Abstract
In normal-tension glaucoma (NTG), optic nerve damage occurs despite a normal intraocular pressure. Studies implicating systemic blood pressure or, more recently, arterial stiffness in the pathophysiology of NTG have produced conflicting results. Our aim was to investigate whether NTG is associated with alterations in the macrocirculation or microcirculation, cardiac function, and peripheral and central hemodynamics.
Thirty patients with NTG (mean age 65 years, range 46–79) and 33 healthy subjects (mean age 67 years, range 42–79) matched for age and sex were included in the study. Exclusion criteria (for both cases and controls) were history of cardiovascular disease, diabetes mellitus, severe hypertension, and hypercholesterolemia. Aortic stiffness was measured using carotid–femoral pulse wave velocity (PWV), central hemodynamics using carotid artery applanation tonometry, and diameter, stiffness, and intima-media thickness (IMT) of the carotid and femoral artery using echo-tracking. Total peripheral resistance index (TPRI) was derived from mean arterial pressure and cardiac index, measured using ultrasound.
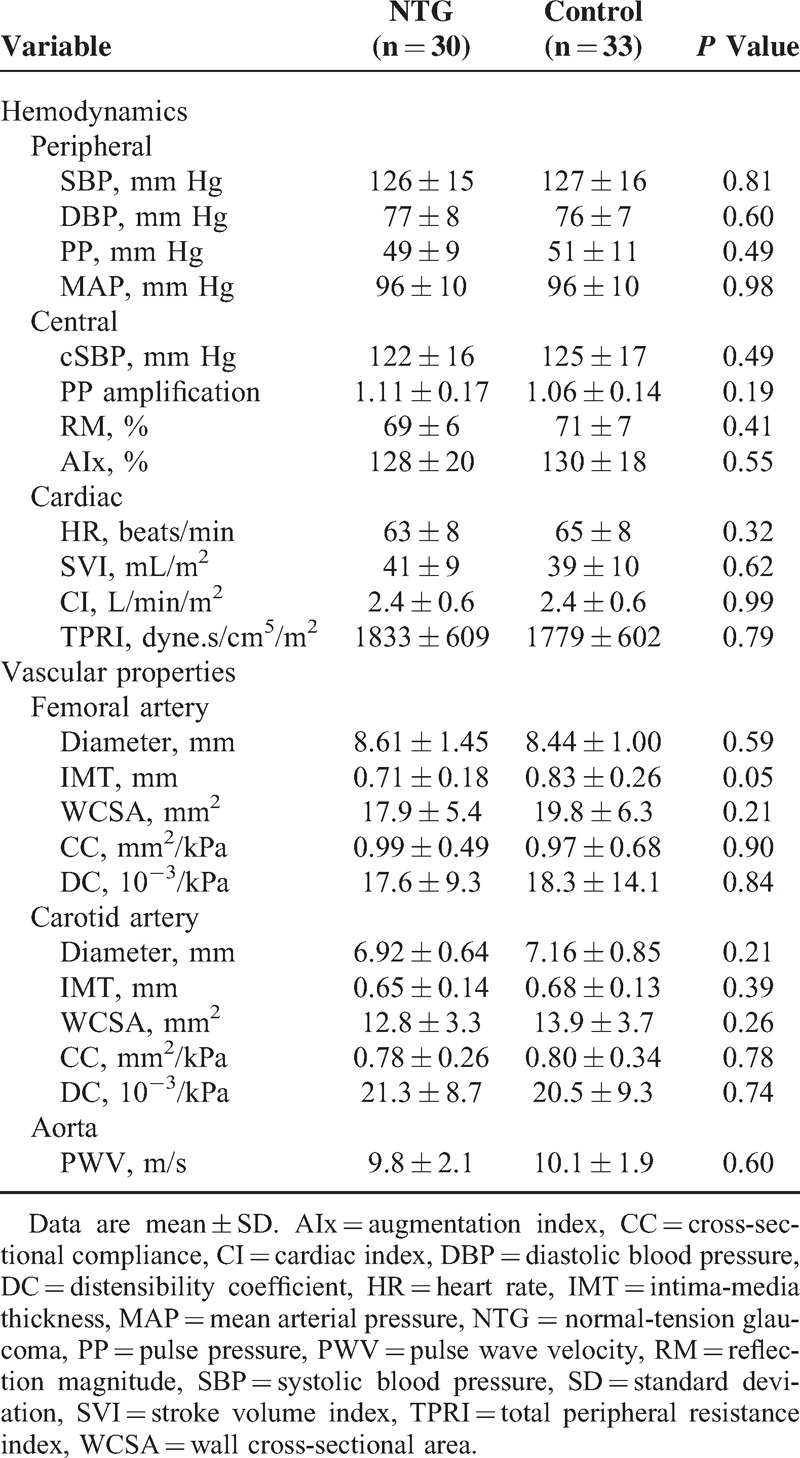
There were no statistically significant differences in arterial structure nor function between NTG patients and age and sex-matched controls. NTG versus controls, respectively: brachial blood pressure 126 ± 15/77 ± 8 versus 127 ± 16/76 ± 7 mm Hg, P = 0.81; carotid–femoral PWV 9.8 ± 2.1 versus 10.1 ± 1.9 m/s, P = 0.60; TPRI 1833 ± 609 versus 1779 ± 602 dyne.s/cm5/m2, P = 0.79; and carotid IMT 0.65 ± 0.14 versus 0.68 ± 0.13 mm, P = 0.39.
