Abstract
The study compares the physical and biologically effective doses (BED) received by the heart and cardiac substructures using three-dimensional conformal RT (3D-CRT), intensity-modulated radiotherapy (IMRT), and simple IMRT (s-IMRT) in postoperative radiotherapy for patients with left-sided breast cancer.
From October 2008 to February 2009, 14 patients with histologically confirmed left-sided breast cancer were enrolled and underwent contrast-enhanced computed tomography (CT) simulation and 18F-FDG positron emission tomography-CT to outline the left cardiac ventricle (LV) and other substructures. The linear-quadratic model was used to convert the physical doses received by critical points of inner heart to BED.
The maximal dose, minimum dose, dose received by 99% of volume (D99) and dose received by 95% of volume (D95) in target areas were significantly better using IMRT and s-IMRT when compared with 3D-CRT (P < 0.05). IMRT and s-IMRT significantly reduced the maximal cardiac dose (5038.98 vs 5346.47 cGy, P = 0.002; 5146.66 vs 5346.47 cGy, P = 0.03). IMRT reduced the maximal dose to LV by 4% (P = 0.05) in comparison with 3D-CRT. The average doses to heart and LV in 3D-CRT plan were significantly lower than those in IMRT plan (P < 0.05). The average cardiac volumes receiving ≥25 Gy (V25 Gy) in IMRT, s-IMRT, and 3D-CRT plans were 73.98, 76.75, and 60.34 cm3, respectively. The average LV volumes receiving ≥25 Gy (V25 Gy) in IMRT, s-IMRT and 3D-CRT plans were 23.37, 24.68, and 17.61 cm3, respectively. In the IMRT plan, the mean BED to the critical points of inner heart located within the high physical dose area were substantially lower than in 3D-CRT or s-IMRT.
Compared with 3D-CRT technique, IMRT and s-IMRT had superior target dose coverage and dose uniformity. IMRT significantly reduced the maximal RT dose to heart and LV. IMRT and s-IMRT techniques did not reduce the volume of heart and LV receiving high doses.
INTRODUCTION
With recent developments in radiotherapy techniques, postoperative adjuvant therapy for breast cancer has evolved from merely pursuing improved local control and survival to reducing late complications and improving quality of life while maintaining the same local control. An important late complication that can afflict patients in adjuvant radiotherapy is cardiovascular injuries, which require making critical treatment decisions to balance the therapeutic effect and injury to normal tissues, especially when internal mammary lymph nodes (IMN) are treated with radiotherapy.
Because of stable therapeutic effect and the anatomical position of breast and chest wall, tangential chest wall fields were regarded as the standard breast cancer radiotherapy technique in most radiotherapy centers for a long time. With the development in the fields of radiation physics and radiation technology, several new techniques have been developed to better cover the breast and IMN drainage area, or reduce exposure dose to heart. However, currently there are no optimal techniques to completely replace the traditional chest wall tangential fields.1–4 This is especially the case for irradiation to IMN drainage area, where optimal target coverage and reduction of exposure dose to heart are major challenges for patient care.
Recently, several research centers have discussed the clinical application of multiple-field inverse planned intensity modulated radiotherapy (IMRT) in breast and chest wall irradiation to reduce the cardiac volume in the radiation field and reduce the cardiac dose exposure.5–10 Most researchers use IMRT techniques with more than 9 treatment fields. Considering the therapeutic equipment usage of our research center, this study planned to compare the radiation doses to target areas, heart and cardiac substructures using 6-field IMRT, 6-field simple IMRT (s-IMRT),11 and tangential-field three-dimensional conformal plan (3D-CRT) techniques. On this basis, the study converted the physical dose to biological effective dose (BED) and compared them to intuitively explain the differences of each cardiac area's biological effects after radiotherapy.
MATERIALS AND METHODS
Patients
This study was approved by the local ethical committee and a pilot study that focused on the differences in volumes between the positron emission tomography (PET)-computed tomography (CT)-based left ventricles (LV) and contrast-enhanced CT-based LV has been published.11 The patients and methods were identical in the current study. From October 2008 to February 2009, 14 patients with pathologically confirmed primary left-sided breast cancer were enrolled with written informed consent. We excluded patients with a history of coronary heart disease, myocarditis, and congestive heart failure. Echocardiography was employed to assess the parameters of LV anatomy and function in patients receiving radiotherapy according to the recommendation by the Breast Multi-Disciplinary Team of Fudan University Cancer Center. A normal LV ejection fraction (50%–75%) was required in all patients before study enrollment or treatment.
