Abstract
Currently, there are no robust models for predicting the outcome of acute-on-chronic hepatitis B liver failure (ACHBLF). We aimed to establish and validate a new prognostic scoring system, named ALPH-Q, that integrates electrocardiography parameters that may be used to predict short-term mortality of patients with ACHBLF.
Two hundred fourteen patients were included in this study. The APLH-Q score was constructed by Cox proportional hazard regression analysis and was validated in an independent patient cohort. The area under the receiver-operating characteristic curve was used to compare the performance of different models, including APLH-Q, Child–Pugh score (CPS), model of end-stage liver disease (MELD), and a previously reported logistic regression model (LRM).
The APLH-Q score was constructed with 5 independent risk factors, including age (HR = 1.034, 95% CI: 1.007–1.061), liver cirrhosis (HR = 2.753, 95% CI: 1.366–5.548), prothrombin time (HR = 1.031, 95% CI: 1.002–1.062), hepatic encephalopathy (HR = 2.703, 95% CI: 1.630–4.480), and QTc (HR = 1.008, 95% CI: 1.001–1.016). The performance of the ALPH-Q score was significantly better than that of MELD and CPS in both the training (0.896 vs 0.712, 0.896 vs 0.738, respectively, both P < 0.05) and validation cohorts (0.837 vs 0.689, 0.837 vs 0.585, respectively, both P < 0.05). Compared with LRM, APLH-Q also showed a better performance (0.896 vs 0.825, 0.837 vs 0.818, respectively).
We have developed a novel APLH-Q score with greater performance than CPS, MELD, and LRM for predicting short-term mortality of patients with ACHBLF.
INTRODUCTION
In recent years, acute-on-chronic liver failure (ACLF) has been increasingly recognized as a specific clinical entity that occurs in patients with acute deterioration of diagnosed or undiagnosed chronic liver disease.1,2 In China, due to the high prevalence of hepatitis B virus (HBV), acute-on-chronic hepatitis B liver failure (ACHBLF) accounts for the majority of ACLF.3 ACHBLF has a rapidly progressive course and may lead to a high short-term mortality.4,5 The most effective approved treatment for ACHBLF is liver transplantation.6,7 However, due to the limited number of liver donors, an optimized prognostic scoring index is urgently needed to evaluate the patient's condition so that transplantation can be performed on patients with the greatest need.
Currently, several known models, such as the Child–Pugh score (CPS) and the model for end-stage liver disease (MELD), were used to assess the severity of liver disease. Unfortunately, they were mainly established on the basis of alcoholic-abuse population, rather than the viral-infected population and consequently have a poor predictive accuracy for ACHBLF patients,8,9 which ranged from 0.601 to 0.760 for CPS and from 0.521 to 0.683 for MELD. To further increase the accuracy of prediction, we have established a logistic regression model (LRM), which was mainly based on available parameters, and it had shown better performance in predicting short-term mortality than existing scoring systems.10 Recently, the robustness of the LRM model has been further validated elsewhere.8,11
As is well known, the liver and the heart are intimately related in both health and disease states. Patients with chronic heart failure may have liver damage; conversely, patients with end-stage liver disease may have heart failure or electrophysiological abnormalities, even in those who do not have a history of cardiac disease.12,13 Recently, several electrophysiological abnormalities, such as chronotropic incompetence, electromechanical uncoupling, and electrocardiographic QT interval prolongation, have frequently been reported in the patients with liver disease, of which the QT prolongation interval is the most widely recognized.14 The QT interval on the electrocardiogram is an indirect measure of the ventricular electrical depolarization and repolarization and should be corrected for the heart rate (named QTc).15 Several studies had found that the presence of prolonged QTc might lead to a higher mortality in patients with liver diseases.6,16 The QTc may be an additional prognostic means to identify patients with high-mortality risk.17 Therefore, the establishment of an early accurate prediction of the prognosis combining with QTc in ACHBLF patients listed for liver transplantation is both necessary and feasible.
