 14.8%, P = 0.50).
Landmark Analysis
Accordingly to the statistic consideration, patient (UPN 8) was excluded from analysis for statistical hypothesis rejection or acceptance because a bone marrow remission was documented before the FLAG-IDA chemotherapy. Among first 14 patients (UPN 1–15, UPN 8 excluded) enrolled in the study, 8 patients relapsed or died from either relapse or transplantation-related toxicity within 2 years after allo-HSCT. One patient (UPN 13) remained leukemia-free up to the last follow-up but did not complete the 2-year landmark. Importantly, the remaining 5 patients completed the designed follow-up for at least 2 years after allo-HSCT without relapse or other lethal event, thus based on the statistical analysis, the null hypothesis of the trial is rejected and the FLAG-IDA/Flu-Bu transplantation protocol is considered being possible to result a potential 2-year LFS around 45%, which is of clinical interest and required confirmation in further large-scale clinical trial.
DISCUSSION
Treatment options for adult patients with refractory AML are extremely limited.1,2,22 Although HSCT is generally the only curative option, active disease before HSCT leads to limited survival.4–8 The reported long-term survival in young patients undergoing fully myeloablative regimens approaches 25% at 3 years and <10% with RIC regimens at 5 years.23,24 One strategy to improve the outcomes is to give preconditioning cytoreduction therapy with active chemotherapy regimen and the feasibility of this strategy has been shown prospectively in refractory AML patients with active disease.10–13
Several lines of evidence demonstrated that lower leukemia burden before transplantation was prognostic factor for achievement of CR and improvement of long-term outcomes after allo-HSCT. In an EBMT Registry, analysis of 168 primary refractory AML underwent unrelated donor transplantation, a lower percentage of bone marrow blasts at transplant was associated with improved survival.25 In a retrospective study, 17 patients with active refractory AML and extensive previous therapy received clofarabine as cytoreduction chemotherapy followed by 14–21 days conditioning regimen with fludarabine, alemtuzumab, and melphalan (Flu/Mel/Alem) or busulfan (Flu/BU/Alem). The effective cytoreduction after clofarabine at the hematological nadir in terms of blast clearance (blasts <10% in bone marrow) was important prognostic factor. The PFS was 6.4 months for patients who achieved cytoreduction after initial induction, compared with 3.8 months (P = .035) for those who did not. OS was 16.6 months for patients who achieved cytoreduction compared with 5.1 months (P = 0.053) for patients who did not.11
In our historical control series with conventional conditioning regimen such as Bu-Cy ± Vp16 or Cy-TBI ± Vp16, although remission can be achieved after allo-HSCT in these patients with refractory AML with median LFS of 3.7 months and OS of 4.5 months which was comparable to the group without success cytoreduction by clofarabine in the study reported by Locke et al.11 Most patients relapsed rapidly within 6 months (70.0% ± 10.2% as shown in Figure 3C) after transplantation, which was before the development of potential allogeneic graft-versus leukemia (GVL) effect, which is more likely associated with cGVHD and believed to have greater impact on the long-term disease control.10,26–28
In this prospective study, the goal was to evaluate whether the new strategy to give conditioning regimen at the nadir of cytoreductive chemotherapy could result in significantly better disease control after transplantation in refractory AML. The enrolled patients with primary or relapsed/refractory AML were heavily pre-treated with extreme high risk because of a median marrow blast of 55%. Of note, 7 days after FLAG-IDA chemotherapy, almost all evaluable patients achieved an extreme hypocellular bone marrow with significant clearance of blasts. Moreover, 8 of 16 patients remained in CR at last follow-up with a median follow-up of 29.5 months (9.5–40.5) without prophylactic DLI. Actually 5 of them remained leukemia-free for >2 years (including 2 for >3 years). Interestingly, 7 of 8 patients who remained alive in remission had either aGVHD or cGVHD. Based on these observations, we speculated that for refractory AML the sequential administration of Flu-Bu conditioning at the aplasia phase of intensive FLAG-IDA cytoreductive chemotherapy, which significantly depleted the leukemia burden, may lead to improved early disease control (7 of 15 evaluable patients remained in CR at the first 6 months after allo-HSCT). This benefit can be possibly enhanced or maintained by the subsequent GVL effect associated with aGVHD or cGVHD and eventually translates into better long-term disease control.10
The major challenge of this protocol was the toxicity such as prolonged myelo-suppression and GI toxicity associated with intensive chemotherapy and conditioning regimen given with a short interval. Indeed, we observed significant myelosuppression, which leads to prolonged neutropenia and increased risk of infection in which all patients had at least 1 episode of neutropenic fever, including 2 patients with septic shock. Aggressive supportive care such as transfusion blood products and TPN were required in most patients. Fortunately, the overall accumulated NRM of patients following HCT was comparable to the historical control.