Clinicopathological data collected included age at diagnosis, preoperative treatment, and type of surgery and radiation target. Patient age at diagnosis ranged from 33 to 67 years (median, 44 years). Neoadjuvant chemotherapy was applied in 4 patients with histological confirmation (core needle biopsy) of large inoperable or locally advanced breast cancer and the protocol is published elsewhere.12 All patients received 4 cycles of paclitaxel 80 mg/m2 in a 1-hour infusion immediately followed by carboplatin at an area under the curve (AUC) of 2 mg/min × min in a 30-minute infusion given on days 1, 8, and 15 of a 28-day cycle, then underwent surgery within 4 weeks from the last scheduled chemotherapy cycle. Twelve patients (85.7%) underwent modified radical mastectomy and 2 (14.3%) received breast-conserving surgery.
The enrolled patients received contrast-enhanced CT scan and PET-CT left ventricular myocardial scanning followed by the adjuvant radiotherapy.
Contrast-Enhanced CT Scan
All patients were immobilized using a Med-Tec 350 breast board (Med-Tech Corporation, Orange, IA) with both arms raised fully upward and abducted. CT images were acquired with 8-mm thickness from the thyroid cartilage to the costophrenic angles using a Philips wide bore CT scanner (Philips Medical, Fitchburg, WI). Intravenous contrast was given during CT scanning. All images were exported to Pinnacle treatment planning system (Pinnacle3 version 7.6; Philips Radiation Oncology Systems, Andover, MA) for target and normal tissue delineations and radiotherapy planning.
PET-CT Left Ventricular Myocardial Scanning
After 4 to 6 hours of fasting time, the blood glucose level of each patient, as measured before an injection of 18F-FDG, was required to be 8 to 10 mmol/L. Subsequently, an 18F-FDG PET-CT scan was performed on the LV using a SIEMENS Biography 16 HR PET/CT (Siemens Medical Solutions, Malvern, PA).
The scan was performed following an intravenous injection of 18F-FDG (7.4 MBq/kg) and as approximately 60-minute rest. The details of the PET-CT scanning were identified in previous article.11
Delineation of Target Area and Normal Tissues
The delineation of clinical target volume (CTV) for target area after modified radical mastectomy and breast-conserving surgery was based on the guidelines recommended by RTOG expert panel consensus.13 For a tumor in the central area and medial side, the CTV after modified radical mastectomy included the chest wall, ipsilateral IMN, and supraclavicular lymph node drainage areas; otherwise, the delineation of CTV followed published guidelines. According to the combined delineation guidelines of IMN,14 the CTV for IMN drainage area was defined from the upper edge of the first to fourth ribs. The center of internal mammary vessels was chosen as a reference point with a margin of 10 mm as the CTV of IMN.
Heart delineation was initiated from the level of pulmonary artery root, including pulmonary artery root, ascending aorta, right atrium, left atrial, both left and right ventricles, and mediastinal fat in mediastinal CT window of CT images.
18F-FDG PET-CT images were used to contour the LVs in all patients. The 18F-FDG PET-CT LV myocardial scans were registered with CT contrast-enhanced scans to delineate the LV, which was defined as LV-PET, as detailed in the previous article.11
CT contrast-enhanced images clearly showed the location of left anterior descending coronary artery (LAD) along the anterior interventricular groove of the heart. LAD-planning organ at-risk volume (PRV) was acquired by creating a cylinder with LAD at the center, with the radius of 10 mm and the height of 4.5 cm, as previously reported.11
Definition of Key Points in the Heart and the Conversion of Physical Dose to Biological Dose
The formula of BED was applied to convert the physical dose to biological dose for all the critical points within the heart and listed as below.
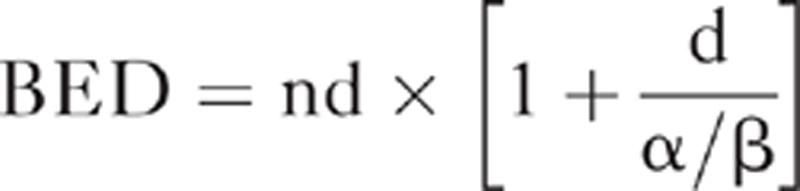 |
In which, n refers to the fractionation number and d stands for the daily fractionation dose. According to the study from Gagliardi which investigated the late complications of the heart after irradiation, the α/β ratio could be cited as 3 Gy.15
The points of interest (POI) that were used to measure the critical point doses received by the heart are outlined below.
