Supplemental Digital Content is available in the text
Abstract
Infliximab is an anti-tumor necrosis factor (TNF) used for treatment of inflammatory bowel disease (IBD) as well as rheumatoid arthritis, psoriasis, and other inflammatory conditions. Antibodies to infliximab (ATI) develop in approximately 45% of infliximab-treated IBD patients and are correlated with loss of clinical response. Scarce data exist as to factors which predict infliximab immunogenicity.
To investigate factors that may predict formation of antibodies to infliximab (ATI) and infliximab therapy failure an observational study of consecutive IBD patients treated with infliximab between 2009 and 2013 was performed. Trough levels of ATI were measured. Patients were monitored for disease activity using clinical activity indexes and were classified according to ATI formation and clinical response. All clinical and demographic parameters were analyzed for association with the designated outcomes.
One hundred fifty-nine patients were included and 1505 sera were tested. On multivariate analysis, Jewish Ashkenazi ethnicity was protective against both development of ATI (odds ratio [OR] 0.35, 95% confidence interval [CI] 0.17–0.7, P = 0.005) and treatment failure (OR 0.29, 95% CI 0.13–0.66, P = 0.003). Concomitant immunomodulator therapy was also negatively associated with immunogenicity and infliximab therapy failure (OR 0.31, 95% CI 0.15–0.65, P = 0.002; OR 0.42 95% CI 0.18−0.99, p = 0.04, respectively), whereas episodic therapy was positively associated with both outcomes (OR 4.2 95% CI 1.07−16.1, p = 0.04, OR 4.45 95% CI 1.2−16.6, p = 0.026 respectively). All other variables, including IBD type, gender, weight, age, smoking status and disease duration, were not predictive of ATI formation or clinical failure. However, among Crohn's disease patients, a non-stricturing non-penetrating phenotype was protective against ATI formation (OR 0.4, 95% CI 0.14−0.96, p = 0.04). P = 0.04, respectively), whereas episodic/interrupted therapy was positively associated with both outcomes (OR 4.2, 95% CI 1.07–16.1, P = 0.04; OR 4.45, 95% CI 1.2–16.6, P = 0.026, respectively). All other variables, including IBD type, sex, weight, age, smoking status, and disease duration, were not predictive of ATI formation or clinical failure. However, among Crohn disease patients, a nonstricturing nonpenetrating phenotype was protective against ATI formation (OR 0.4, 95% CI 0.14–0.96, P = 0.04).
Jewish Ashkenazi ethnicity is protective of ATI formation and infliximab therapy failure. These findings suggest a role for ethnicity, and implicitly for genetic predisposition, in modulating the risk of anti-TNF immunogenicity and treatment unresponsiveness.
INTRODUCTION
Infliximab is a chimeric mouse–human monoclonal immunoglobulin G1 (IgG1) antibody against tumor necrosis factor α (TNFα), which is effective in Crohn's disease (CD) and ulcerative colitis (UC),1–3 as well as rheumatoid arthritis, psoriasis, and other inflammatory conditions. Recent data suggest that antibodies to infliximab (ATI) develop in approximately 45% of inflammatory bowel disease (IBD) patients even when receiving scheduled infliximab therapy.4,5 The formation of ATI is associated with lower serum infliximab levels, infusion reactions, and loss of clinical response (LOR).6,7 Preinfusion steroids and concomitant immunomodulator therapy are known to decrease the risk of immunogenicity, whereas episodic therapy increases ATI formation rate.8–10
Several parameters related to disease severity, including TNFα tissue burden, C-reactive protein (CRP) level and serum albumin concentration, have been associated with increased infliximab clearance and consequently lower serum levels.6,11 Additional patient-specific factors, such as body weight, sex, age, and smoking status have also been linked with infliximab trough levels and clinical response, albeit with conflicting findings in the different studies.6,12–15 However, only scarce data exist regarding individual patient's factors specifically predicting ATI formation and clinical outcome.16 Identification of such patient-specific characteristics could assist in tailoring personalized treatment regimens, which would minimize future immunogenicity and loss of response.
Hence, the aim of the present study was to systematically map out clinical and demographic patient-related factors, which predict ATI formation. Predictors for infliximab therapy failure were sought as a secondary outcome.
