Abstract
One of the most important adverse effects of zoledronic acid (ZA) is osteonecrosis of the jaw (ONJ). In previous literature, several risk factors have been identified in the development of ONJ. In this study, we aimed to determine the role of trastuzumab, an antiangiogenic agent, as an independent risk factor for the development of this serious side effect.
Our study included 97 patients (mean age: 54 ± 10 years) with breast cancer, recorded in the archives of the Istanbul Florence Nightingale Breast Study Group, who received ZA therapy due to bone metastases between March 2006 and December 2013. We recorded the patients’ ages, weights, duration of treatment with ZA, number of ZA infusions, dental procedures, anticancer treatments (chemotherapy, aromatase inhibitor, trastuzumab), the presence of diabetes mellitus or renal dysfunction, and smoking habits.
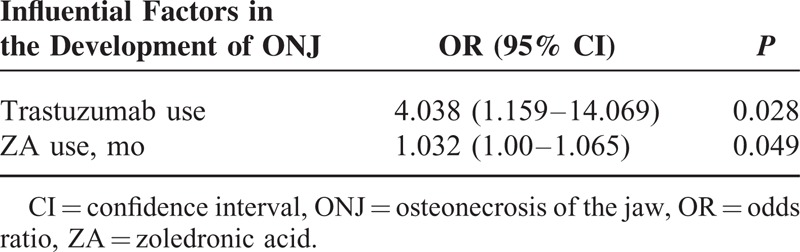
Thirteen patients (13.40%) had developed ONJ. Among the patients with ONJ, the mean time of exposure to ZA was 41 months (range: 13–82) and the mean number of ZA infusions was 38 (range: 15–56). The duration of treatment with ZA and the use of trastuzumab were observed to be 2 factors that influenced the development of ONJ (P = 0.049 and P = 0.028, respectively).
The development of ONJ under ZA treatment may be associated solely with the duration of ZA treatment and the concurrent administration of trastuzumab. These findings show that patients who are administered trastuzumab for metastatic breast cancer while undergoing ZA treatment are prone to developing ONJ. Therefore, we recommend intense clinical observation to avoid this particular condition in patients receiving ZA and trastuzumab.
INTRODUCTION
Breast cancer is the most frequently observed type of invasive cancer, affecting approximately 1 million women worldwide and causing bone metastases in 65% to 75% of patients.1 Bisphosphonates are some of the most effective treatments for preventing complications related to bone metastases. Zoledronic acid (ZA) is the most effective molecule in reducing skeletal-related events (SREs) in patients with breast cancer.2 Bisphosphonates inhibit bone resorption and protect bone structure by inhibiting the differentiation of osteoclastic precursors, promoting apoptosis of osteoclasts, and stimulating the secretion of osteoclast inhibitory factor from osteoblasts.3 One of the most important adverse effects that limit its clinical use is osteonecrosis of the jaw (ONJ).4 According to the American Oral and Maxillofacial Surgery Association, current therapy or a history of therapy with bisphosphonates, no radiotherapy to the head and neck area, and the presence of exposed necrotic bone in the maxilla and/or mandible for at least 8 weeks support the diagnosis of ONJ.5 Studies have found the incidence of ONJ to be 1% to 10%.6,7 Following the first scientific report published by Marx8 in 2003 that pointed to a link between bisphosphonates and ONJ, the number of studies focusing on this subject has rapidly increased.9,10 Moreover, numerous risk factors for the development of ONJ have also been described (cancer and anticancer therapy, dental risk factors, corticosteroids, alcohol and tobacco abuse, anemia, diabetes, obesity, and renal impairment).11,12
Trastuzumab is one of the most widely used agents for the management of all metastatic breast cancers with human epidermal growth factor receptor 2 (HER2) overexpression as indicated by 3+ HER2 immunostaining or gene amplification on the fluorescence in situ hybridization test.13 The development of ONJ has been reported to have occurred in 2 patients with concurrent use of trastuzumab and bisphosphonates.14,15 However, in both these reports, the authors did not correlate the occurrence of ONJ in their patients with the combined use of these 2 agents. In the present study, we made an attempt to analyze the use of trastuzumab as an independent risk factor for the development of ONJ in metastatic breast cancer patients undergoing ZA treatment.