This study could not show an association of NTG with altered IMT, arterial stiffness, total peripheral resistance, cardiac output, and peripheral or central hemodynamics at rest. Although the majority of these NTG patients do exhibit symptoms of vascular dysregulation, in the present study this was not translated into alterations in the microcirculation or macrocirculation at rest.
INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide1 and is characterized by typical damage to the optic nerve head, termed “glaucomatous optic neuropathy.” According to the vascular theory, this damage results from either low or fluctuating ocular blood flow, causing ischemia and reperfusion injury at the optic nerve head, respectively.2 Although ocular blood flow is often reduced because of elevated intraocular pressure (IOP), the existence of normal-tension glaucoma (NTG, with IOP < 21 mm Hg) suggests that other factors are also involved. Indeed, dysregulation of vascular resistance is now considered a key pathogenic factor, particularly in NTG.3 Moreover, dysregulation often manifests itself systemically as the “primary vascular dysregulation syndrome.” NTG patients often suffer from this syndrome4 or its hallmarks (eg, cold extremities,5–8 migraine,9,10 reduced sensation of thirst, and others). However, the exact role of systemic dysregulation in the pathophysiology of NTG remains to be identified.
Historically, NTG has been linked with low arterial blood pressure, either diurnally11,12 or only at night.13,14 Many studies, however, did not find an association between NTG and low blood pressure15–24 or show overdipping.17,25–27 Focusing on more integrative measures of vascular health did not solve these discrepancies. Augmentation index (AIx) (a measure of wave reflections) in NTG patients was found increased by Mroczkowska et al,21 but unaltered by Graham et al.23 Pulse wave velocity (a measure of arterial stiffness) in NTG was found increased in one study28 whereas not different from controls in other studies.18,29 Since an association between increased stiffness of the carotid artery30 and aorta31 and retinal arteriolar narrowing has been shown, it is very likely that these factors may also play a role in the pathophysiology of NTG. Therefore, it was hypothesized that NTG is associated with systemic vascular abnormalities in ≥1 arterial beds.
However, at present, not all hemodynamic variables, such as muscular artery properties and total peripheral resistance, have been investigated in NTG. However, compliance of a muscular (the brachial) artery was found decreased in patients with migraine,32 whose condition might share a common etiology with NTG.33 Similarly, total peripheral resistance may be an interesting parameter to examine in NTG, as it can be altered in case of systemic microvascular abnormalities.34
Therefore, the aim of this study was to gain more insight into the function of the systemic microcirculation and macrocirculation in NTG, by comparing NTG patients with healthy age and sex-matched controls. To this aim, noninvasive measurements of arterial structure and function were performed: diameter, intima-media thickness (IMT), and stiffness of elastic (carotid) and more muscular (femoral) arteries; aortic stiffness (carotid-to-femoral pulse wave velocity [PWV]); total peripheral resistance; and peripheral and central hemodynamics.
METHODS
Study Design
A cross-sectional case–control study was carried out at the Heymans Institute of Pharmacology of the Ghent University, Ghent, Belgium. The study consisted of a screening visit (between June 2012 and April 2013) and a study visit (no later than 3 months after study visit). At screening, a fasted blood sample was drawn (to determine total cholesterol, low-density lipoproteins, high-density lipoproteins, creatinine, glucose, and triglycerides), brachial blood pressure was measured, and a questionnaire was completed (medical history, lifestyle habits, medication use, and signs of vascular dysregulation; Table 1). The study visit included all hemodynamic measurements. Subjects who were on vasoactive drugs were asked to stop treatment 3 days before study visit. NTG subjects were asked not to use eye drops on the day of the examinations (or only after the examinations were over). The study was approved by the Ethics Committee of Ghent University and conducted according to the ICH Good Clinical Practice and in compliance with the Declaration of Helsinki. All participants gave written informed consent.