METHODS
Patient Population
This was an observational noninterventional retrospective study of patients with IBD attending the Gastroenterology Department of the Sheba medical center and receiving infliximab infusions between February 2009 and November 2013. Clinical parameters were retrieved from the charts. Patients’ ethnicity was either retrieved from chart review or – when unavailable – inquired through direct interview with patients. The Israeli Jewish population consists of 2 major groups: Ashkenazi Jews from central and Eastern Europe and Sephardic Jews from the Mediterranean, North Africa, and Asia. Patients’ ethnicity was determined by both parents’/grandparents’ place of birth. If parents had different origins, ethnicity was designated as “mixed.” Non-Jewish patients were categorized accordingly.
Inclusion criteria were as follows:
The patient gave informed consent to participate in the study.
The patient had sequential consecutive pre-infusion sera obtained and analyzed for infliximab and ATI levels, starting from the first infusion of infliximab onward.
Demographic and clinical data, including ethnic origin, were available.
Exclusion criteria:
The patient was lost to follow-up.
Sequential sera were unavailable or the patient started infliximab infusions before study initiation, unless he/she entered the study period receiving the standard 5 mg/kg/8 week regimen without evidence of ATI.
The study was approved by the Sheba Medical Center Ethics committee.
Outcome Definitions
Clinical response was determined by the Harvey-Bradshaw index (HBI) and by the simple clinical colitis activity index (SCCAI) on the day of infliximab infusion, for Crohn's disease and ulcerative colitis patients, respectively. A response to therapy required a drop >3 points in either the HBI or SCCAI and remission was defined as a HBI of ≤4 or SCCAI ≤2.17–19 When unavailable, clinical response was determined by the documented physician global assessment of the patient.
Primary nonresponse was defined as lack of improvement (no decrease in HBI or SCCAI) at the end of the induction period coupled with the physician's decision to stop infliximab therapy. Secondary loss of response was defined by re-emergence of disease activity (a rise of >3 points in HBI score or of >2 points in SCCAI score) after achieving an appropriate induction response, coupled with a need for alteration of therapy (dose intensification or addition of an immunomodulator) or a need for infliximab discontinuation.20 Infliximab failure was defined as cessation of infliximab therapy due to primary nonresponse, secondary loss of response or due to a severe infusion reaction. Antibody formation was defined as positive when a patient tested positive for ATI on >2 consecutive time points. Transient antibodies were defined as measurable ATI on up to 2 consecutive infusions, which disappeared on the next infusions without any alteration of therapy.4,5
Measurement of ATI
ATI were determined as previously described,21,22 before each infliximab infusion prospectively. Briefly, infliximab (0.1 mg/mL) was added to pre-plated TNFα (500 ng/mL) in 100-μL wells of ELISA plates (Nunc, Roskilde, Denmark). After drying, 100 μL of serum was added and incubated for 90 min at room temperature. Plates were then washed and goat anti-human λ chain HRP-labeled antibody (Sertec, Oxford, UK) was added at a dilution of 2.5 × 104 for 60 min and reacted with tetramethylbenzidine substrate. The results were read by an ELISA reader EL-800 (Biotek Instruments, Winooski, VT) and expressed as μg/mL-equivalent (μg/mL-e) after normalization versus results obtained using additions of graded concentrations between 9 and 600 ng/mL of horseradish peroxidase (HRP)-labeled goat anti-human F(ab’)2 fragment antibody (MP Biomedical, Santa-Ana, CA, 92707 USA). The lower limit of detection was 2.5 μg/mL-eq.
Statistical Analysis
Clinical and demographic parameters were analyzed for their association with the designated outcomes. Variables that were found to be statistically significant on univariate analysis (P ≤ 0.1) were incorporated into the multivariate analysis with backwards logistic regression. Continuous variables were analyzed by a 2-tailed Student t test or by Mann–Whitney test, as appropriate. Categorical variables were analyzed by Fisher's exact test. Odds ratio (OR) and 95% confidence intervals (CI) were computed for all variables compared. Kaplan–Meier survival curves were plotted to assess the temporal rate of events and log rank test was computed for the comparison between survival free durations. All statistics were performed using MedCalc software (version 12.2.1.0, Mariakerke, Belgium). A 2-tailed P < 0.05 was considered statistically significant.