PATIENTS AND METHOD
Patient data were identified retrospectively from the archives of the Florence Nightingale Breast Study Group, Istanbul, between March 2006 and December 2013. In this study, we included 97 consecutive patients with metastatic breast cancer who had bone metastases and underwent treatment with ZA. Patients with <12 months of follow-up and radiotherapy to the head and neck area were excluded from the study. Patients were analyzed according to their characteristics (age, weight, number of ZA infusions, time of exposure to ZA [months], smoking habits, dental procedures, receiving aromatase inhibitors [AI], receiving chemotherapy [CT], trastuzumab treatment, and renal dysfunction). The diagnosis of bone metastasis was based on radiologic methods such as direct radiography, bone scintigraphy, and positron emission tomography-computed tomography. The standard therapy involved intravenous infusion of 4 mg every 3 to 4 weeks (in 150 cc of saline within 15 minutes).
Patients were examined by a dentist every 6 to 12 months and all dental procedures performed before the initiation of therapy with ZA and during the therapy period were recorded. Patients with suspected ONJ were referred to a maxillofacial surgeon. The diagnosis of the ONJ was made through clinical and radiologic examinations, and biopsies were performed when necessary. The study was approved by the Bilim University ethics committee (Decision no: 27-200).
Statistical Evaluation
Statistical analysis was performed using the Statistical Package for Social Sciences Social Sciences (SPSS Inc., Chicago, IL)for Windows 17.0 software. During the evaluation of the study data, in addition to the descriptive statistical methods (mean, median, number, and percentage), χ2 was employed for the qualitative comparison of the development of ONJ along with the patient and disease-related characteristics, whereas quantitative comparisons were made through the independent samples t test. Evaluation of the independent parameters related to the development of ONJ was based on the multiple logistical regression (forward stepwise) model. The results were assessed within a 95% confidence interval, and a value of P < 0.05 was accepted as statistically significant.16