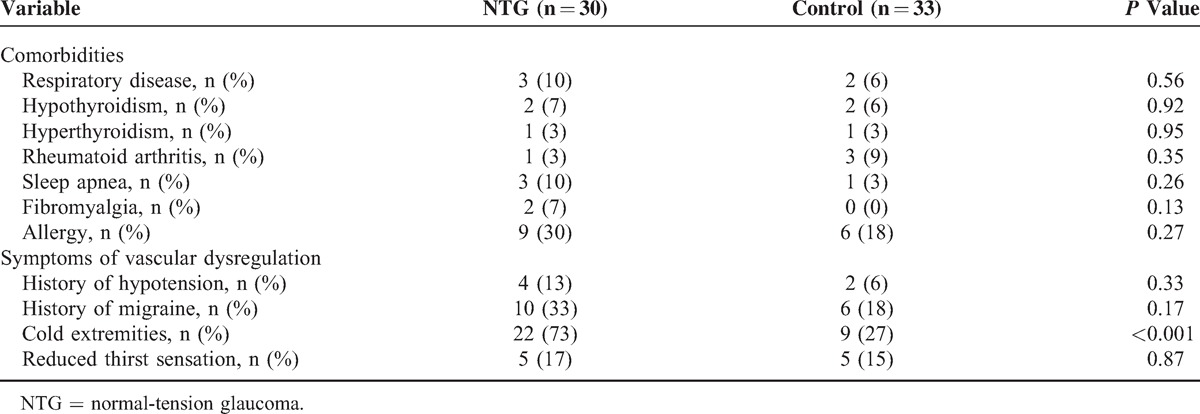
Table 1.
Results of the Study Questionnaire
Participants
Thirty-two patients diagnosed with NTG were recruited from the Department of Ophthalmology of the University hospital. NTG was defined as neuroretinal rim loss assessed by stereo disc assessment and photography, with a typical visual field defect, despite normal IOP <21 mm Hg. Thirty-three healthy control subjects were recruited from the local community and matched with cases for age and sex. Exclusion criteria were history of cardiovascular disease (CVD), modest or severe arterial hypertension (ie, systolic blood pressure [SBP] >160 and/or diastolic blood pressure [DBP] >100 mm Hg), diabetes mellitus, severe hypercholesterolemia (defined as total cholesterol >290 mg/dL), and pregnancy or lactation.
Control and NTG subjects underwent the following examinations: visual acuity assessment, slit-lamp examination, Goldmann applanation tonometry, fundoscopy, Haag-Streit Octopus 311 perimeter, spectral domain optical coherence tomography (Heidelberg): nerve fiber layer thickness, and central corneal thickness measurement.
Hemodynamic Measurements
Hemodynamic measurements were done in supine position and under standardized conditions.35 Supine brachial SBP and DBP and heart rate (HR) were recorded with a validated semiautomated oscillometric device (OMRON M6; OMRON Healthcare, Hoofddorp, The Netherlands). Mean arterial pressure (MAP) was calculated by taking the area under the curve of scaled brachial artery pressure waveforms (PWFs) obtained by applanation tonometry (Sphygmocor; AtCor Medical, Sydney, Australia).
Carotid and femoral artery diameter (D), distension, and wall thickness (IMT) were measured on the right common carotid artery and the right common femoral artery, at diastole, 2 cm proximal to the bifurcation, with a 10-MHz pulsed ultrasound echotracking system (Wall Track system; AU5, Esaote Pie Medical, Maastricht, The Netherlands). Wall cross-sectional area (WCSA) was calculated by subtracting luminal area [π∗(D/2 − IMT)2] from arterial cross-sectional area [π∗(D/2)2]. Reproducibility, expressed as the coefficient of variation between 2 measurement series, was 2.4% for femoral IMT, 2.0% for femoral diameter, 1.4% for carotid IMT, and 1.9% for carotid diameter.