The left ventricle (LV), right ventricle (RV), left atrium (LA), and right atrium (LA) were contoured on enhanced CT.
The POIs were defined on the second CT image level after the emergence of the LV. Eight points have been defined as LV-a to LV-h in the wall of LV. Two points in right ventricle defined as RV-a and RV-b, 2 in left atrium as LA-a and LA-b, 2 in right atrium as RA-a and RA-d. The example was showed in Figure 1. The physical dose of the POIs was measured 1 by 1.
FIGURE 1.
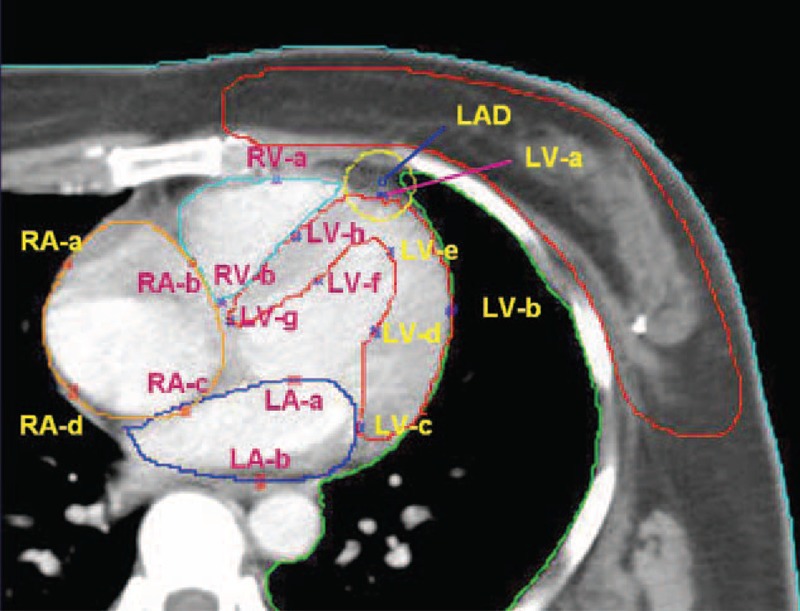
The definition of points of interest (POI) in heart.
Radiation Treatment Planning
Three different radiotherapy techniques were applied for each patient: 3D-CRT, IMRT, and s-IMRT. 3D-CRT was consisted of a pair of tangential beams with or without IMN irradiation. The mixed modality beam was used for IMN irradiation which consisted of photon and electron beam with 1:2 ratio and the total dose was 50 Gy, 2 Gy/fraction.
IMRT was defined as an inverse planning technique using 6 beams with maximum segments of 100. For each segment, the minimum area was 4 cm2 and minimum monitor units (MUs) were 4 MU.
Similarly with IMRT, s-IMRT was used 6 beams with maximum segments of 35. For each segment, the minimum area was 10 cm2 and minimum monitor units (MUs) were 10 MU.
All the treatments were delivered with 6 MV photon beams of the Elekta linear accelerator (Elekta Precise; Elekta Oncology Systems, Crawley, UK). The dose prescription was 50 Gy with 2 Gy/fraction. For the patients with breast-conserving surgery, 10 Gy boost to the tumor cavity in 5 fractions. The final dose distributions met the following constrains: more than 95% dose covered the entire CTV, <30% heart volume received doses of 15 Gy and <30% of ipsilateral lung received 20 Gy.
Statistical Analysis
All statistical tests were done in SPSS 16.0 statistical software (SPSS, Inc, Chicago, IL) and graphics were created with Graphpad Prism 5 (GraphPad Software Inc., CA, USA) in this study. The datasets were described with statistical parameters of median, mean, and standard error (SD) for the dose and volume parameters. Paired t test was used to test the difference of the dose and volume parameters of the target area, heart, and LV between different radiotherapy techniques. The significant level of 0.05 was adopted.