RESULTS
The median age of the patients was 55 years (range: 33–74). The mean time of exposure to ZA was 37 ± 18 months (range: 13–87) and the mean number of ZA infusions was 35 ± 16 (range: 10–73) (Table 1).
TABLE 1.
Patient Characteristics
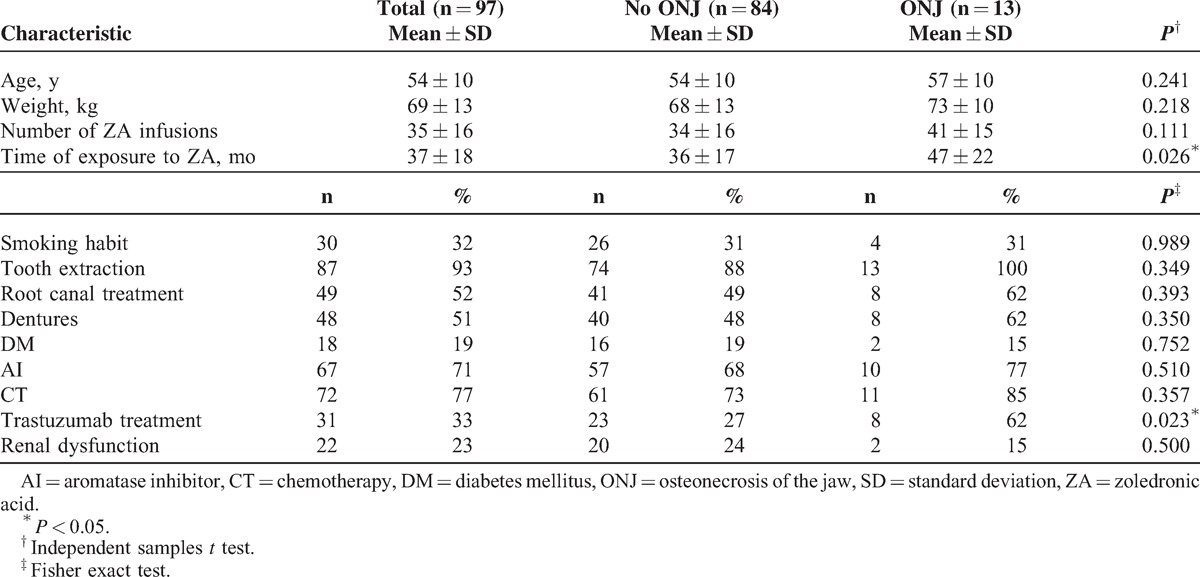
Thirteen patients (13.40%) developed ONJ. The median age of the patients who developed ONJ was 61 years (range: 39–73). The mean time of exposure to therapy with ZA was 47 ± 22 months (range: 13–87), and the mean number of intravenous ZA infusions was 41 ± 15 (range: 15–66). The 8 (62%) of 13 patients developed ONJ had received transtuzumab, but 5 (38%) of them had not received. Among these patients, 1 patient (7.69%) was asymptomatic, whereas 9 patients (69.24%) were diagnosed through clinical and radiologic examinations. In 4 patients (30.76%), the probability of metastasis was ruled out by biopsy. There were 15 lesions in total; 11 patients had single lesions whereas 2 patients had double lesions. Six of the lesions were detected in the mandible and 5 in the maxilla, whereas 2 involved both the maxilla and the mandible (Table 2).
TABLE 2.
Characteristics of Patients With ONJ
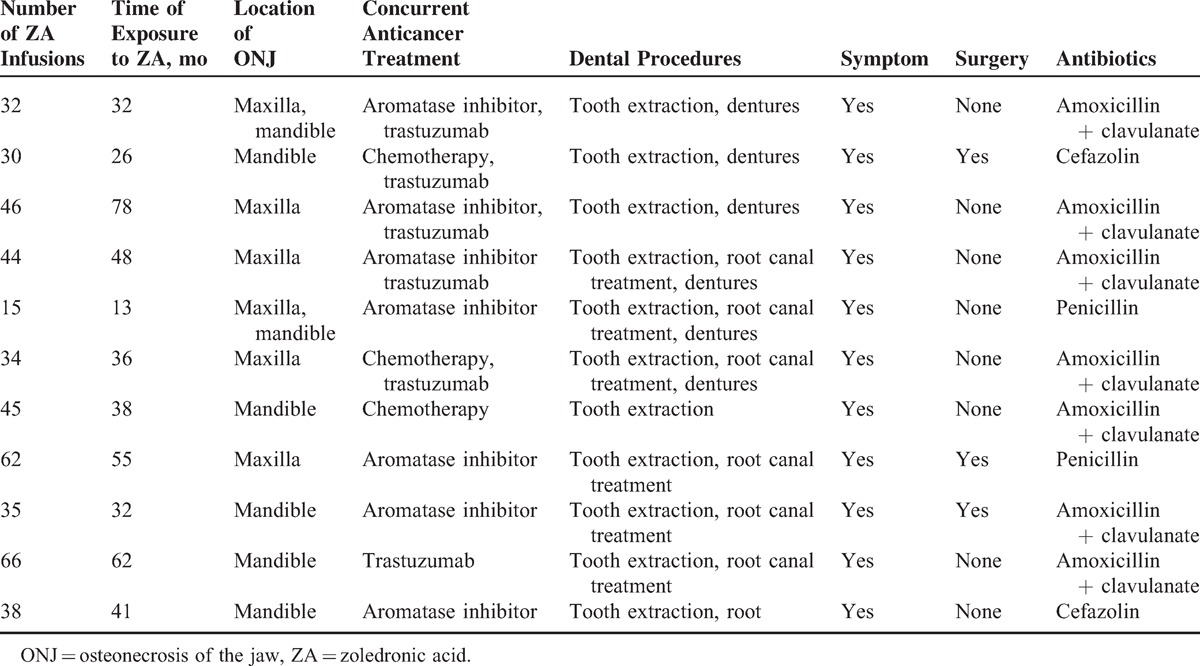
None of the patients received chronic corticosteroid therapy. When the diagnosis of ONJ was made, 9 patients (69.20%) were under treatment with AIs, 8 patients (62%) were taking trastuzumab, and 3 patients (23%) were receiving systemic CT. All of the patients with ONJ had undergone dental procedures. The dental procedures included tooth extractions in all patients, root canal treatment in 8 patients, and dentures in 7 patients. Following conservative treatment, 4 patients (30.76%) needed surgery (Table 2).