Femoral and carotid arterial cross-sectional compliance (CC, a measure of the buffering capacity) and distensibility coefficient (DC, the inverse of the stiffness) were calculated as CC = ΔA/PP = π × (Ds2 − Dd2)/(4 × PP), and DC = (ΔA/Ad)/PP = (Ds2 − Dd2)/(Dd2 × PP), where ΔA is the systolic–diastolic change in arterial cross section, Ds is the arterial diameter at end systole, Dd is the arterial diameter at end diastole, Ad is the arterial cross section at end diastole, and PP is the local pulse pressure.36 Local PP was obtained by recording local PWFs with applanation tonometry (Sphygmocor), calibrated using brachial artery DBP and MAP.37 Pulse-pressure amplification was calculated as brachial/carotid PP. Reproducibility of femoral and carotid DC was 9.0% and 8.5%, respectively.
Aortic stiffness was measured along the carotid–femoral path, using applanation tonometry (Sphygmocor). Carotid-to-femoral PWV was calculated using the 80% rule.38 Reproducibility of PWV was 4.3%.
Wave reflections were assessed by the AIx, which was calculated from the carotid PWFs as P2/P1, in which P2 indicates the amplitude of the late systolic peak and P1 indicates the amplitude of the early systolic peak.39 As a more accurate estimation of the amount of wave reflection, reflection magnitude (RM) was calculated using an average physiologic flow waveform as described by Kips et al.40
Cardiac function was measured using echocardiography (AU5; Esaote, Genoa, Italy). Stroke volume index (SVI) was calculated from aortic cross-sectional area multiplied by the flow velocity integral, divided by body surface area.41 Cardiac index (CI) was calculated as SVI∗HR. Total peripheral resistance index (TPRI) was calculated as MAP/CI. Reproducibility of cardiac output was 4.4%.
Statistical Analysis
Continuous variables were compared between the groups by an independent samples t test when normally distributed, or by Mann–Whitney U test when nonnormally distributed. Categorical variables were compared between groups by Pearson χ2 test. Values of P < 0.05 were considered significant. Data are reported as mean ± standard deviation or frequencies (percentages). Cases and controls were matched by keeping age and sex distributions as close as possible (ie, statistically not significantly different from each other; P > 0.05). All analyses were done using PASW18 (SPSS Inc, Chicago, IL).
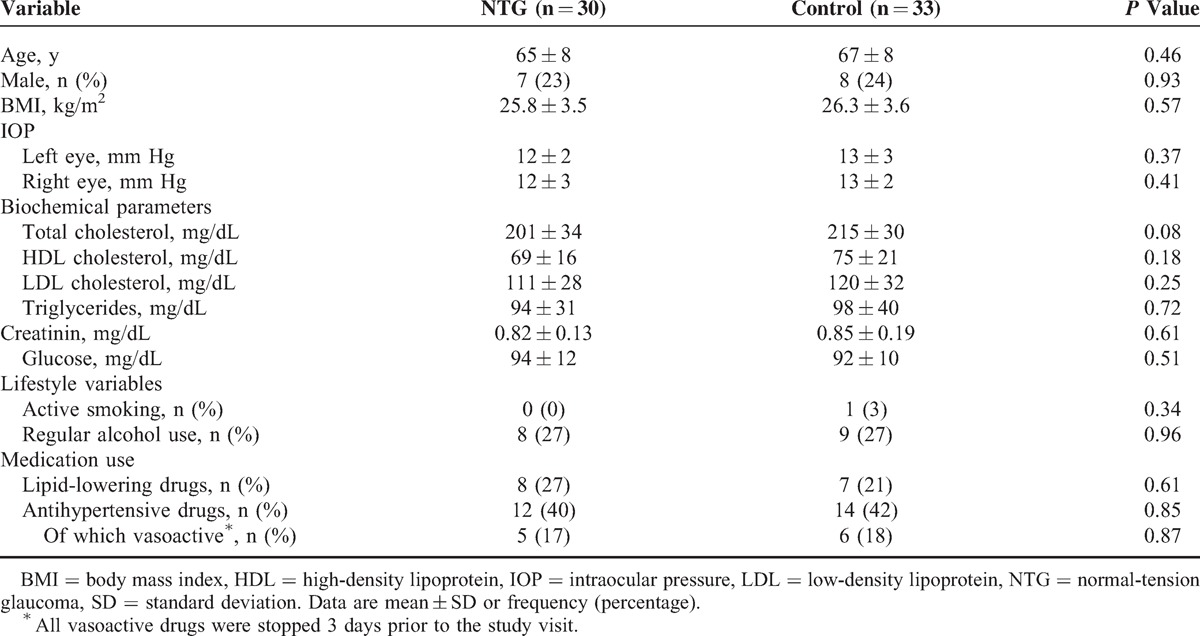
RESULTS
Of all screened NTG subjects (n = 32), 2 participants were excluded because of type II diabetes mellitus and history of CVD, respectively. No cases of optic disc hemorrhages have been observed. Baseline characteristics of subjects are summarized in Table 2. There were no significant differences between NTG and control subjects for age, sex, body mass index, lifestyle habits, or any of the biochemical variables. When asked, all subjects taking vasoactive medication (NTG 17% versus controls 18%, P = 0.87) stopped treatment 3 days prior to the study visit. Survey data (Table 1) revealed that significantly more NTG patients suffered from cold hand and/or feet (73% versus 27%, P < 0.001). This effect was maintained after excluding patients with migraine in the control group (P < 0.001). There were also trends toward an increased prevalence of migraine (P = 0.17), fibromyalgia (P = 0.13), and sleep apnea (P = 0.26) in the NTG group.