RESULTS
The Parameters Comparison of Target Dose Coverage
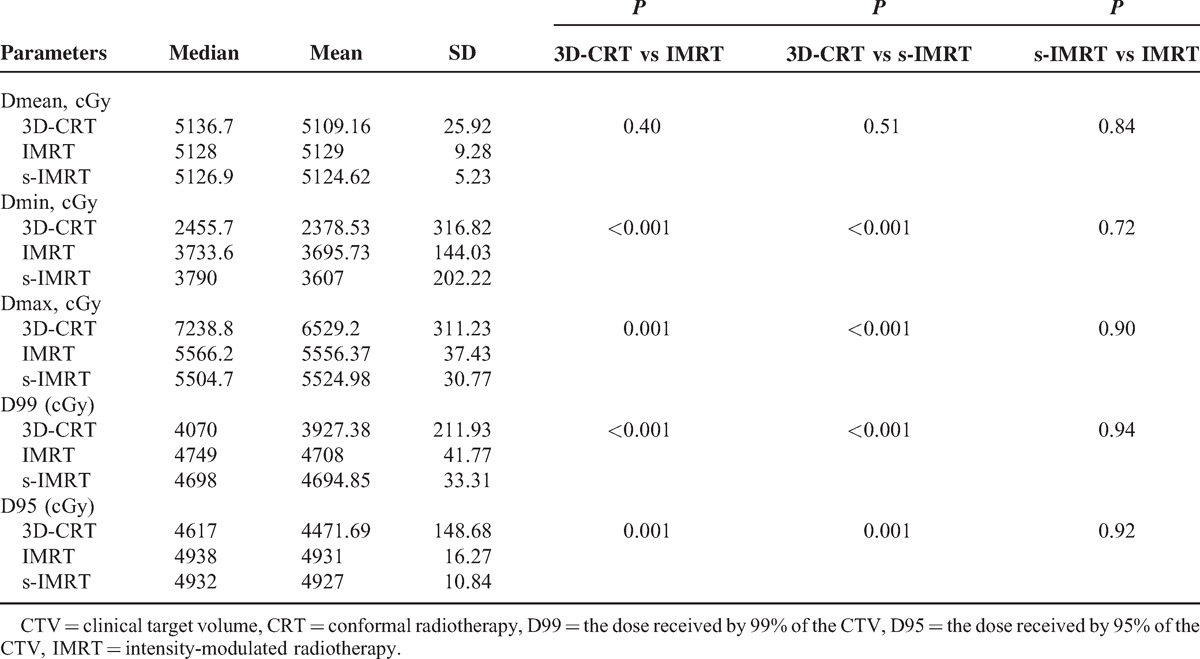
The results of the dose and volume parameters of the CTV in the 3 radiotherapy techniques are listed in the Table 1. Compared with 3D-CRT technique, IMRT, and s-IMRT were superior with better target dose coverage. The maximal dose, minimum dose, dose received by 99% of the CTV (D99) and dose received by 95% of the CTV (D95) in target areas were all statistically different (P < 0.05).
TABLE 1.
The Comparison of the Parameters of CTV in the 3 Radiotherapy Techniques