RESULTS
Patient Population
Forty two of 216 patients who received infliximab during the study period were excluded due to missing serum samples (19 had started infliximab before the study was initiated and 23 received infusions outside our center). Additional 10 patients were lost to follow-up and 5 patients refused to consent. Thus, 159 patients were included (125 CD, 34 UC, median follow-up time 11.5, interquartile range = 3.5–23 months) and 1505 consecutive sera samples were analyzed for ATI levels during the 4-year study (Table 1).
TABLE 1.
Background Disposition and Clinical Characteristics

Predictors of ATI Formation
Demographic and clinical parameters were analyzed for their association with ATI formation by univariate and multivariate analysis (Table 2, Figure 1). Concomitant immunomodulator therapy protected against ATI formation (OR 0.31, 95% CI 0.15–0.65, P = 0.002), whereas episodic/interrupted therapy increased the risk for immunogenicity (OR 4.2, 95% CI 1.07–16.1, P = 0.04). Jewish Ashkenazi, as opposed to Jewish Sephardic ethnicity, was independently protective of ATI formation (OR 0.35, 95% CI 0.17–0.7, P = 0.005). Accordingly, survival free of ATI formation was significantly longer among the Ashkenazi patients (log rank test, P = 0.0086, Figure 2).
TABLE 2.
Demographic and Clinical Factors Associated With Sustained ATI Formation
FIGURE 1.
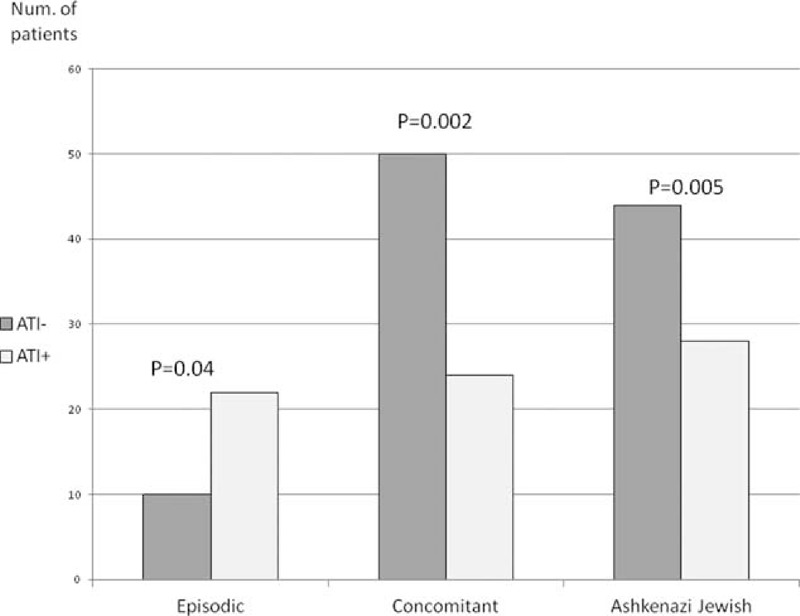
Prevalence of episodic/interrupted therapy, concomitant IMM therapy, and Jewish Ashkenazi ethnicity among patients who developed ATI versus those who did not. ATI = antibodies to infliximab, IMM = immunomodulators.
FIGURE 2.
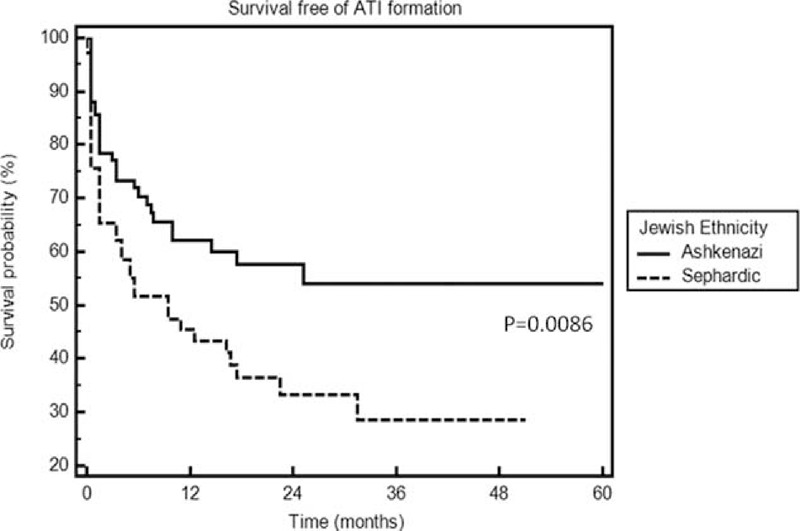
Survival free of ATI formation in Jewish Ashkenazi versus Sephardic patients. ATI = antibodies to infliximab.