Table 2.
Baseline Characteristics of the Study Population
None of the cardiovascular parameters were different between NTG and control subjects (Table 3). Femoral IMT was borderline significant (P = 0.05), and lower in the NTG subjects. However, when this parameter (IMT) was corrected for differences in arterial diameter (WCSA), this near statistical significance disappeared (P = 0.21).
Table 3.
Hemodynamic Measurements
DISCUSSION
A comprehensive assessment of the macrocirculation and microcirculation at rest did not reveal any difference between NTG patients and age and sex-matched healthy controls. This finding confirms those of others who observed no difference in blood pressure and/or waveform parameters, and PWV.18,23 In addition, we showed that muscular artery stiffness, RM, and total peripheral resistance, which to our knowledge constitute a blind spot in NTG research, were also not different from the controls. However, questionnaire reports do suggest that vascular dysregulation is present in the majority of NTG patients, and not restricted to the eye. To summarize, despite arguments for a systemic involvement, no systemic differences in cardiovascular structure and function were found at rest.
There are several possible explanations for this paradox.
Vascular dysregulation represents a defective response to a certain stressor, whereas all cardiovascular parameters were measured at rest. As symptoms of vascular dysregulation occur only episodically (eg, at night, after cold exposure, and others), provocative tests may be needed to unmask alterations in cardiovascular function. Indeed, Su et al42 observed no differences in brachial artery blood flow at baseline, but an impaired response following ischemia in NTG patients. Similarly, Nicolela et al8 found no difference in plasma endothelin-1 levels at baseline, but a significantly higher endothelin-1 concentration in glaucoma patients after cold exposure.
Although it is evident to consider improper cardiovascular function as a direct cause of inadequate ocular blood flow, the pathophysiology of NTG may involve defects in other organ systems as well. Glaucoma is a multifactorial disease, having an immunological, endocrine, and neurological component, which may make it difficult to isolate a single (cardiovascular) profile.43–47
This is a cross-sectional study. Therefore, we cannot exclude the possibility that cardiovascular alterations were present long before diagnosis, but were in the meantime influenced by other factors, such as lifestyle changes, medication, course of disease, and others. To illustrate, glaucoma patients often recall having low blood pressure in youth,48 but this effect may disappear with aging.
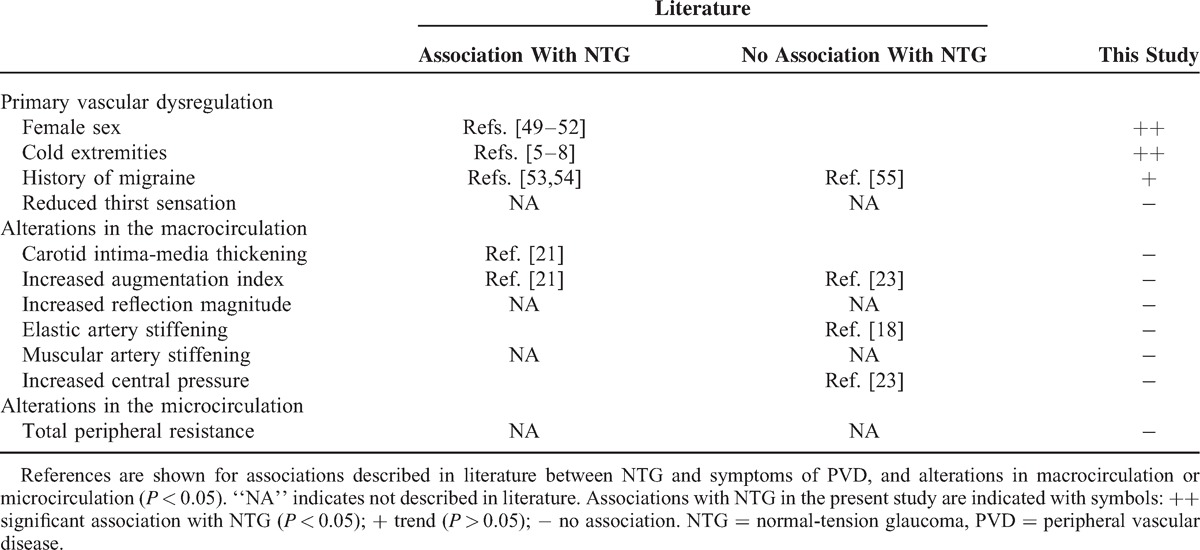
Table 45–8,18,21,23,49–55 gives an overview of literature data and associations with NTG tested in the present study. From this Table, it is clear that the vast majority of studies find associations between NTG and signs of vascular dysregulation, but not consistently with vascular alterations at rest, while no literature data exists on muscular artery stiffness, total peripheral resistance, and RM.
