Abstract
To characterize serum IgG subclass levels in several autoimmune diseases, including primary Sjogren syndrome (pSS), systemic sclerosis (SSc), systemic lupus erythematosus (SLE), and primary biliary cirrhosis (PBC). We aimed to analyze serum IgG subclass distribution and to test whether serum IgG4 levels are elevated in these diseases.
Serum IgG subclass levels from 102 pSS, 102 SSc, 100 SLE, and 59 PBC patients, as well as 40 healthy controls (HCs), were measured using the immunonephelometric assay. The distribution of IgG subclasses among these autoimmune diseases was analyzed.
In this cross-sectional study, serum IgG1 (IgG1/IgG) and/or IgG3 (IgG3/IgG) were significantly increased, compared with those in HCs. Only 6.34% of patients had levels of serum IgG4 >135 mg/dL. There were no significant differences in the frequency of elevated serum IgG4 levels between patients and HC. In pSS, serum IgG1 levels were much higher than those in other disease groups, whereas serum IgG2 and IgG3 levels were most prominently increased in PBC.
A strikingly different serum IgG subclass distribution was detected in patients with autoimmune diseases compared with HCs. Serum IgG subclass levels also showed distinct characteristics among different autoimmune diseases. Serum IgG4 levels in these patients were lower or not much higher than those in HCs, which differed from IgG4-related diseases.
INTRODUCTION
Primary Sjogren syndrome (pSS), systemic lupus erythematosus (SLE), systemic sclerosis (SSc), and primary biliary cirrhosis (PBC) are common systemic or organ-specific autoimmune diseases. Autoimmune diseases usually develop when the balance between pathogen recogniton and avoidance of self-attack is impaired, especially to environmental triggering of genetically susceptible individuals.1
Various autoantibodies have been detected in the serum of these patients, and most of the autoantibodies are immunoglobulin G (IgG). There are 4 distinct subclasses of IgG termed IgG1, IgG2, IgG3, and IgG4. The 4 IgG subclasses show considerable differences in their bioactivities, including the ability to fix complements (IgG3 > IgG1 > IgG2 > IgG4) and to bind to Fc receptors.2 It has also been reported that 50% of IgG4 “halves” can disassociate and reassociate with other IgG4 antibodies, which makes them incapable of cross-linking antigens, thereby down-modulating the immune response.3,4 These IgG subclasses could contribute to the immunopathogenesis of autoimmune diseases by regulating immunoglobulin, FcγR, and complement interactions.5,6
Significant attention has been foucused on detecting serum IgG subclasses since IgG4-related disease (IgG4-RD) was classified in 2010 as a novel disease entity.7 IgG4-RD is considered to be a systemic, chronic, and inflammatory disorder. IgG4-RD has the following features in common: diffuse organ swelling or focal mass formation with fibrosis, IgG4-positive plasma cell infiltrates in involved tissues, and elevated levels of serum IgG4.7 Currently, one of the diagnostic criteria of IgG4-RD is serum IgG4 >135 mg/dL.8 However, some studies have indicated that this criterion was not specific because other clinical situations, including some autoimmune diseases, were also associated with increased serum IgG4 levels.9–11
Few studies have systemically analyzed serum IgG subclass levels in conventional autoimmune diseases and limited data are available about IgG4 levels in large patient cohorts for diseases other than IgG4-RD.12–14 To better understand autoimmune diseases, this study characterized the levels of these serum IgG subclasses in 4 common autoimmune diseases and investigated whether serum IgG4 levels were elevated or not.
MATERIALS AND METHODS
Subjects
A total of 102 pSS, 102 SSc, 100 SLE, and 59 PBC patients were randomly selected from our clinical information library at the Department of Rheumatology, Peking Union Medical College Hospital (PUMCH), China; all patients were presented at PUMCH between October 2009 and March 2012. An additional 40 healthy individuals were randomly selected from the Center of Health Examination, PUMCH, who presented between February 2012 and March 2012. The study was approved by the Ethical Committee of PUMCH and written informed consent was obtained from each participant.