Predictors of Infliximab Therapy Failure
Next, demographic and clinical parameters were analyzed for their association with infliximab therapy failure (Table 3). ATI formation was significantly more prevalent among patients who failed infliximab therapy (OR 5.6, 95% CI 2.2–14.4, P = 0.0003). Again, Jewish Ashkenazi ethnicity was protective against infliximab therapy failure (OR 0.35, 95% CI 0.15–0.83, P = 0.019) and survival free of infliximab failure was longer among the Ashkenazi patients (log rank test, P = 0.0046, Figure 3). Because the existence of ATI serves as an outcome itself and is an immunogenic rather than a clinical parameter, we performed an additional multivariate analysis removing excluding ATI formation from the analysis. After removal of the ATI variable, episodic/interrupted therapy became significantly predictive of infliximab therapy failure (OR 4.45, 95% CI 1.2–16.6, P = 0.026), whereas concomitant immunomodulator therapy became protective of this outcome (OR 0.42, 95% CI 0.18–0.99, P = 0.04). Jewish Ashkenazi ethnicity retained its statistical significance (OR 0.3, 95% CI 0.13–0.67, P = 0.003).
TABLE 3.
Demographic and Clinical Factors Associated With Infliximab Therapy Failure
FIGURE 3.
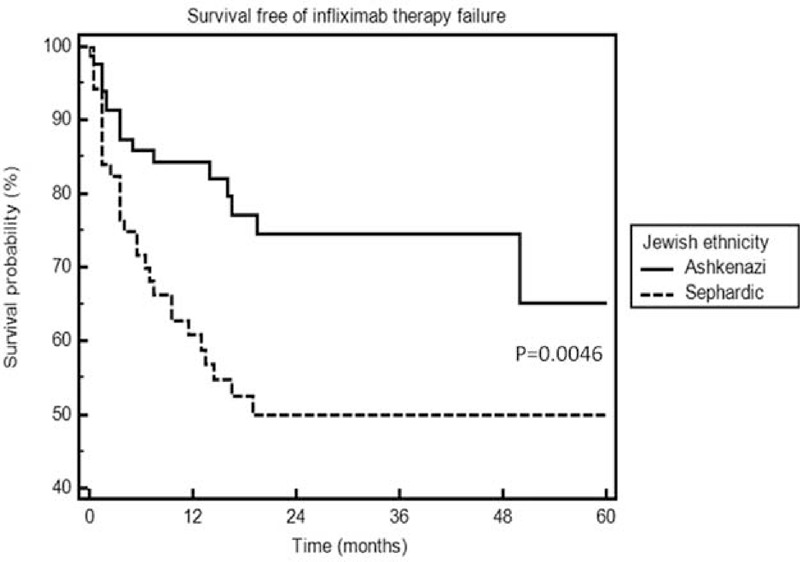
Survival free of infliximab therapy failure in Jewish Ashkenazi versus Sephardic patients.
Of patients experiencing infliximab therapy failure, 16 were primary nonresponders and 56 experienced secondary loss of response. Among Spheradic jews, 11 of 70 were primary nonresponders compared to patients 5 of 72 Ashkenazi (P = 0.12). Thirty-four of 70 Sephardic experienced secondary nonresponse compared with 22 of 72 among Ashkenazis (P = 0.04).
Ten of 16 primary nonresponders developed ATI by the first measurement point compared to 32 of 56 secondary nonresponders (P = 0.78). The primary nonresponders (11 patients) were mostly Sephardic and developed higher median ATI levels than patients who experienced secondary loss of response (median 3.7 versus 7.75, respectively, P = 0.028).