Table 4.
Associations With NTG Tested in Literature and/or in This Study
Strengths and Limitations
The strength of this study is that the influence of confounders is limited by matching subjects for age and gender, which was successful and resulted in similar levels of biochemical (eg, cholesterol, fasting glucose, and others) and physical (eg, height, weight, and others) variables between the case and the control group. However, this study has some limitations as well. First, this study suffers from its cross-sectional design. Second, because of low prevalence of NTG, the sample size was small. However, to detect a difference in CC of 20%, as was found in patients with migraine,32 this sample size was deemed adequate (power 80%, α = 0.05). Third, vasoactive drugs were stopped 3 days prior to the measurement visit, which may not be sufficient to cancel out all its hemodynamic effects. However, even if there are residual effects, it is not likely that this has affected our conclusions, since use of vasoactive medication was not different between glaucoma and control subjects. Fourth, cases and controls with history of CVD, hypercholesterolemia, or severe hypertension (causing increased levels of arterial stiffness and wave reflections) were excluded, since we aimed to investigate NTG in its purest form. Fifth, the TPRI is a calculated parameter, constituting a rough index of the systemic microcirculation.
CONCLUSION
To conclude, our data show no alterations of the microcirculation or macrocirculation in NTG at rest, despite a history of clinical symptoms of systemic vascular dysregulation. In particular, vascular dysregulation did not lead to statistically significant alterations in vascular tone as evidenced by no differences in function of the muscular femoral artery, total peripheral resistance, MAP, and measures of wave reflection. Provocative tests may be needed to reveal alterations in cardiovascular function in NTG patients.
Footnotes
Abbreviations: AIx = augmentation index, AUC = area under the curve, CC = cross-sectional compliance, CI = cardiac index, CVD = cardiovascular disease, DBP = diastolic blood pressure, DC = distensibility coefficient, HR = heart rate, IMT = intima-media thickness, IOP = intraocular pressure, MAP = mean arterial pressure, NTG = normal-tension glaucoma, PP = pulse pressure, PWF = pressure waveform, PWV = pulse wave velocity, RM = reflection magnitude, SBP = systolic blood pressure, SVI = stroke volume index, TPRI = total peripheral resistance index, WCSA = wall cross-sectional area.