The Comparison of the Parameters in the Heart
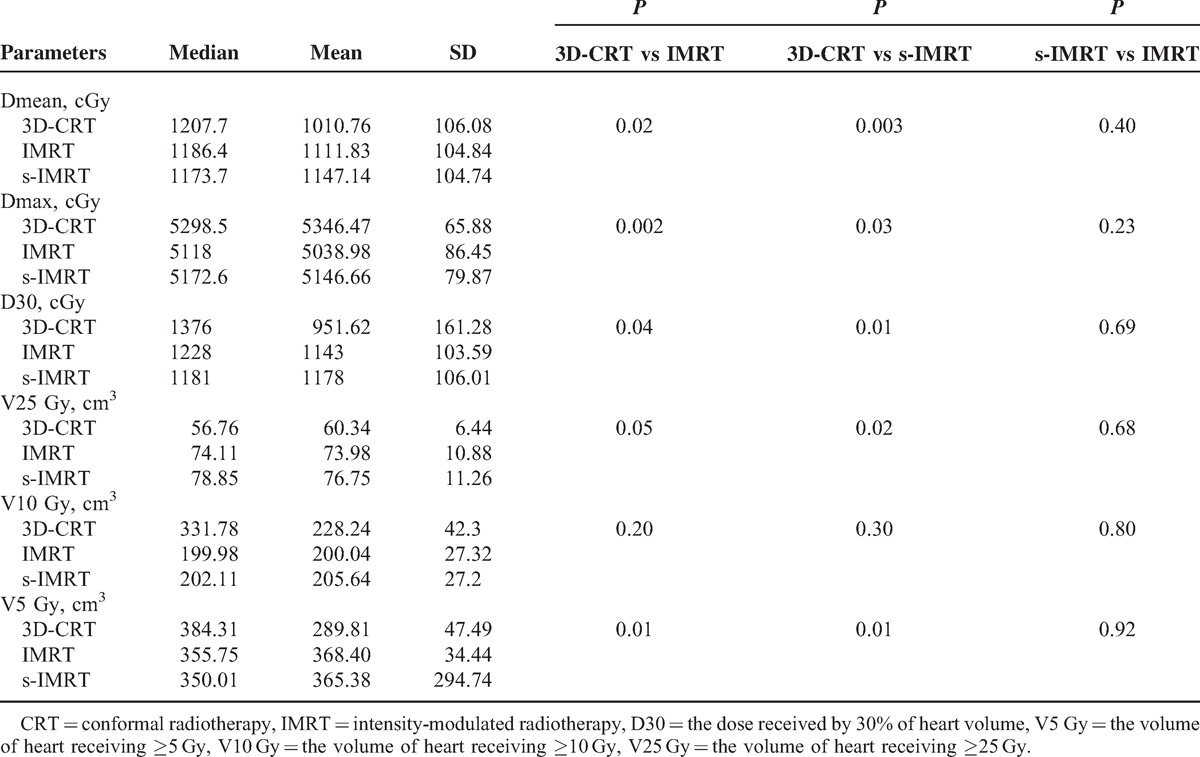
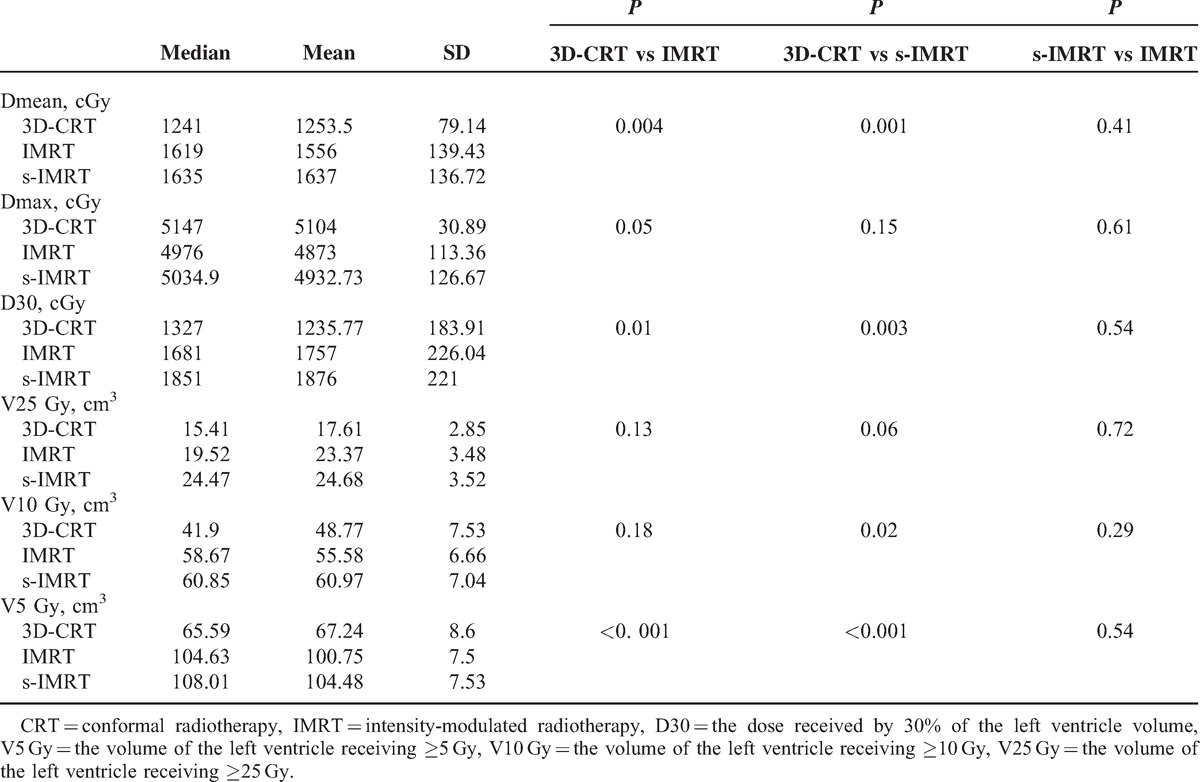
The results of the dose and volume parameters for the heart between the 3 radiotherapy techniques are listed in the Table 2. The maximal dose in IMRT and s-IMRT were substantially reduced (P < 0.05), but the average dose of heart in 3D-CRT were the lowest and show the statistically differences (P < 0.05). Low-dose volume of heart in IMRT was significant larger than 3D-CRT (P < 0.05).