Overall, we present the results of phase II trial of conditioning regimen Flu-Bu sequentially given at the hematological nadir of previous cytoreductive chemotherapy. FLAG-IDA was feasible and can be considered as effective salvage treatment for patients with refractory AML. The small number of patients studied in the trial precludes any definitive conclusions, but this strategy has sufficient activity to warrant further investigation prospectively with a larger patient sample.
Uncited reference
20.
Acknowledgments
The authors thank Dr Ming Wan and Dr Jun-Cai Xu from Shanghai Clinical Research Center (SCRC) for assistance in the sample size estimation and statistics analysis.
Footnotes
Abbreviations: aGVHD = acute GVHD, allo-HSCT = allogeneic hematopoietic stem cell transplantation, AML = acute myeloid leukemia, ATG = antithymocyte globulin, cGVHD = chronic GVHD, CIMBTR = Center for International Blood and Marrow Transplant Research, CR = complete remission, Crp = bone marrow remission without platelet recovery, CsA = cyclosporine, DLI = donor lymphocytes infusion, FLAG-IDA = fludarabine, cytarabine and idarubicin, Flu-Bu = busulfan and fludarabine, GI = gastric-intestine, GVHD = graft-versus-host disease, GVL = graft-versus leukaemia, LFS = leukemia-free survival, MDS = myelodysplasia syndrome, MMF = mycophenolate mofetil, MNC = mononuclear cells, MSD = HLA-matched sibling donor, MTX = methotraxate, MUD = HLA-matched unrelated donor, NRM = nonrelapse mortality, OS = overall survival, PFS = progression-free survival, PIF = primary induction failure, PTLD = post-transplant lymphoproliferative disorder, RIC = reduced-intensity conditioning, TPN = total parenteral nutrition.
WT and XF contributed equally to the manuscript.
The authors declare no conflict of interest.
REFERENCES
- 1.Craddock C, Tauro S, Moss P, et al. Biology and management of relapsed acute myeloid leukaemia. Br J Haematol 2005; 129:18–34. [DOI] [PubMed] [Google Scholar]
- 2.Mato AR, Morgans A, Luger SM. Novel strategies for relapsed and refractory acute myeloid leukemia. Curr Opin Hematol 2008; 15:108–114. [DOI] [PubMed] [Google Scholar]
- 3.Revesz D, Chelghoum Y, Le QH, et al. Salvage by timed sequential chemotherapy in primary resistant acute myeloid leukemia: analysis of prognostic factors. Ann Hematol 2003; 11:684–690. [DOI] [PubMed] [Google Scholar]
- 4.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant 2003; 12:766–771. [DOI] [PubMed] [Google Scholar]
- 5.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM). Bone Marrow Transplant 2000; 26:1157–1163. [DOI] [PubMed] [Google Scholar]
- 6.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood 1992; 4:1090–1093. [PubMed] [Google Scholar]
- 7.Mehta J, Powles R, Horton C, et al. Bone marrow transplantation for primary refractory acute leukaemia. Bone Marrow Transplant 1994; 3:415–418. [PubMed] [Google Scholar]
- 8.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA matching, and marrow cell dose. Blood 1997; 89:4226–4235. [PubMed] [Google Scholar]
- 9.Duval M, Klein JP, He WS, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28:3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108:1092–1099. [DOI] [PubMed] [Google Scholar]
- 11.Locke FL, Artz A, Rich E, et al. Feasibility of clofarabine cytoreduction before allogeneic transplant conditioning for refractory AML. Bone Marrow Transplant 2010; 45:1692–1698. [DOI] [PubMed] [Google Scholar]