There was no association of the development of ONJ with age (P = 0.241), weight (P = 0.218), number of ZA infusions (P = 0.111), smoking habits (P = 0.989), dental procedures (tooth extraction [P = 0.349], root canal treatment [P = 0.393], dentures [P = 0.350]), diabetes mellitus (P = 0.752), receiving AI (P = 0.510), receiving CT (P = 0.357), or renal dysfunction (P = 0.500).
Duration of exposure to ZA and the use of trastuzumab were associated with the development of ONJ (P = 0.049 and P = 0.028, respectively) (Table 3). No patient who received ZA for <13 months developed ONJ.
TABLE 3.
Multivariate Logistic Regression Analysis (Forward Stepwise)
DISCUSSION
Unless there is an interfering condition, bisphosphonates are currently regarded as the standard therapy for SREs in the treatment of bone metastases.17 ONJ is one of the most important complications associated with bisphosphonate therapy used in patients who have breast cancer with bone metastases. This condition is due to accumulation of bisphosphonates in great amounts both in the alveolar bone and the surrounding soft tissue. This increases the risk of avascular necrosis, which, in addition to disruption of the mucosal barrier mediated by stimulating the apoptosis of keratinocytes, delays wound healing and tissue repair by inhibiting the formation of blood vessels through antiangiogenic effects.18 Trastuzumab is another antiangiogenic agent particularly indicated in metastatic breast cancers with HER2 overexpression.13 Trastuzumab has been demonstrated to inhibit angiogenesis and this effect is believed to occur through the expression of antiangiogenic factors and inhibition of proangiogenic factors.19,20 Combining bisphosphonates with antiangiogenic agents has been suggested to induce ONJ more frequently than using bisphosphonates alone.21 In this article, we focused on the impact of trastuzumab as well as the other factors in the development of ONJ in metastatic breast cancer patients receiving ZA.
Although ONJ is mostly associated with dental procedures, other factors that play a role in its pathogenesis are listed in some studies: duration of exposure to ZA, number of infusions, type of bisphosphonate, route of administration (oral, intravenous), concurrent CT, chronic use of corticosteroids, poor oral hygiene, smoking, and poorly fitting dentures.9 Although symptoms including orofacial pain, puffy face, and malodorous discharge during treatment with bisphosphonates support the diagnosis of ONJ, it may be necessary to rule out any metastases to the orofacial bones. However, because of the risk of diagnostic biopsy of the bone that may lead to a compromise in wound healing, the diagnosis is usually based on clinical and radiologic examinations.22
In a retrospective study by Bamias et al,12 the most important risk factors suggested to increase the risk of development of ONJ were found to be duration of exposure to treatment, the number of infusions, dental procedures, and the type of bisphosphonate used. The duration of bisphosphonate treatment has also been marked as a risk factor for the development of ONJ in other clinical studies.9,11,23 In our study, duration of ZA treatment was detected as a significant risk factor in the development of ONJ, which strengthens the outcomes of the above studies.
Although a relationship between dental procedures and ONJ was observed in certain studies,24 no statistically significant correlation was observed between dental procedures and ONJ in our study. The fact that the majority of the patients in which no ONJ occurred had also undergone dental procedures may have led to this result.