Predictors of Immunogenicity Among Patients Followed for 1 Year
It was recently demonstrated that ATIs seldom develop after the first year of infliximab therapy.4 Hence, to exclude a potential caveat whereby some ATI-negative patients may develop ATI later on during the course of their treatment, an additional subanalysis was performed comprising only of the 119 patients followed for at least 54 weeks (unless ATI developed beforehand). Even in this more stringent cohort, concomitant immunomodulator therapy and Ashkenazi Jewish ethnicity retained their statistically significant association with ATI formation on multivariate analysis (OR 0.4, 95% CI 0.17–1, P = 0.05; OR 0.29, 95% CI 0.12–0.7, P = 0.008, respectively).
Predictors of Immunogenicity and Therapy Failure Among CD Patients
Because the above analyses consisted of both CD and UC patients, we sought to examine whether clinical characteristics specific to CD may predict ATI formation and clinical response (Supplementary Tables 1 and 2). Even within the CD cohort, Jewish Ashkenazi ethnicity remained significantly protective against ATI formation (OR 0.23, 95% CI 0.1–0.55, P = 0.0013) and survival free of ATI formation was significantly longer among Ashkenazi Jewish patients compared with Sephardic-origin Jews (log rank test, P = 0.029, supplementary Figure 1, http://links.lww.com/MD/A237). In addition, non-stricturing nonpenetrating CD, as opposed to stricturing or penetrating disease, was found to be protective of ATI formation (OR 0.4, 95% CI 0.14–0.96, P = 0.04) and survival free of ATI formation was significantly longer among patients with nonstricturing nonpenetrating disease (log rank test, P = 0.0037, supplementary Figure 2, http://links.lww.com/MD/A237).
DISCUSSION
Immunogenicity has emerged as an important determinant of anti-TNF treatment failure in IBD. Scheduled therapy and concomitant immunomodulator therapy were shown to be associated with lower risk of infliximab immunogenicity.9,23,24 Several genetic studies, particularly in rheumatology patients, identified different genetic factors associated with reduced response to infliximab and/or infusion reactions.25–26 However, to the best of our knowledge, patient-specific predictors of infliximab immunogenicity have not been identified so far. This knowledge gap has precluded a rational tailoring of individualized therapy, namely, prescribing combination immunomodulator-infliximab therapy to those patients with high risk of infliximab immunogenicity, while sparing it from patients with low risk of developing antibodies to infliximab (ATI).
This study aimed to identify factors predicting formation of ATI and therapy failure in IBD patients treated with infliximab. A novel negative association of Jewish Ashkenazi ethnicity with ATI formation and with infliximab failure was identified. This finding was statistically significant for the entire cohort, and became even more pronounced on a subanalysis of CD patients only. Scarce data exist regarding the effect of ethnic background on response rate to infliximab. In a single study, Parsi et al13 have found no difference in response rate in Afro-Americans versus white IBD patients, although only 9 Afro-Americans patients were included. Interestingly, features of CD may be somewhat different in Jewish Ashkenazi patients, who are afflicted less frequently by perianal disease and by extraintestinal manifestations.27,28 Shaoul et al29 reported that pediatric Jewish Ashkenazi patients manifested a less complicated disease pattern. Moreover, a higher response rate to azathioprine was reported in Ashkenazi Jews with CD, as opposed to Sephardic CD patients.30 Taken together, these features of CD in Jewish Ashkenazi patients might suggest a less aggressive disease. The present findings extend these observations by indicating that an Ashkenazi Jewish origin also confers a favorable clinical response to infliximab. This was predominantly mediated by lower immunogenicity rate in Ashkenazi Jews compared with Sephardic ones. However, the fact that clinical outcome was better even when including ATI formation itself as a variable in the analysis (Table 3), indicates that other, yet to be defined mechanisms, independent of ATI formation, also play a role in improving the response of this ethnic group to infliximab. In contrast, episodic/interrupted and concomitant immunomodulator therapies were not correlated with treatment failure when presence of ATI was included as a variable (Table 3), but regained their significant independent correlation when ATI was removed from the analysis. This may suggest that their impact on treatment success is predominantly mediated via their effect on immunogenicity.