- 12.Platzbecker U, Thiede C, Fussel M, et al. Reduced intensity conditioning allows for up-front allogeneic hematopoietic stem cell transplantation after cytoreductive induction therapy in newly-diagnosed high-risk acute myeloid leukemia. Leukemia 2006; 20:707–714. [DOI] [PubMed] [Google Scholar]
- 13.Buchholz S, Dammann E, Stadler M, et al. Cytoreductive treatment with clofarabine/ara-C combined with reduced-intensity conditioning and allogeneic stem cell transplantation in patients with high-risk, relapsed, or refractory acute myeloid leukemia and advanced myelodysplastic syndrome. Eur J Haematol 2012; 88:52–60. [DOI] [PubMed] [Google Scholar]
- 14.Liu QF, Fan ZP, Zhang Y, et al. Sequential intensified conditioning and tapering of prophylactic immunosuppressants for graft-versus-host disease in allogeneic hematopoietic stem cell transplantation for refractory leukemia. Biol Blood Marrow Transplant 2009; 15:1376–1385. [DOI] [PubMed] [Google Scholar]
- 15.Martin MG, Augustin KM, Uy GL, et al. Salvage therapy for acute myeloid leukemia with fludarabine, cytarabine, and idarubicin with or without gemtuzumab ozogamicin and with concurrent or sequential G-CSF. Am J Hematol 2009; 84:733–737. [DOI] [PubMed] [Google Scholar]
- 16.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: Study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant 2002; 8:468–476. [DOI] [PubMed] [Google Scholar]
- 17.Estey E. Treatment of refractory AML. Leukemia 1996; 10:932–936. [PubMed] [Google Scholar]
- 18.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 1974; 18:295–304. [DOI] [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11:945–956. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Lee TY, Kim KM, et al. Admissible two-stage designs for phase II cancer clinical trials. Stat Med 2004; 23:561–569. [DOI] [PubMed] [Google Scholar]
- 21.Lee ET, editor. Statistical Methods for Survival Data Analysis. Belmont, CA: Lifetime Learning; 1980. p. 355. [Google Scholar]
- 22.Ofran Y, Rowe JM. Treatment for relapsed acute myeloid leukemia: what is new? Curr Opin Hematol 2012; 19:89–94. [DOI] [PubMed] [Google Scholar]
- 23.Horowitz MM, Rowlings PA. An update from the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry on current activity in hematopoietic stem cell transplantation. Curr Opin Hematol 1997; 4:395–400. [DOI] [PubMed] [Google Scholar]
- 24.Sierra J, Storer B, Hansen JA, et al. Unrelated donor marrow transplantation for acute myeloid leukemia: an update of the Seattle experience. Bone Marrow Transplant 2000; 26:397–404. [DOI] [PubMed] [Google Scholar]
- 25.Craddock C, Labopin M, Pillai S, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia 2011; 25:808–813. [DOI] [PubMed] [Google Scholar]
- 26.Schmid C, Schleuning M, Ledderose G, et al. Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 2005; 23:5675–5687. [DOI] [PubMed] [Google Scholar]
- 27.Weisdorf D, Zhang MJ, Arora M, et al. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant 2012; 18:1727–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka I, Kami M, Takahashi S, et al. Clinical impact of graft-versus-host disease against leukemias not in remission at the time of allogeneic hematopoietic stem cell transplantation from related donors. The Japan Society for Hematopoietic Cell Transplantation Working Party. Bone Marrow Transplant 2004; 34:711–719. [DOI] [PubMed] [Google Scholar]