Antiangiogenic agents that are used with increasing frequency may enhance the risk of ONJ, especially when used concurrently with bisphosphonates. For instance, there have been recent reports of patients with ONJ caused by antiangiogenic agents such as sunitinib (multikinase inhibitors), bevacizumab (a monoclonal antibody that targets vascular endothelial growth factor), and everolimus (inhibitor of mammalian target of rapamycin), with or without bisphosphonates.25–27
Trastuzumab is also an antiangiogenic agent that is especially indicated in breast cancer treatment.13 There have been no reports in the literature on development of ONJ solely due to trastuzumab treatment. There have been a few cases of development of ONJ during concurrent treatment with bisphosphonates and trastuzumab; however, an association of trastuzumab with the occurrence of ONJ has not been clearly stated in these reports.14,15 Moreover, in a study by Hoff et al,28 a large number of patients were evaluated for ONJ incidence and risk factors regarding the development of ONJ. No relationship was observed between ONJ and treatment with trastuzumab, anthracycline, tamoxifen, taxane, or AIs.29–30 In agreement with our findings, the rate of treatment with trastuzumab in patients with ONJ was observed to be significantly higher than in those without ONJ (P = 0.028).
The combination of ZA and other antiangiogenic agents (sunitinib, everolimus, bevacizumab) has recently been revealed to be associated with an ONJ rate of up to 16%.21,31–36 In addition, treatment with bevacizumab alone has been correlated to ONJ in a few case presentations.35 We suggest that our study brings up the matter of the effect of trastuzumab on ONJ when it is combined with ZA, as we observed a 13.6% occurrence rate of ONJ, which matches the previous reports.6,7 The outcomes of our study may indicate that trastuzumab has antiangiogenic potency similar to that of other agents.
CONCLUSION
This study evaluated data obtained from patients with isolated metastatic breast cancer, whose files and treatments were regularly followed up. In this study, the development of ONJ was associated with longer treatment and higher cumulative doses of intravenous ZA therapy, and with concurrent treatment of ZA and trastuzumab in breast cancer patients. ZA is widely used in the treatment of bone metastases due to breast cancer. The increased risk of ONJ should be kept in mind and all preemptive measures should be taken, especially when ZA is used with trastuzumab. The retrospective nature of the study and the statistical analysis of a small number of cases of ONJ are limitations of our study. We suggest that prospective studies should be performed to confirm these results, and more careful studies are also needed to define the minimum dose and duration of therapy with bisphosphonates necessary to prevent skeletal complications of malignancy.
Acknowledgments
The authors would like to thank David Chapman for the medical writing and editing assistance provided in the preparation of this article, and Atilla Bozdogan, PhD, for performing the statistical analysis.
Footnotes
Abbreviations: AI = aromatase inhibitor, CT = chemotherapy, HER2 = human epidermal growth factor receptor 2, ONJ = osteonecrosis of the jaw, SREs = skeletal-related events, ZA = zoledronic acid.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001; 27:165–176. [DOI] [PubMed] [Google Scholar]
- 2.Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. A randomized, double-blind, multicenter, comparative trial. Cancer 2003; 98:1735–1744. [DOI] [PubMed] [Google Scholar]
- 3.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000; 88 (suppl 12):2961–2978. [DOI] [PubMed] [Google Scholar]
- 4.Tanvetyanon T, Stiff PJ. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol 2006; 17:897–907. [DOI] [PubMed] [Google Scholar]
- 5.Colella G, Campisi G, Fusco V. American Association of Oral and Maxillofacial Surgeons position paper: bisphosphonate related osteonecrosis of the jaws—2009 update: the need to refine the BRONJ definition. J Oral Maxillofac Surg 2009; 67:2698–2699. [DOI] [PubMed] [Google Scholar]
- 6.Badros A, Weikel D, Salama A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 2006; 24:945–952. [DOI] [PubMed] [Google Scholar]
- 7.Wang EP, Kaban LB, Strewler GJ, et al. Incidence of osteonecrosis of the jaw in patients with multiple myeloma and breast or prostate cancer on intravenous bisphosphonate therapy. J Oral Maxillofac Surg 2007; 65:1328–1331. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 2003; 61:1115–1117. [DOI] [PubMed] [Google Scholar]