TABLE 2.
The Comparison of the Parameters in the Heart
The Comparison of the Parameters in the Left Ventricle
The results of the dose and volume parameters for left ventricle (LV) in 3 radiotherapy techniques in 3 radiotherapy techniques are listed in the Table 3. Compared with 3D-CRT, IMRT reduced the maximal dose to LV by 4% (P = 0.05). Same as heart, the average doses to LV and the low-dose volume of LV in 3D-CRT plan are the lowest (P < 0.05).
TABLE 3.
The Comparison of the Parameters in the Left Ventricle
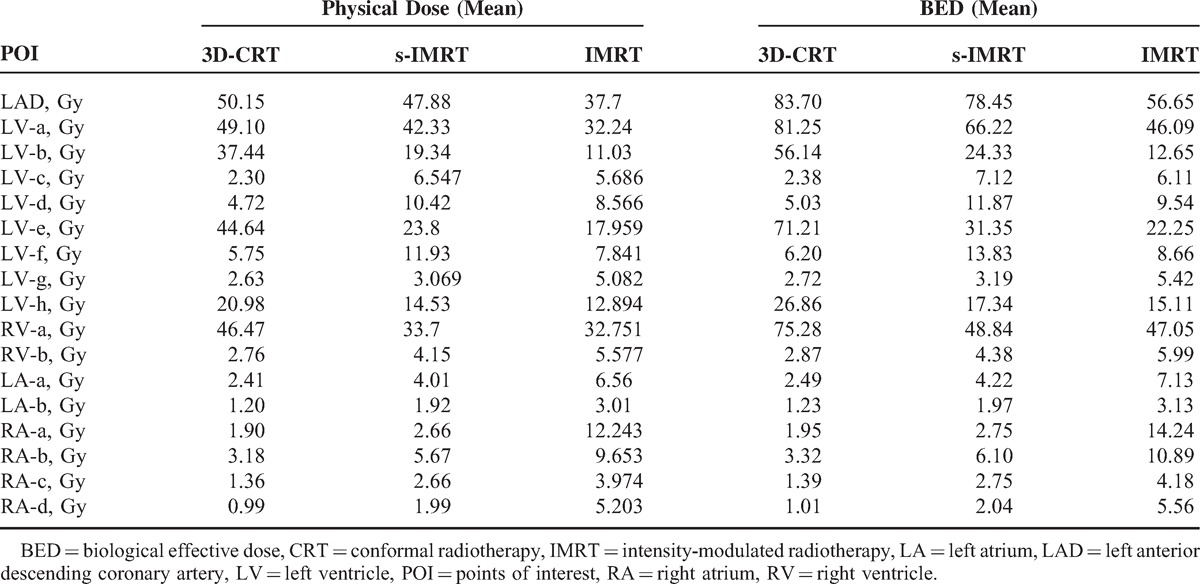
The Comparison of POI Biological Dose
After defining the cardiac critical dose points as POIs, the results of physical dose and biological dose were calculated and these ranges listed in Table 4. For the LAD, IMRT has a 24% reduction of physical dose compared with 3D-CRT, and significant 32% reduction of biological dose calculated. However regarding low dose, all of physical dose and biological dose in IMRT were higher.
TABLE 4.
The Physical Dose and Biological Dose for Each POI
DISCUSSION
The study made a further comparison and assessment for the different radiotherapy techniques most commonly used in current breast cancer radiotherapy. The study observed from the cases that compared with 3D-CRT technique, IMRT and s-IMRT have superior target dose coverage and dose uniformity, and IMRT significantly reduces the maximal dose to heart and LV. However, IMRT and s-IMRT techniques were unable to reduce the volume of heart and LV receiving high doses. Comparing IMRT with s-IMRT, there are no significant statistical difference in target area coverage and normal tissue restrictions, but IMRT is slightly superior to s-IMRT.