In this study, we integrated electrocardiography parameters including QTc with other clinical and biochemical variables to establish a new prognostic score, named APLH-Q score. We tested the accuracy of the APLH-Q score's prediction ability and compared it with existed scores in a prospective separated patient cohort.
MATERIALS AND METHODS
Study Protocol
We established and validated a clinical scoring system in two separate medical centers (training cohort: the First Affiliated Hospital of Wenzhou Medical University from March 2009 to December 2012; prospective validation cohort: Ningbo No. 2 Hospital from April 2012 to April 2013) with the same medical record systems.
The start date of the followup was the date of diagnosis of ACHBLF. All the patients were followed up for at least 3 months. An informed consent was obtained from each patient included in the study, and the research protocol of the study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and the Ningbo No. 2 Hospital. The study was performed according to Standards for the Reporting of Diagnostic Accuracy Studies.18
Patients Definition and Observation
ACLF was diagnosed according to the guidelines and recommendations of the Asian Pacific Association for the Study of the Liver in 2009.1 In brief, ACLF is defined as acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease. ACHBLF is defined as ACLF caused solely by hepatitis B virus. Patients who meet the following criteria were excluded: infected and/or coinfected with non-B hepatitis virus; alcohol abuse; autoimmune diseases; toxic or other causes that might lead to liver failure; past or current hepatocellular carcinoma; pregnancy; hematologic disorders; confirmed cardiovascular diseases and/or taking any agents contributing to a prolonged QTc interval, such as cordarone, β-blockers, quinolones; and liver transplantation, or serious diseases in other organ systems.
We took a detailed history of all the patients when they were in a hospital. Medical history was recorded upon admission. The basic characteristics of the patients were detected within the first 24 hours after the established diagnosis of ACHBLF. Physical examination, laboratory tests, and abdominal ultrasound scanning were performed. Meanwhile, a routine 12-lead electrocardiographic examination (ECG) made at a paper speed of 25 mm/s was performed for all the patients.
The care that was provided to the patients at both centers was the same and in accordance with the Asia-Pacific consensus recommendations.1 This routinely included antiviral treatment, absolute bed rest, energy supplements and vitamins, intravenous drop infusion albumin maintenance water, electrolyte and acid–base equilibrium, and prevention and treatment complications, etc.
Clinical Parameters
Clinical parameters included age, gender, body mass index, blood pressure, hepatic encephalopathy (HE), liver cirrhosis (LC), ascites, and hepatorenal syndrome (HRS). We reclassified the HE grade into 0: non-HE, 1: mild (grades 1–2), and 2: severe (grades 3–4) according to the West-Haven criteria.19 LC was defined by the following combined parameters: a score greater than 2 according to the aspartate aminotransferase (AST) to platelet ratio using the formula: [AST/upper limit of normal]/platelet count (×109/L) × 10020, ultrasonographic evidence of a small-sized liver with and without splenomegaly/ascites, and an albumin level less than 35 g/L without other identifiable causes of hypoalbuminemia such as renal loss or gastrointestinal loss. HRS was defined as the low glomerular filtration rate, as indicated by serum creatinine of >1.5 mg/dL or 24-hour creatinine clearance <40 mL/min, without the presence of chronic kidney diseases.21 The detection of ascites includes history, physical examination, abdominal ultrasound, and laboratory assessment of liver function, renal function, serum, and urine electrolytes. We reclassified ascites grade into 0: nonascites, 1: mild (grade 1), and 2: moderate to severe (grades 2–3).22 Also, the diagnosis of bacterial or fungal infection was based on infection-positive cultures of blood, ascites, urine or sputum, and/or clinical findings suggestive of infections.