A total of 102 pSS patients (96 females, 6 males) fulfilled the classification criteria for pSS that was proposed by the American-European Consensus Group15; patients ranged in age from 15 to 73 years and the mean age was 44.7 ± 12.1 years. There were 102 SSc patients (93 females, 9 males) who fulfilled the scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee16; patients ranged in age from 16 to 76 years and the mean age was 44.3 ± 12.0 years. A total of 100 SLE patients (92 females, 8 males) fulfilled the 1997 American College of Rheumatology revised criteria for SLE17; patients ranged in age from 15 to 69 years and the mean age was 30.7 ± 13.0 years. Finally, 59 PBC patients (57 females, 2 males) fulfilled the 2009 AASLD revised criteria for PBC18; patients ranged in age from 20 to 71 years and the mean age was 47.1 ± 9.0 years. Patients affected by another autoimmune disease were not included.
For the healthy controls (HCs) group, 40 healthy individuals (37 females, 3 males) who ranged in age from 24 to 60 years were selected and the mean age was 30.6 ± 8.5 years. There were no significant differences in sex or age between the patients and HCs. The laboratory test results of HCs were in the normal range, and there was no history of any specific allergic or autoimmune diseases in the healthy individuals.
Measurements of Serum IgG Subclass Concentrations
Sera from patients and HCs were collected and stored at −80°C before testing. Serum IgG subclass levels were measured using the immunonephelometric assay19,20 with molecular biology kits (Siemens, Germany, including N Latex IgG1, N Latex IgG2, N Latex IgG3, N Latex IgG4 and N Supplementary Reagent/Precipitation) and the automatic protein analyzer (Siemens BN prospec-I, Germany) according to the manufacturer's instructions. Serum IgG levels represent the sum of IgG1, IgG2, IgG3, and IgG4.
Statistical Analysis
Data are presented as means ± standard deviation (SD). Differences between groups were compared by nonparametric Mann–Whitney U test or Student t test. Ratios between 2 groups were calculated by Fisher exact probabilities in 4-fold tables. A P value <0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS v.19.0 statistical package (SPSS, Chicago, IL) and GraphPad Prism version 6.0 (GraphPad, San Diego, CA).
RESULTS
Serum IgG Subclass Levels and IgG 1–4/IgG Ratios Between Patients and HCs
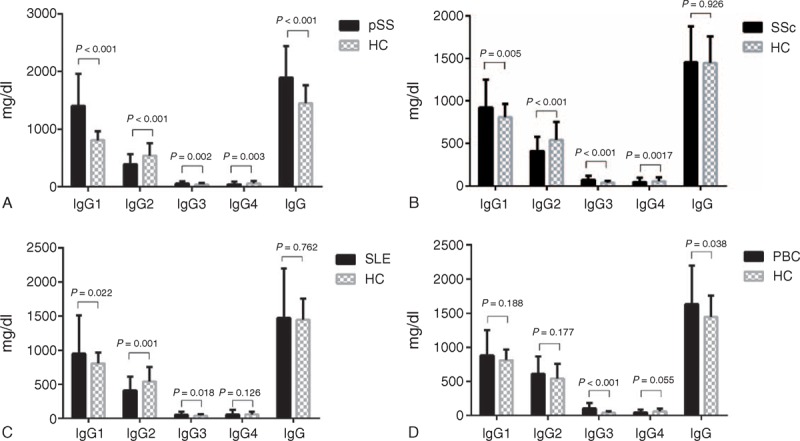
Compared with HCs, serum IgG1 (IgG1/IgG) and/or IgG3 (IgG3/IgG) were significantly increased in the pSS, SSc, SLE, and PBC groups. Serum IgG2 (IgG2/IgG) were much lower in pSS, SSc, and SLE patients, whereas serum IgG2 (IgG2/IgG) were slightly higher in PBC patients, although there was no significant difference between PBC patients and HCs. Serum IgG4 and/or IgG4/IgG were significantly reduced in pSS, SSc, and PBC patients, whereas no significant difference in IgG4 (IgG4/IgG) between SLE patients and HCs was detected (Table 1, Figure 1 and Figure 2).
TABLE 1.