- 9.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 2004; 62:527–534. [DOI] [PubMed] [Google Scholar]
- 10.Stumpe MR, Chandra RK, Yunus F, et al. Incidence and risk factors of bisphosphonate-associated osteonecrosis of the jaws. Head Neck 2009; 31:202–206. [DOI] [PubMed] [Google Scholar]
- 11.Fehm T, Beck V, Banys M, et al. Bisphosphonate-induced osteonecrosis of the jaw (ONJ): incidence and risk factors in patients with breast cancer and gynecological malignancies. Gynecol Oncol 2009; 112:605–609. [DOI] [PubMed] [Google Scholar]
- 12.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. Clin Oncol 2005; 23:8580–8587. [DOI] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25:118–145. [DOI] [PubMed] [Google Scholar]
- 14.Kubo N, Katayama K, Ishizaki A, et al. A case of the lower jaw due to bisphosphonates in a breast cancer patient with bone metastasis. Gan to Kagaku Ryoto 2008; 35:1973–1975. [PubMed] [Google Scholar]
- 15.Mouri Y, Yoshida M, Nakano S, et al. A case of osteonecrosis of the jaw in a breast cancer patient with bone metastases receiving long-term treatment with bisphosphonates. Breast Cancer 2009; 16:147–150. [DOI] [PubMed] [Google Scholar]
- 16.Cox DR. Regression models and life tables. J R Stat Soc Ser B 1972; 34:187–220. [Google Scholar]
- 17.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003; 21:4042–4057. [DOI] [PubMed] [Google Scholar]
- 18.Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonates-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 2005; 63:1567–1575. [DOI] [PubMed] [Google Scholar]
- 19.Izumi Y, Xu L, di Tomaso E, et al. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 2002; 416:279–280. [DOI] [PubMed] [Google Scholar]
- 20.Wen XF, Yang G, Mao W, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenetic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene 2006; 25:6986–6996. [DOI] [PubMed] [Google Scholar]
- 21.Christodoulou C, Pervena A, Klouvas G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology 2009; 76:209–211. [DOI] [PubMed] [Google Scholar]
- 22.Stockmann P, Hinkmann FM, Lell MM, et al. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig 2010; 14:311–317. [DOI] [PubMed] [Google Scholar]
- 23.Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 2006; 91:968–971. [PubMed] [Google Scholar]
- 24.Badros A, Terpos E, Katodritou E, et al. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol 2008; 26:5904–5909. [DOI] [PubMed] [Google Scholar]
- 25.Santos-Silva AR, Belizário Rosa GA, Castro Júnior GD, et al. Osteonecrosis of the mandible associated with bevacizumab therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115:e32–e36. [DOI] [PubMed] [Google Scholar]
- 26.Fleissig Y, Regev E, Lehman H. Sunitinib-related osteonecrosis of jaw: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 113:e1–e3. [DOI] [PubMed] [Google Scholar]
- 27.Kim DW, Jung YS, Park HS, et al. Osteonecrosis of the jaw related to everolimus: a case report. Br J Oral Maxillofac Surg 2013; 51:e302–e304. [DOI] [PubMed] [Google Scholar]
- 28.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 2008; 23:826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beuselinck B, Wolter P, Karadimou A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer 2012; 107:1665–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoefert S, Eufinger H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: presentation of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110:463–469. [DOI] [PubMed] [Google Scholar]
- 31.Brunello A, Saia G, Bedogni A, et al. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone 2009; 44:173–175. [DOI] [PubMed] [Google Scholar]
- 32.Ayllon J, Launay-Vacher V. Osteonecrosis of the jaw under bisphosphonate and antiangiogenic therapies: cumulative toxicity profile? Ann Oncol 2009; 20:600–601. [DOI] [PubMed] [Google Scholar]
- 33.Bozas G, Roy A, Ramasamy V, et al. Osteonecrosis of the jaw after a single bisphosphonate infusion in a patient with metastatic renal cancer treated with sunitinib. Onkologie 2010; 33:321–323. [DOI] [PubMed] [Google Scholar]
- 34.Agrillo A, Nastro Siniscalchi E, Facchini A, et al. Osteonecrosis of the jaws in patients assuming bisphosphonates and sunitinib: two case reports. Eur Rev Med Pharmacol Sci 2012; 16:952–957. [PubMed] [Google Scholar]
- 35.Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol 2008; 26:4037–4038. [DOI] [PubMed] [Google Scholar]
- 36.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4:335–348. [DOI] [PubMed] [Google Scholar]