Eighty-five percent of the patients in this study received chest wall treatment after modified radical mastectomy. Currently internationally, most centers still adopt tangential field therapy for chest wall treatments. IMRT technique is mainly applied for the radiotherapy patients after breast-conserving surgery. Beckham et al16 compared the differences between IMRT and 3D-CRT plans of the patients receiving whole breast irradiation and IMN irradiation after breast-conserving surgery. The technique adopted by IMRT is to design plans in 11 equally divided main fields within the angle range of 190 degrees; the 3D-CRT plan is similar to the study. Beckham's study finds that IMRT can improve the dose uniformity and conformality of target area and can significantly reduce the volume of normal tissues receiving high doses such as heart and lung. Similarly, Lohr et al17 compare the dose with normal tissues of 14 patients receiving 3D-CRT and semiautomatic IMRT after breast-conserving surgery. IMRT adopts 9-field technique. Lohr's research results show that IMRT technique significantly reduces the maximal dose to heart and LV; however, in 3D-CRT, the average dose to heart, the average dose to 60% volume and the average dose to 30% volume are all lower than those in IMRT. Lohr's research results are similar to the results of the study. The study shows that IMRT significantly reduces the maximal dose to heart and LV. But the V5 volume of heart and LV in IMRT is significantly larger than that in 3D-CRT.
The incident angles of fields in the IMRT technique adopted by Beckham et al are limited to a fan-shaped area of 190 degrees. But the incident angles of the main fields in Lohr's study and this study are relatively dispersed. Such dispersed incident angles are better for dose coverage for target areas and dose uniformity, but the volume of normal tissues receiving low-dose radiation is larger, which is a common “paradox” in IMRT technique. Beckham's study showed that the doses to contralateral mammary gland and normal tissues in IMRT were relatively higher than those in 3D-CRT technique. The clinical significance of relatively large-volume low-dose area in IMRT technique needs to be further deeply studied. In the first place, such doses may not necessarily cause acute clinical symptoms, but for the patients with the breast cancer, a good prognosis can help them achieve a long-term survival. The largest risk after low-dose radiotherapy lies in the likelihood of inducing second primary tumor.16,18,19
In functional subunits theory, heart is considered as a tissue having both parallel and serial functional subunits. Although the heart requires different dose-limiting methods in parallel and serial organs, most laboratory findings show that the main limiting factor is the maximal dose to the heart.20,21 This study compared the doses with heart and cardiac substructures in three different plans. The assessment and evaluation of different radiotherapy plans mainly include 2 aspects: the evaluation of physical doses including dose coverage and dose uniformity in target area; the evaluation of dose and volume of normal tissues around target area. When designing plans, the restricted conditions for normal tissues’ dose-volume generally depend on long-term laboratory and clinical experience. As a result, the evaluation of doses to normal tissues is limited to the assessment of the physical dose. However, it is difficult to predict the biological and clinical effects of normal tissues by physical dose alone. The reasons are that the possible acute or later biological effects appearing in normal tissues after radiotherapy cannot be directly evaluated by physical dose; the emergence of biological effects is related to various factors such as the inherent radiosensitivity of normal tissues, radiation volume and so on, and biological effects caused by combined action of multiple factors.
Physical dose is an indispensable factor in the emergence of biological effects. Laboratory and clinical research may develop a series of theoretical models. These models link physical dose to biological effects by calculating Normal Tissue Complication Probability (NTCP). The relative seriality model (NTCP-RSM) developed by Kallman et al22 is most commonly used in studying cardiac toxicity. The reason is that in functional subunits theory, heart is considered as a dissecting tissue having both parallel and serial functional subunits. The advantage of applying the model is enabling us to recognize and understand the dose and volume factors related to the damages to heart caused by radiotherapy, and enabling us to calculate and compare the cardiac NTCP in different treatment plans. However, the model is based on a phenomenological basis, and its theoretical basis is an assumption that the cytoctony method of the treatment is extrapolated from the Poisson distribution; as a result, the model requires complete clinical treatment and follow-up data. However, when studying the complications of normal tissue after long-term radiotherapy, there are usually no follow-up data with sufficient duration of follow-up or clinical complications incidence, which limits the application of the model. The study compares and predicts NTCP by adopting the most basic linear-quadratic model to calculate the biological dose to points of inner heart (POI). By exploring the case on key levels, the study proposes that among the three different plans of 3D-CRT, IMRT, and s-IMRT, IMRT significantly reduced the biological dose to LAD, LV anterior wall vessels and myocardial tissue, from which it can be extrapolated that the NTCP of cardiac tissues probably receiving high dose in IMRT is significantly lower than that in 3D-CRT and even in s-IMRT.