Serum IgG Subclass, IgG Levels, and IgG 1–4/IgG Ratios
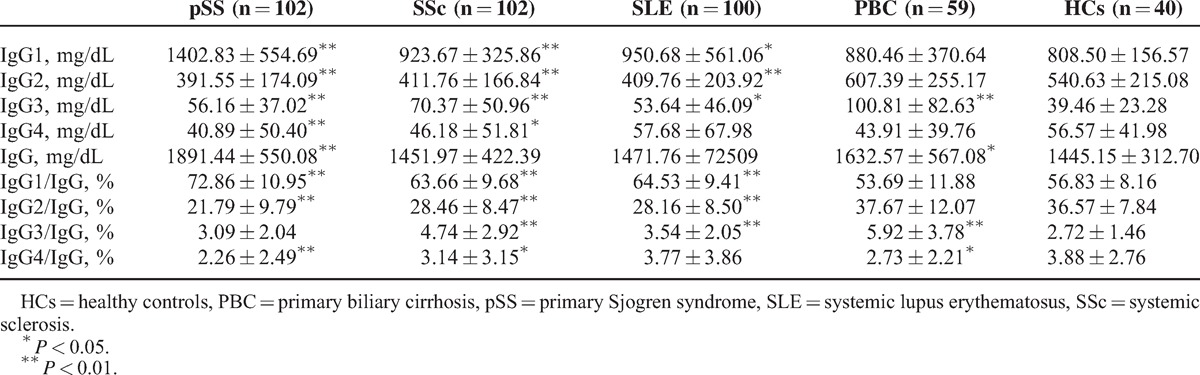
FIGURE 1.
Serum IgG subclass levels and IgG levels of pSS, SSc, SLE, and PBC patients, as well as HCs. HCs = healthy controls, PBC = primary biliary cirrhosis, pSS = primary Sjogren syndrome, SLE = systemic lupus erythematosus, SSc = systemic sclerosis.
FIGURE 2.
Serum IgG1–4/IgG ratios of pSS, SSc, SLE, and PBC patients, as well as HCs. HCs = healthy controls, PBC = primary biliary cirrhosis, pSS = primary Sjogren syndrome, SLE = systemic lupus erythematosus, SSc = systemic sclerosis.
Additionally, serum IgG levels were significantly higher in pSS and PBC patients than those in HCs (Table 1, Figure 1).
Serum IgG Subclass Levels Among Patients With Different Autoimmune Diseases
Serum IgG1 levels in pSS patients were 1402.83 ± 554.69 mg/dL, which were significantly increased compared with those in SSc (923.67 ± 325.86 mg/dL, P < 0.001), SLE (950.68 ± 561.06 mg/dL, P < 0.001), and PBC (880.46 ± 370.64 mg/dL, P < 0.001) patients (Table 1, Figure 3A).
FIGURE 3.
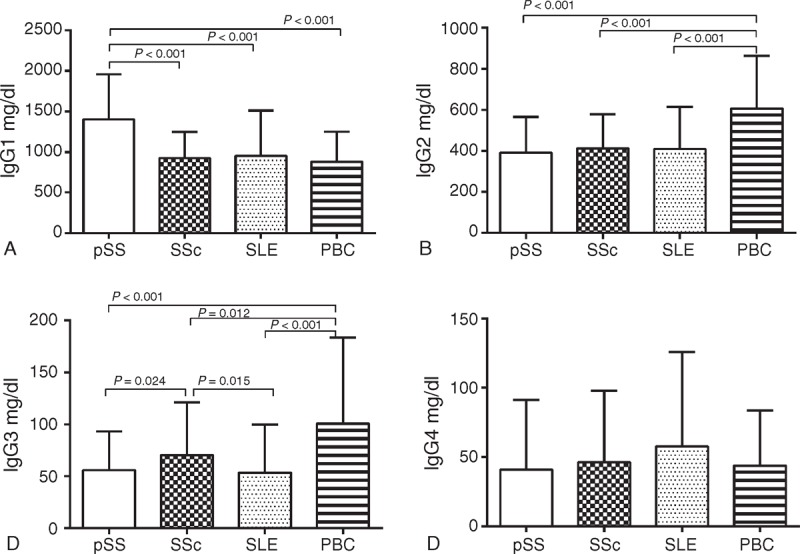
Serum IgG subclass levels among patients with different autoimmune diseases. PBC = primary biliary cirrhosis, pSS = primary Sjogren syndrome, SLE = systemic lupus erythematosus, SSc = systemic sclerosis.