Laboratory Parameters
Laboratory parameters included alanine aminotransferase (ALT), aspartate aminotranferase (AST), total bilirubin (TB), albumin (ALB), platelet count, hemoglobin (Hb), serum creatinine (Cr), prothrombin time (PT), prothrombin time activity (PTA), international normalized ratio (INR), serum sodium, and potassium. HBV serologic markers were collected for each patient (Abbott, AXSYM). Serum HBV DNA was measured by quantitative PCR assay (Roche Amplicor, limit of detectability of 100 IU/mL) after admission. Hepatitis C virus antibody and human immunodeficiency virus antibody were detected using ELISA (IEGAN, Freedom evolyzer/150). Antinuclear antibody (ANA) was evaluated using indirect immunofluorescence, and soluble liver antigen/liver pancreas antigen (SLA/LP), anti-liver/kidney microsomal antibody Type 1 (anti-LKM-1), and anti-liver cytosol antibody Type 1 (anti-LC-1) were evaluated using immunoblot analysis (Euroimmun, Lubeck, Germany).
Electrocardiography Parameters
All ECG data were reviewed visually, and any segments containing signal loss, noise, or extra-systole were discarded. The ECG parameters, including P-wave, PR interval, QRS interval, QT interval, and RR interval, were evaluated in Adobe Photoshop CS5 (Adobe Systems, San Jose, CA) with a scan version. Magnification of the ECG enabled a fine determination of the measurement points. The ECG traces were reviewed by two of the investigators (Zai-Xin Zheng and Ke-Qing Shi), who were blinded to the clinical parameters of the patients. The QT interval was also manually assessed and corrected according to the Bazett formula (QTc = QT interval/RR interval).15 QTc ≥ 440 milliseconds was classified as prolonged.
Scoring Systems and Prognostic Models
MELD scores were calculated according to the Malinchoc formula: R = 9.57 × ln(creatinine [mg/dL]) + 3.78 × ln(bilirubin [mg/dL]) + 11.2 × ln(INR) + 6.43 × (aetiology: 0 if cholestatic or alcoholic, 1 otherwise).23 CPS, which included HE, PT, ascites, total bilirubin, and serum albumin, was assessed according to the standard criteria.24 In addition, an LRM constructed by our group was also calculated: P = −1.343 + 0.772 × HE + 2.279 × HRS + 0.85 × LC + 1.026 × HBeAg − 2.117 × PTA/age.10
Construction of the ALPH-Q Score
For the training cohort, we performed univariate Cox proportional hazards regression analysis for determining the association of clinical and laboratory parameters with prognosis and survival time. Covariables with a P value of <0.01 as per univariate regression analyses were included in a forward-conditional step-wise Cox proportional hazards regression to identify independent predictors for the prognosis of the patients with ACHBLF. For this analysis, the conditional probabilities for stepwise entry and removal of a factor were 0.05 and 0.10, respectively. The hazard or instantaneous risk of death h(t) at time t after randomization for a patient with variables xl,. . .,xn has the form h(t) = h0(t) exp(b1x1 + b2x2 + … + bnxn).
A prognostic index (PI = b1x1 + b2x2 + … + bnxn) can be calculated for each patient on the basis of the final model. Higher values for PI mean worse prognosis, and lower values mean better prognosis.25 We then defined the PI as a new prognosis score, named ALPH-Q score, that was based on the final included five parameters (age, liver cirrhosis, PT, HE, and QTc).
To assess differences in prognosis efficiency between the ALPH-Q score and other models, area under the receiver-operating characteristic curve (auROC), which is a measure of discrimination, was calculated. The 3-month mortality was used to evaluate diagnostic performance of scoring systems for patients with ACHBLF in majority of studies26–28, in addition to this study. Furthermore, the standard index of validity, such as Youden index, sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value, and negative predictive value, was calculated according to the ROC results.
Statistical Analysis
The Kolmogorov–Smirnov test was applied to determine whether sample data were likely to be derived from a normal distribution population. Continuous variables of normal and skewed distributions are expressed as mean ± standard deviation (SD) and median (interquartile range), respectively. Categorical values were expressed by absolute and relative frequencies. Differences in variables were analyzed using Student t tests (for normally distributed data) or Wilcoxon signed rank test (for skewed distributed data). The chi-square test or the Fisher exact test was used for categorical data, as appropriate. For all the analyses, a P value of <0.05 was considered statistically significant. A statistical analysis was performed using SPSS version 20.0 (SPSS, Chicago, IL) and MedCalc version 12.7 (MedCalc Software, Ostend, Belgium).