Multiple prior studies have examined various demographic and clinical factors, such as BMI, smoking status, and disease location, and have linked them with clinical response, however with contradictory findings.11–14 Smoking and increased BMI were associated with attenuated response to infliximab,12,13,15 whereas Vermeire et al14 identified younger age as an independent variable favoring short-term response to infliximab. In our study, no significant difference in ATI formation or response rate was noted with respect to sex, weight, smoking status, or age. Higher C-reactive protein (CRP) levels have been shown to increase infliximab clearance rate, thereby decreasing serum levels and clinical response.6,11,31 Moreover, it has recently been reported that a significant decrease in CRP soon after induction may be indicative of durable sustained response to infliximab therapy.32
Seow et al33 reported that ATI development rates were significantly higher in UC patients in comparison with previous reports concerning CD patients, but in another study, no statistically significant difference in therapy outcome between the groups was found.34 In our study, no difference was discernible as to either ATI formation or infliximab therapy failure in UC versus CD patients, although the number of UC patients was limited (n = 34) so the study may be underpowered to detect such difference.
The SONIC and the UC SUCCESS trials demonstrated that combination therapy with thiopurines was more effective than infliximab monotherapy.23,35 Feagan et al36 recently reported that the combination of infliximab and methotrexate was no more effective than infliximab alone, although this study differed in design from the SONIC and SUCCESS trials, in particular with respect to corticosteroids use during the induction phase. Indeed, in the present study, although inversely related to immunogenicity and loss of response, the type of immunomodulator (methotrexate versus thiopurines) had no effect on ATI formation rate or clinical outcome. However, lack of difference between the groups could have resulted from the small sample size (only 9 patients were treated with methotrexate). Along the same line, the dosage of concomitant thiopurine had no effect on both outcomes.
Certain limitations of this study need to be acknowledged. The treating physicians were not kept blinded to the ATI results, so one cannot exclude that the results may have influenced clinical management. However, our primary outcome was the emergence of ATI and clinical response was only a secondary outcome. In addition, although our sample is one of the largest so far, 159 patients, some of the subgroups were small (only 34 UC patients, 15 episodic/interrupted therapy patients), which might have affected the ability to detect statistically significant differences in multivariate analysis.
In conclusion, in addition to previously described predictors of immunogenicity, such as episodic therapy and monotherapy, Jewish Sephardic ethnicity (compared with Jewish Ashkenazi descendents) was found as a novel factor associated withATI formation and infliximab therapy failure. These findings suggest a role for ethnic origin, and implicitly for background genetics, in predisposing to immune-reactivity to anti-TNF. Future studies should explore these correlations in other ethnic groups as well as delineate the genetic background responsible for these observations.
Footnotes
Abbreviations: ATI = antibodies to infliximab, CD = Crohn's disease, HBI = Harvey-Bradshaw index, IBD = inflammatory bowel diseases, LOR = loss of response, SCCAI = simple clinical colitis activity index, UC = ulcerative colitis.
This study was financially supported in part by the ’Talpiot’ Medical Leadership program of the Sheba Medical Center (to SBH) and the Helmsley Charitable Trust (SB-H, RE, and YC).
YC has served as a consultant to AbbVie, Takeda, Janssen, pharmacosmos and Schering-Plough and has received unrestricted educational grant from Janssen. SB-H received consultancy fees and/or research support from AbbVie, Schering Plough, Janssen, and Takeda.