Serum IgG2 levels in PBC patients were 607.39 ± 255.17 mg/dL, which were significantly higher compared with those in pSS (391.55 ± 174.09 mg/dL, P < 0.001), SSc (411.76 ± 166.84 mg/dL, P < 0.001), and SLE (409.76 ± 203.92 mg/dL, P < 0.001) patients (Table 1, Figure 3B).
Levels of serum IgG3 in PBC patients were 100.81 ± 82.63 mg/dL, which were much higher compared with those in pSS (56.16 ± 37.02 mg/dL, P < 0.001), SSc (70.37 ± 50.96 mg/dL, P = 0.012), and SLE (53.64 ± 46.09 mg/dL, P < 0.001) patients. Furthermore, serum IgG3 in SSc patients were much higher than those in pSS or SLE patients (P = 0.024 and P = 0.015, respectively), but were significantly lower than those in PBC patients (Table 1, Figure 3C).
There were no significant differences in serum IgG4 levels among the pSS (40.89 ± 50.40 mg/dL), SSc (46.18 ± 51.81 mg/dL), SLE (57.68 ± 67.98 mg/dL), and PBC patients (57.68 ± 67.98 mg/dL) (pSS vs SSC: P = 0.398, pSS vs SLE: P = 0.162, pSS vs PBC: P = 0.274, SSc vs SLE: P = 0.572, SSc vs PBC: P = 0.719, and SLE vs PBC: P = 0.643; Table 1, Figure 3D).
The Frequency of Elevated Serum IgG4 levels in Patients and HCs
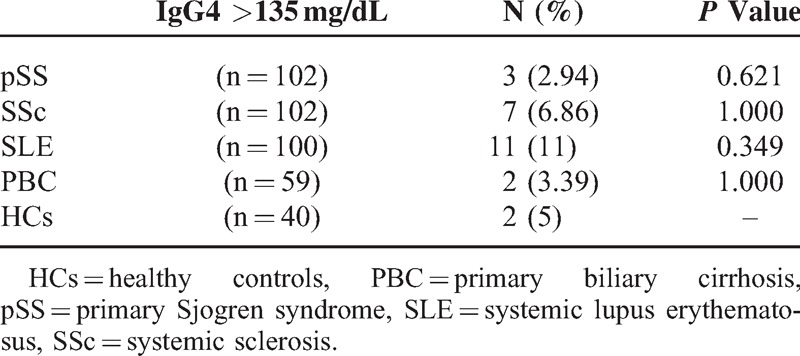
Serum IgG4 >135 mg/dL is considered to be one of the diagnostic criteria for IgG4-RD.21 We calculated the numbers and frequency of patients and HCs whose serum IgG4 levels were >135 mg/dL. There were only 23 (6.34%) patients with elevated serum IgG4 levels among the 363 patients who suffered from these autoimmune diseases. We detected no significant difference in the frequency of elevated serum IgG4 levels between patients and HCs (Table 2).
TABLE 2.
The Frequency of Elevated Serum IgG4 Levels in Patients and HCs
DISCUSSION
SLE, pSS, SSc, and PBC are relatively common autoimmune diseases. As an integral component of the adaptive immune response, IgG can drive the pathogenesis of these disorders.22 These 4 IgG subclasses have different physical and biological properties, and could play an important role in the development of autoimmune disorders.3
Previous studies of the IgG subclasses focused on identifying specific antibodies for particular antigens in autoimmune diseases, such as IgG subclasses of anti-mitochondrial antibodies in PBC or anti-cyclic citrullinated peptide/mutated citrullinated vimentin antibodies in rheumatoid arthritis,23,24 or instead focused on IgG subclasses deposited in the skin or kidney.25,26 Moreover, the assays used to measure serum IgG subclasses were radioimmunodiffusion or enzyme-linked immunosorbent assay. They are not very accurate or convenient compared with the immunonephelometric assay.20
Overall, the serum IgG subclass distribution in autoimmune diseases remains somewhat unclear. In this cross-sectional study, we chose 4 conventional autoimmune diseases in which to analyze the serum IgG subclass levels. Our findings suggested that serum IgG subclass levels in these autoimmune diseases were selectively increased or decreased and we identified distinct characteristics in each autoimmune disease group. Only 6.34% of patients with autoimmune diseases exhibited serum IgG4 >135 mg/dL, which was not significantly different from HCs.