This study has received grants from the Funds for Research in Ophthalmology (FRO) and the Belgian Glaucoma Society (Research Award 2008).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 2004; 82:887–888. [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol 2013; 13:43–49. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Harris A. Ocular Blood Flow in Glaucoma: The 6th Consensus Report of the World Glaucoma Association. Amsterdam, The Netherlands: Kugler Publications; 2009. [Google Scholar]
- 4.Flammer J, Konieczka K, Flammer AJ. The primary vascular dysregulation syndrome: implications for eye diseases. EPMA J 2013; 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension and high-tension glaucoma. Am J Ophthalmol 1991; 111:585–588. [DOI] [PubMed] [Google Scholar]
- 6.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol 1998; 82:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’brien C. Vasospasm and glaucoma. Br J Ophthalmol 1998; 82:855–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolela MT, Ferrier SN, Morrison CA, et al. Effects of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci 2003; 44:2565–2572. [DOI] [PubMed] [Google Scholar]
- 9.Corbett JJ, Phelps CD, Eslinger P, et al. The neurologic evaluation of patients with low-tension glaucoma. Invest Ophthalmol Vis Sci 1985; 26:1101–1104. [PubMed] [Google Scholar]
- 10.Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci 1985; 26:1105–1108. [PubMed] [Google Scholar]
- 11.Demailly P, Cambien F, Plouin PF, et al. Do patients with low tension glaucoma have particular cardiovascular characteristics? Ophthalmologica 1984; 188:65–75. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser HJ, Flammer J, Graf T, et al. Systemic blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol 1993; 231:677–680. [DOI] [PubMed] [Google Scholar]
- 13.Graham SL, Drance SM, Wijsman K, et al. Ambulatory blood pressure monitoring in glaucoma: the nocturnal dip. Ophthalmology 1995; 102:61–69. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol 1996; 80:864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leighton DA, Phillips CI. Systemic blood pressure in open-angle glaucoma, low tension glaucoma, and the normal eye. Br J Ophthalmol 1972; 56:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol 1996; 80:864–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plange N, Kaup M, Daneljan L, et al. 24-h blood pressure monitoring in normal tension glaucoma: night-time blood pressure variability. J Hum Hypertens 2005; 20:137–142. [DOI] [PubMed] [Google Scholar]
- 18.Hulsman CAA, Vingerling JR, Hofman A, et al. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch Ophthalmol 2007; 125:805–812. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-K, Oh WH, Park KH, et al. Circadian blood pressure and intraocular pressure patterns in normal tension glaucoma patients with undisturbed sleep. Korean J Ophthalmol 2010; 24:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na K-S, Lee NY, Park S-H, et al. Autonomic dysfunction in normal tension glaucoma: the short-term heart rate variability analysis. J Glaucoma 2010; 19:377–381. [DOI] [PubMed] [Google Scholar]
- 21.Mroczkowska S, Ekart A, Sung V, et al. Coexistence of macro- and micro-vascular abnormalities in newly diagnosed normal tension glaucoma patients. Acta Ophthalmol (Copenh) 2012; 90:e553–e559. [DOI] [PubMed] [Google Scholar]
- 22.Wierzbowska J, Wierzbowski R, Stankiewicz A, et al. Cardiac autonomic dysfunction in patients with normal tension glaucoma: 24-h heart rate and blood pressure variability analysis. Br J Ophthalmol 2012; 96:624–628. [DOI] [PubMed] [Google Scholar]
- 23.Graham SLM, Butlin M, Lee M, et al. Central blood pressure arterial waveform analysis, and vascular risk factors in glaucoma. J Glaucoma 2013; 22:98–103. [DOI] [PubMed] [Google Scholar]
- 24.Mroczkowska S, Benavente-Perez A, Negi A, et al. Primary open-angle glaucoma vs normal-tension glaucoma: the vascular perspective. JAMA Ophthalmol 2013; 131:36–43. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwagi K, Hosaka O, Kashiwagi F, et al. Systemic circulatory parameters comparison between patients with normal tension glaucoma and normal subjects using ambulatory monitoring. Jpn J Ophthalmol 2001; 45:388–396. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y-K, Oh WH, Park KH, et al. Circadian blood pressure and intraocular pressure patterns in normal tension glaucoma patients with undisturbed sleep. Korean J Ophthalmol 2010; 24:23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wierzbowska J, Wierzbowski R, Stankiewicz A, et al. Cardiac autonomic dysfunction in patients with normal tension glaucoma: 24-h heart rate and blood pressure variability analysis. Br J Ophthalmol 2012; 96:624–628. [DOI] [PubMed] [Google Scholar]