BU and OH-N took part in data acquisition; BU drafted the manuscript; MY, EF, and OP performed laboratory analysis of data; UK, RL, AL, YM, BA, AL, BW, YC, and RE assisted in critical revision of the manuscript for important intellectual content; SB-H designed the study and drafted the manuscript.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med 2004; 350:876–885. [DOI] [PubMed] [Google Scholar]
- 3.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353:2462–2476. [DOI] [PubMed] [Google Scholar]
- 4.Ungar B, Chowers Y, Yavzori M, et al. The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2013; 63:1258–1264. [DOI] [PubMed] [Google Scholar]
- 5.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol 2013; 108:962–971. [DOI] [PubMed] [Google Scholar]
- 6.Ordas I, Mould DR, Feagan BG, et al. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012; 91:635–646. [DOI] [PubMed] [Google Scholar]
- 7.Ainsworth MA, Bendtzen K, Brynskov J. Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol 2008; 103:944–948. [DOI] [PubMed] [Google Scholar]
- 8.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348:601–608. [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2004; 2:542–553. [DOI] [PubMed] [Google Scholar]
- 10.Kamm MA. Review article: biological drugs in Crohn's disease. Aliment Pharmacol Ther 2006; 24 Suppl 3:80–89. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Feagan BG, Sandborn WJ, et al. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis 2012; 18:349–358. [DOI] [PubMed] [Google Scholar]
- 12.Arnott ID, McNeill G, Satsangi J. An analysis of factors influencing short-term and sustained response to infliximab treatment for Crohn's disease. Aliment Pharmacol Ther 2003; 17:1451–1457. [DOI] [PubMed] [Google Scholar]
- 13.Parsi MA, Achkar JP, Richardson S, et al. Predictors of response to infliximab in patients with Crohn's disease. Gastroenterology 2002; 123:707–713. [DOI] [PubMed] [Google Scholar]
- 14.Vermeire S, Louis E, Carbonez A, et al. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn's disease. Am J Gastroenterol 2002; 97:2357–2363. [DOI] [PubMed] [Google Scholar]
- 15.Harper JW, Sinanan MN, Zisman TL. Increased body mass index is associated with earlier time to loss of response to infliximab in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19:2118–2124. [DOI] [PubMed] [Google Scholar]
- 16.Moss AC, Brinks V, Carpenter JF. Review article: immunogenicity of anti-TNF biologics in IBD - the role of patient, product and prescriber factors. Aliment Pharmacol Ther 2013; 38:1188–1197. [DOI] [PubMed] [Google Scholar]
- 17.Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005; 54:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980; 1:514. [DOI] [PubMed] [Google Scholar]
- 19.Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010; 8:357–363. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther 2011; 33:987–995. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 2011; 60:41–48. [DOI] [PubMed] [Google Scholar]
- 22.Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis 2012; 18:1628–1633. [DOI] [PubMed] [Google Scholar]
- 23.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010; 362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013; 11:444–447. [DOI] [PubMed] [Google Scholar]
- 25.Criswell LA1, Lum RF, Turner KN, et al. The influence of genetic variation in the HLA-DRB1 and LTA-TNF regions on the response to treatment of early rheumatoid arthritis with methotrexate or etanercept. Arthritis Rheum 2004; 50:2750–2756. [DOI] [PubMed] [Google Scholar]
- 26.Dubinsky MC1, Mei L, Friedman M, et al. Genome wide association (GWA) predictors of anti-TNFalpha therapeutic responsiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2010; 16:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karban A, Itay M, Davidovich O, et al. Risk factors for perianal Crohn's disease: the role of genotype, phenotype, and ethnicity. Am J Gastroenterol 2007; 102:1702–1708. [DOI] [PubMed] [Google Scholar]
- 28.Karban A, Waterman M, Panhuysen CI, et al. NOD2/CARD15 genotype and phenotype differences between Ashkenazi and Sephardic Jews with Crohn's disease. Am J Gastroenterol 2004; 99:1134–1140. [DOI] [PubMed] [Google Scholar]
- 29.Shaoul R, Karban A, Reif S, et al. Disease behavior in children with Crohn's disease: the effect of disease duration, ethnicity, genotype, and phenotype. Dig Dis Sci 2009; 54:142–150. [DOI] [PubMed] [Google Scholar]
- 30.Koifman E, Karban A, Mazor Y, et al. Thiopurine effectiveness in patients with Crohn's disease: a study of genetic and clinical predictive factors. Inflamm Bowel Dis 2013; 19:1639–1644. [DOI] [PubMed] [Google Scholar]
- 31.Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther 2012; 35:568–576. [DOI] [PubMed] [Google Scholar]
- 32.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut 2014; 63:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 2010; 59:49–54. [DOI] [PubMed] [Google Scholar]
- 34.Alzafiri R, Holcroft CA, Malolepszy P, et al. Infliximab therapy for moderately severe Crohn's disease and ulcerative colitis: a retrospective comparison over 6 years. Clin Exp Gastroenterol 2011; 4:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014; 146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 36.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology 2014; 146:681–688.e1. [DOI] [PubMed] [Google Scholar]