This preliminary study showed that serum IgG1 (IgG1/IgG) and/or IgG3 (IgG3/IgG) were much higher in these autoimmune diseases. Serum IgG2 (IgG2/IgG) levels were significantly lower in pSS, SLE, and SSc patients than those in HCs. Generally, most autoantigens are protein antigens (T cell-dependent antigens) that can stimulate the production of IgG1-autoantibodies or IgG3-autoantibodies, and a few autoantibodies are carbohydrate antigens (T cell-independent antigens) that can stimulate IgG2-antoantibodies.27 Our findings were different from previous studies. Liu et al and Lin et al13,14 reported that significant increases in serum IgG1, IgG2, and IgG3 levels were observed in pSS and SLE patients compared with normal controls. This might be explained by differences in patient selection and further research will be needed to resolve these discrepancies.
Our data also suggested that the serum IgG1 (IgG1/IgG) and/or IgG3 (IgG3/IgG) were significantly higher in these patients than those in healthy individuals, even though their IgG levels were in a normal range (data not shown). This finding indicated that serum IgG subclasses were preferentially produced during the pathogenesis of these autoimmune diseases. Therefore, we speculated affected individuals not only lost tolerance to autoantigens, but also exhibited aberrant immune regulation. Kawasaki et al28 found that there was Th1/Th2 cell skewing in patients with autoimmune diseases. Th1/Th2 cells can stimulate different IgG subclass synthesis and suppress other IgG subclasses by the secretion of different cytokines. Th1 cell subset is characterized by the production of interferon-γ, interleukin (IL)-2, and tumor necrosis factor (TNF)-α. Th1 cell responses induce macrophage and cytotoxic T cell activation, as well as IgG subclass switching, predominantly IgG1 and IgG3, to favor complement fixation and opsonization. Conversely, Th2 cells, defined by their propensity to secrete IL-4, IL-5, and IL-10, are important in allergy and mast cell/IgE-mediated immediate-type hypersensitivity responses.
Autoimmune disease can result in the production of various polyclonal autoantibodies. In this study, we found that serum IgG1 levels of pSS patients were much higher than those in the other three diseases groups. Serum IgG2 and IgG3 levels of PBC patients were prominently increased compared with those in the other diseases groups. This finding might indicate that serum IgG subclass distribution in each autoimmune disease has unique characteristics, and elucidating these characteristics could yield a better understanding of their pathogenic roles of IgG in disease development. Erikkson et al and Kang et al29,30 reported that the main autoantibodies of Ro/La antigen were IgG1 in pSS. IgG1 secretion might be regulated by some cytokines, such as IL-18 and IL-21, and this immune disorder can mediate exocrine gland injury. Rigopolou et al31 reported that IgG3 were the main antibodies to mitochondria in PBC. PBC patients with specific anti-nuclear antibody staining patterns, including membranous or multiple nuclear dots (in particular of the IgG3 type), are associated with a more severe disease course.32 Hence, serum IgG3 might contribute to the development of PBC. We only studied the serum IgG subclass distribution characteristics among common systemic or organ-specific autoimmune diseases in this report, and in the future, we aim to understand the correlation between serum IgG subclasses and clinical manifestations in each autoimmune disease.
Increasing attentions have been given to studies of serum IgG4 concentrations since IgG4-RD was established as a clinical entity across a wide range of organ systems.7 These diseases share a set of consistent pathological features including a dense lymphocyte and plasma cell infiltrate that is rich in IgG4-positive plasma cells, storiform fibrosis and elevated serum IgG4 concentrations.21 Some conventional autoimmune diseases share several similarities with IgG4-RD. For example, lacrimal and salivary damage can occur in both pSS and Mikulicz disease (MD) patients, and MD was formerly considered to be a subtype of pSS.33 Interestingly, SSc and PBC are also characterized by fibrosis and lymphoplasmacytic infiltrates.34,35 There are also various autoantibodies and multi-organ damages in SLE. Yamamoto et al9 indicated that elevated IgG4 could be observed in several clinical situations other than IgG4-RD. Ebbo et al10 reported that 13.6% of patients who suffered from autoimmune diseases were among those patients whose serum IgG4 levels were above the cutoff value of 135 mg/dL. Herein, serum IgG4 (IgG4/IgG) levels were prominently reduced or not significantly different in patients with autoimmune diseases compared with those in healthy individuals. Only 6.34% of patients had elevated serum IgG4 levels (>135 mg/dL), which was much lower than the results of previous studies. Mavragani et al36 recently reported that raised serum IgG4 levels were detected in 7.5% of pSS patients and they fulfilled the criteria of possible or definite IgG4-RD. However, only 2.94% of pSS patients showed elevated serum IgG4 levels in our study and they did not have a tendency to IgG4-RD. As this was a cross-sectional study, we should follow-up these patients with elevated IgG4 to observe their clinical manifestations and serum IgG subclass levels.