CONCLUSION
Compared with 3D-CRT technique, IMRT and s-IMRT have more sufficient dose coverage for target area and the dose uniformity is better. Meanwhile, IMRT significantly reduces the maximal radiotherapy dose to heart and LV. However, IMRT and s-IMRT techniques are unable to reduce the volume of heart and LV receiving high doses. Comparing IMRT with s-IMRT, there are no significant statistical difference in target area coverage and normal tissue restrictions, but IMRT slightly wins over s-IMRT. The calculation of BED to critical points of inner heart predicts that the cardiac NTCP in IMRT technique is significantly lower than that in 3D-CRT. More samples are required in the future to compare the doses to target areas, heart and cardiac substructures of patients in different subgroups.
Footnotes
Abbreviations: 3D-CRT = three-dimensional conformal plan, BED = biological effective dose, CTV = clinical target volume, IMN = internal mammary lymph nodes, IMRT = intensity modulated radiotherapy, LAD = left anterior descending coronary artery, LV = left ventricle, NTCP = Normal Tissue Complication Probability, POI = points of interest, s-IMRT = simple IMRT.
LZ and XM contributed equally to the work.
This work was partly supported by the National Natural Science Foundation of China (Grant No. 81402525).
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997; 337:956–962. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999; 353:1641–1648. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997; 337:949–955. [DOI] [PubMed] [Google Scholar]
- 4.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378:1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JM, Ribeiro GG. Mortality patterns over 34 years of breast cancer patients in a clinical trial of post-operative radiotherapy. Clin Radiol 1989; 40:204–208. [DOI] [PubMed] [Google Scholar]
- 6.Houghton J, Baum M, Haybittle JL. Role of radiotherapy following total mastectomy in patients with early breast cancer. The Closed Trials Working Party of the CRC Breast Cancer Trials Group. World J Surg 1994; 18:117–122. [DOI] [PubMed] [Google Scholar]
- 7.Rutqvist LE, Johansson H. Mortality by laterality of the primary tumour among 55 000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer 1990; 61:866–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutqvist LE, Lax I, Fornander T, et al. Cardiovascular mortality in a randomized trial of adjuvant radiation therapy versus surgery alone in primary breast cancer. Int J Radiat Oncol Biol Phys 1992; 22:887–896. [DOI] [PubMed] [Google Scholar]
- 9.Corn BW, Trock BJ, Goodman RL. Irradiation-related ischemic heart disease. J Clin Oncol 1990; 8:741–750. [DOI] [PubMed] [Google Scholar]
- 10.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005; 97:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu XL, Zhang Q, Chen JY, et al. Delineation of the cardiac substructures based on PET-CT and contrast-enhanced CT in patients with left breast cancer treated with postoperative radiotherapy. Technol Cancer Res Treat 2013; 12:99–107. [DOI] [PubMed] [Google Scholar]
- 12.Huang O, Chen C, Wu J, et al. Retrospective analysis of 119 Chinese noninflammatory locally advanced breast cancer cases treated with intravenous combination of vinorelbine and epirubicin as a neoadjuvant chemotherapy: a median follow-up of 63.4 months. BMC Cancer 2009; 9:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions. http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx. [Google Scholar]
- 14.Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys 2009; 73:944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagliardi G, Lax I, Ottolenghi A, et al. Long-term cardiac mortality after radiotherapy of breast cancer—application of the relative seriality model. Br J Radiol 1996; 69:839–846. [DOI] [PubMed] [Google Scholar]
- 16.Beckham WA, Popescu CC, Patenaude VV, et al. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys 2007; 69:918–924. [DOI] [PubMed] [Google Scholar]
- 17.Lohr F, El-Haddad M, Dobler B, et al. Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009; 74:73–80. [DOI] [PubMed] [Google Scholar]
- 18.Krueger EA, Fraass BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2003; 56:1023–1037. [DOI] [PubMed] [Google Scholar]
- 19.Lomax AJ, Cella L, Weber D, et al. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003; 55:785–792. [DOI] [PubMed] [Google Scholar]
- 20.Fajardo LF, Stewart JR, Cohn KE. Morphology of radiation-induced heart disease. Arch Pathol 1968; 86:512–519. [PubMed] [Google Scholar]
- 21.Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol 1970; 59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 22.Kallman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol 1992; 62:249–262. [DOI] [PubMed] [Google Scholar]