- 28.Ikuyo O, Futoshi I, Hiroshi O, et al. Arterial sclerosis grade in normal-tension glaucoma patients. J Eye 2004; 21:397–400. [Google Scholar]
- 29.Chiba T, Chiba N, Kashiwagi K. Systemic arterial stiffness in glaucoma patients. J Glaucoma 2008; 17:15–18. [DOI] [PubMed] [Google Scholar]
- 30.Liao D, Wong TY, Klein R, et al. Relationship between carotid artery stiffness and retinal arteriolar narrowing in healthy middle-aged persons. Stroke J Cereb Circ 2004; 35:837–842. [DOI] [PubMed] [Google Scholar]
- 31.Cheung N, Sharrett AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the multi-ethnic study of atherosclerosis. Hypertension 2007; 50:617–622. [DOI] [PubMed] [Google Scholar]
- 32.Vanmolkot FH, Van Bortel LM, de Hoon J. Altered arterial function in migraine of recent onset. Neurology 2007; 68:1563–1570. [DOI] [PubMed] [Google Scholar]
- 33.Schacknow PN, Samples JR. The Glaucoma Book: A Practical Evidence-Based Approach to Patient Care. New York: Springer; 2010. [Google Scholar]
- 34.Greene AS, Tonellato PJ, Lui J, et al. Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol 1989; 256 (1 Pt 2):H126–H131. [DOI] [PubMed] [Google Scholar]
- 35.Van Bortel LM, Duprez D, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15:445–452. [DOI] [PubMed] [Google Scholar]
- 36.Boutouyrie P, Bussy C, Hayoz D, et al. Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 2000; 101:2601–2606. [DOI] [PubMed] [Google Scholar]
- 37.Van Bortel LM, Balkestein EJ, van der Heijden-Spek JJ, et al. Non-invasive assessment of local arterial pulse pressure: comparison of applanation tonometry and echo-tracking. J Hypertens 2001; 19:1037–1044. [DOI] [PubMed] [Google Scholar]
- 38.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 39.Chen C-H, Ting C-T, Nussbacher A, et al. Validation of carotid artery tonometry as a means of estimating augmentation index of ascending aortic pressure. Hypertension 1996; 27:168–175. [DOI] [PubMed] [Google Scholar]
- 40.Kips JG, Rietzschel ER, De Buyzere ML, et al. Evaluation of noninvasive methods to assess wave reflection and pulse transit time from the pressure waveform alone. Hypertension 2009; 53:142–149. [DOI] [PubMed] [Google Scholar]
- 41.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep 1970; 54:225–235. [PubMed] [Google Scholar]
- 42.Su W-W, Cheng S-T, Hsu T-S, et al. Abnormal flow-mediated vasodilation in normal-tension glaucoma using a noninvasive determination for peripheral endothelial dysfunction. Invest Ophthalmol Vis Sci 2006; 47:3390–3394. [DOI] [PubMed] [Google Scholar]
- 43.Pache M, Flammer J. A sick eye in a sick body? Systemic findings in patients with primary open-angle glaucoma. Surv Ophthalmol 2006; 51:179–212. [DOI] [PubMed] [Google Scholar]
- 44.Cartwright MJ, Grajewski AL, Friedberg ML, et al. Immune-related disease and normal-tension glaucoma. A case-control study. Arch Ophthalmol 1992; 110:500–502. [DOI] [PubMed] [Google Scholar]
- 45.Jämsén K. Thyroid disease, a risk factor for optic neuropathy mimicking normal-tension glaucoma. Acta Ophthalmol Scand 1996; 74:456–460. [DOI] [PubMed] [Google Scholar]
- 46.Kesler A, Haber I, Kurtz S. Neurologic evaluations in normal-tension glaucoma workups: are they worth the effort? Isr Med Assoc J 2010; 12:287–289. [PubMed] [Google Scholar]
- 47.Erb C, Batra A, Lietz A, et al. Psychological characteristics of patients with normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol 1999; 237:753–757. [DOI] [PubMed] [Google Scholar]
- 48.Gherghel D, Orgül S, Gugleta K, et al. Retrobulbar blood flow in glaucoma patients with nocturnal over-dipping in systemic blood pressure. Am J Ophthalmol 2001; 132:641–647. [DOI] [PubMed] [Google Scholar]
- 49.Orgul S, Flammer J, Gasser P. Female preponderance in normal-tension glaucoma. Ann Ophthalmol Glaucoma 1995; 27:355–359. [Google Scholar]
- 50.Levene RZ. Low tension glaucoma: a critical review and new material. Surv Ophthalmol 1980; 24:621–664. [DOI] [PubMed] [Google Scholar]
- 51.Drance SM, Sweeney VP, Morgan RW, et al. Studies of factors involved in the production of low tension glaucoma. Arch Ophthalmol 1973; 89:457–465. [DOI] [PubMed] [Google Scholar]
- 52.Nicolela MT, Drance SM. Various glaucomatous optic nerve appearances: clinical correlations. Ophthalmology 1996; 103:640–649. [DOI] [PubMed] [Google Scholar]
- 53.Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci 1985; 26:1105–1108. [PubMed] [Google Scholar]
- 54.Wang JJ, Mitchell P, Smith W. Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology 1997; 104:1714–1719. [DOI] [PubMed] [Google Scholar]
- 55.Usui T, Iwata K, Shirakashi M, et al. Prevalence of migraine in low-tension glaucoma and primary open-angle glaucoma in Japanese. Br J Ophthalmol 1991; 75:224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]