Our preliminary findings suggested that serum IgG4 levels in pSS, SSc, SLE, and PBC were different from those in IgG4-RD. These data might be used in differential diagnosis, although they share some similarities in underlying pathogenic processes. Additionally, we considered that not only serum IgG4, but also other IgG subclasses should be considered in the differential diagnosis between common autoimmune diseases and IgG4-RD.
CONCLUSIONS
Serum IgG subclass distribution in patients with autoimmune diseases was different from that in healthy people. Serum IgG subclass levels presented distinct patterns in different autoimmune diseases. Levels of serum IgG4 in these autoimmune diseases were lower or not much higher than those in healthy individuals, which was different from IgG4-RD cases. Further studies of serum IgG subclass distribution will help to elucidate the immunopathogenesis of autoimmune diseases.
Footnotes
Abbreviations: AMA = anti-mitochondrial antibodies, HC = healthy controls, IgG = immunoglobulin G, IgG4-RD = IgG4-related disease, IL = interleukin, MD = Mikulicz's disease, PBC = primary biliary cirrhosis, pSS = primary Sjogren syndrome, SLE = systemic lupus erythematosus, SSc = systemic sclerosis, TNF = tumor necrosis factor.
All authors were involved in drafting and revising the article. Study conception and design: FZ, QS. Performed experiments and/or collected clinical data: PL, HZ, ML, DX, XZ. Data analysis and interpretation: HZ, FZ, PL. Contributed reagents/materials/analysis tools: YL, PL, QW, YH. Manuscript preparation: HZ, FZ, DW.
This study was supported by grants from the National Science Technology Pillar Program in the 11th Five-Year Plan (2008BAI59B03) and the Research Special Fund for Public Welfare Industry of Health (201202004).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet 2013; 382:819–831. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. mAbs 2012; 4:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigal LH. Basic science for the clinician 58: IgG subclasses. J Clin Rheumatol 2012; 18:316–318. [DOI] [PubMed] [Google Scholar]
- 4.Kapur R, Einarsdottir HK, Vidarsson G. IgG-effector functions: “the good, the bad and the ugly”. Immunol Lett 2014; 160:139–144. [DOI] [PubMed] [Google Scholar]
- 5.Kapur R, Einarsdottir HK, Vidarsson G. IgG-effector functions: “The Good, The Bad and The Ugly”. Immunol Lett 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716–3725. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Yamamoto M, Suzuki C, et al. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev 2010; 9:591–594. [DOI] [PubMed] [Google Scholar]
- 8.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012; 22:21–30. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Tabeya T, Naishiro Y, et al. Value of serum IgG4 in the diagnosis of IgG4-related disease and in differentiation from rheumatic diseases and other diseases. Mod Rheumatol 2012; 22:419–425. [DOI] [PubMed] [Google Scholar]
- 10.Ebbo M, Grados A, Bernit E, et al. Pathologies Associated with Serum IgG4 Elevation. Int J Rheumatol 2012; 2012:602809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takahashi H, Suzuki C, et al. Analysis of serum IgG subclasses in Churg-Strauss syndrome - the meaning of elevated serum levels of IgG4. Intern Med 2010; 49:1365–1370. [DOI] [PubMed] [Google Scholar]
- 12.Blanco F, Kalsi J, Ravirajan CT, et al. IgG subclasses in systemic lupus erythematosus and other autoimmune rheumatic diseases. Lupus 1992; 1:391–399. [DOI] [PubMed] [Google Scholar]
- 13.Lin GG, Li JM. IgG subclass serum levels in systemic lupus erythematosus patients. Clin Rheumatol 2009; 28:1315–1318. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Li J. Preferentially immunoglobulin (IgG) subclasses production in primary Sjogren's syndrome patients. Clin Chem Lab Med 2012; 50:345–349. [DOI] [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria, Committee. Arthritis Rheum 1980; 23:581–590. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 18.Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology (Baltimore, MD) 2009; 50:291–308. [DOI] [PubMed] [Google Scholar]
- 19.Vlug A, Nieuwenhuys EJ, van Eijk RV, et al. Nephelometric measurements of human IgG subclasses and their reference ranges. Ann Biol Clin 1994; 52:561–567. [PubMed] [Google Scholar]
- 20.Pressac M, Allouche F, Circaud R, et al. Evaluation of human IgG subclass assays on Beckman array. Ann Clin Biochem 1995; 32 (Pt 3):281–288. [DOI] [PubMed] [Google Scholar]
- 21.Guma M, Firestein GS. IgG4-related diseases. Best Pract Res Clin Rheumatol 2012; 26:425–438. [DOI] [PubMed] [Google Scholar]
- 22.Lux A, Nimmerjahn F. Of mice and men: the need for humanized mouse models to study human IgG activity in vivo. J Clin Immunol 2013; 33 Suppl 1:S4–S8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Weetman AP, Jayne DR, et al. Anti-mitochondrial antibody IgG subclass distribution and affinity in primary biliary cirrhosis. Clin Exp Immunol 1992; 88:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelmann R, Brandt J, Eggert M, et al. IgG1 and IgG4 are the predominant subclasses among auto-antibodies against two citrullinated antigens in RA. Rheumatology 2008; 47:1489–1492. [DOI] [PubMed] [Google Scholar]
- 25.Alahlafi AM, Wordsworth P, Wojnarowska F. The distribution of IgG subclasses in the lupus band suggests disease-specific alteration in subclass switching rather than polyclonal B-cell activation. Clin Exp Dermatol 2004; 29:288–292. [DOI] [PubMed] [Google Scholar]
- 26.Kuroki A, Shibata T, Honda H, et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis,membranous lupus nephritis,and idiopathic membranous nephropathy. Intern Med (Tokyo, Japan) 2002; 41:936–942. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol 2010; 125 (2 Suppl 2):S41–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawasaki Y, Suzuki J, Sakai N, et al. Evaluation of T helper-1/-2 balance on the basis of IgG subclasses and serum cytokines in children with glomerulonephritis. Am J Kidney Dis 2004; 44:42–49. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson P, Andersson C, Ekerfelt C, et al. Relationship between serum levels of IL-18 and IgG1 in patients with primary Sjogren's syndrome, rheumatoid arthritis and healthy controls. Clin Exp Immunol 2004; 137:617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang KY, Kim HO, Kwok SK, et al. Impact of interleukin-21 in the pathogenesis of primary Sjogren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res Ther 2011; 13:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigopoulou EI, Davies ET, Bogdanos DP, et al. Antimitochondrial antibodies of immunoglobulin G3 subclass are associated with a more severe disease course in primary biliary cirrhosis. Liver Int 2007; 27:1226–1231. [DOI] [PubMed] [Google Scholar]
- 32.Rigopoulou EI, Davies ET, Pares A, et al. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut 2005; 54:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Q, Wu G, Hoschar A. IgG4-related Mikulicz's disease is a multiorgan lymphoproliferative disease distinct from Sjogren's syndrome: a Caucasian patient and literature review. Clin Exp Rheumatol 2013; 31:289–294. [PubMed] [Google Scholar]
- 34.Reddi DM, Cardona DM, Burchette JL, et al. Scleroderma and IgG4-related disease. Am J Dermatopathol 2013; 35:458–462. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan MM. Primary biliary cirrhosis. N Engl J Med 1996; 335:1570–1580. [DOI] [PubMed] [Google Scholar]
- 36.Mavragani CP, Fragoulis GE, Rontogianni D, et al. Elevated IgG4 serum levels among primary Sjogren's syndrome patients: do they unmask underlying IgG4-related disease? Arthritis Care Res 2014; 66:773–777. [DOI] [PubMed] [Google Scholar]