RESULTS
Baseline Characteristics of the Study Sample
Three hundred eighty three patients with suspected ACLF were enrolled in the study. After exclusion of those who did not meet the inclusive criteria (Figure 1), 214 individuals (121 in the training cohort and 93 in the validation cohort) were finally included. As shown in Table 1, in the training cohort, the mean age was 43.3 ± 12.0 years, and the patients were predominantly men (79.3%), of which 42.1% patients (n = 51) died at the end of the followup. The most common complication of ACHBLF was ascites (71 patients, 58.7%). In the validation cohort, the mean age was 47.8 ± 13.5 years, and male was also predominant (68.8%), of which 32.3% patients (n = 30) died at the end of the followup. The training cohort had a higher mortality rate than that in the validation cohort, but not statistically significant (42.1% vs 32.3%, P = 0.139).
Figure 1.
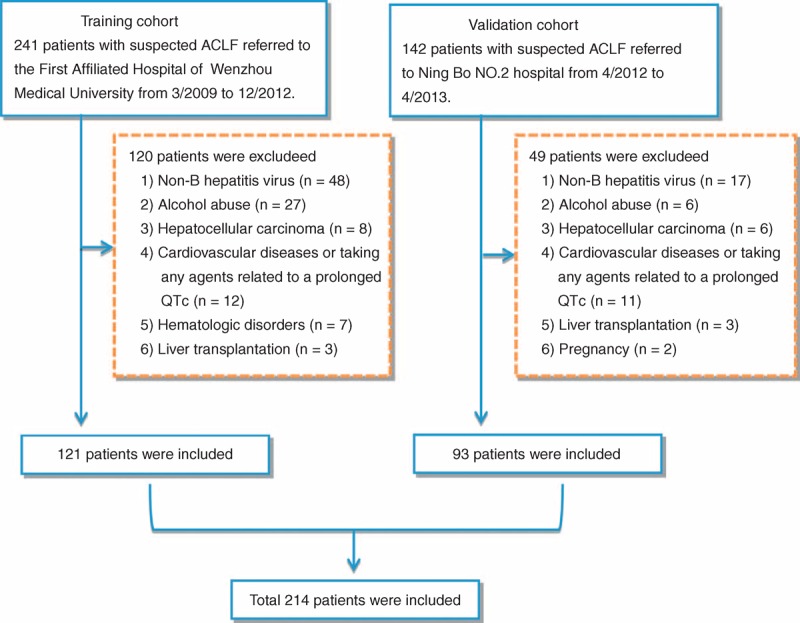
A flow diagram of study participants.
TABLE 1.
Characteristics of Patients With Acute-on-Chronic Hepatitis B Liver Failure, Stratified by Different Cohorts
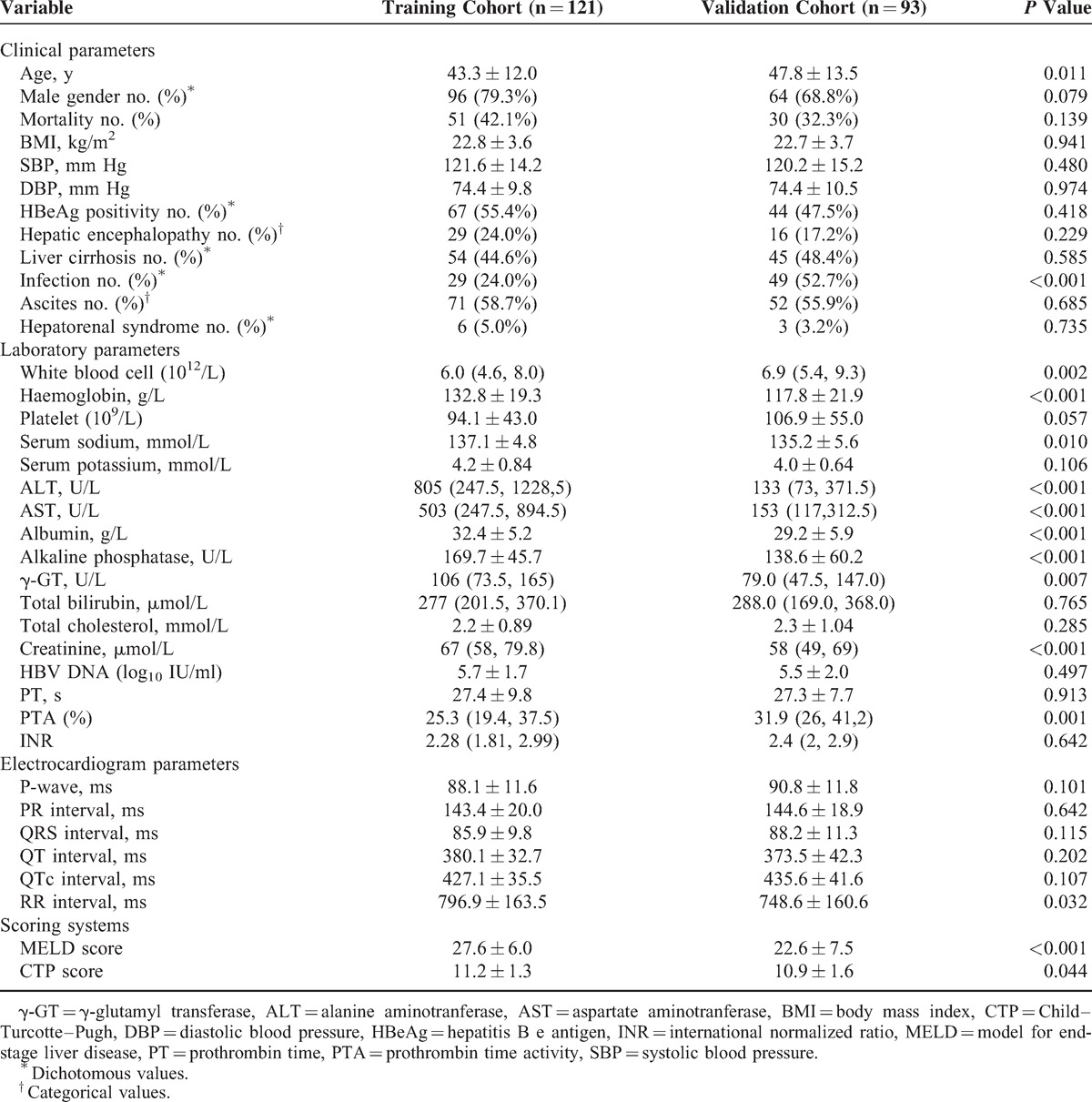
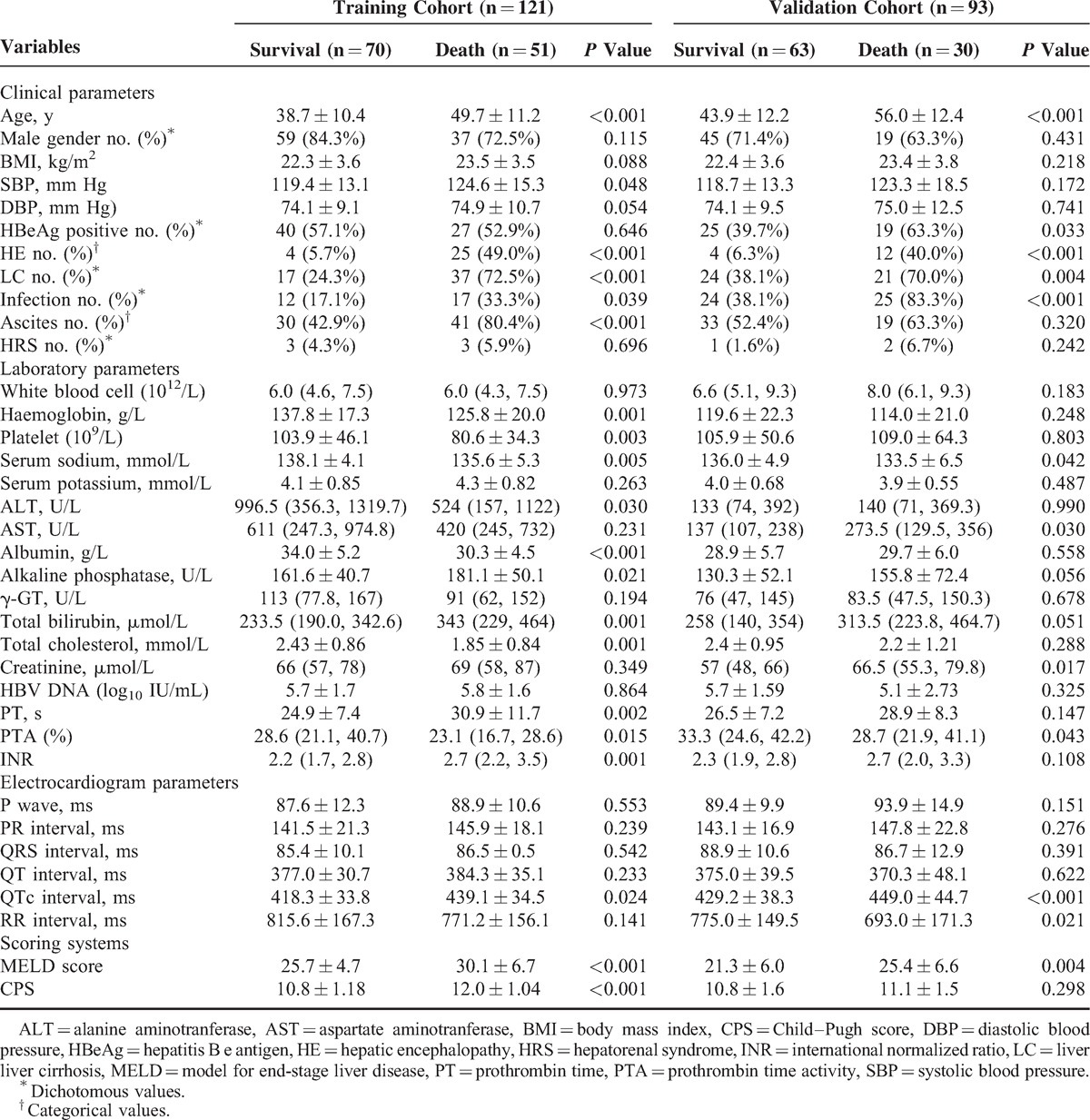
Table 2 shows that patients who survived had a lower MELD score (25.7 vs 30.1, P < 0.001), CPS (10.8 vs 12.0, P < 0.001), systolic blood pressure (SBP, 119.4 mm Hg vs 124.6 mm Hg, P = 0.048), HE (5.7% vs 49.0%, P < 0.001), LC (24.3% vs 72.5%, P < 0.001), infection (17.1% vs 33.3%, P = 0.039), and ascites (42.9% vs 80.4%, P < 0.001) in the training cohort. The patients’ conditions were confirmed in the validation cohort, except for CPS (11.1 vs 10.8, P = 0.298), systolic blood pressure (123.3 mm Hg vs 118.7 mm Hg, P = 0.172), and ascites (63.3% vs 52.4%, P = 0.320). We had also found that the QT interval was not statistically different in the survival group and the death group of both cohorts (377.0 milliseconds vs 384.3 milliseconds, P = 0.233; 375.0 milliseconds vs 370.3 milliseconds, P = 0.622), but it had a longer QTc in the death group (418.3 milliseconds vs 439.1 milliseconds, P = 0.024; 429.2 milliseconds vs 449.0 milliseconds, P < 0.001).
TABLE 2.
Characteristics of Patients With Acute-on-Chronic Hepatitis B Liver Failure, Stratified by Mortality
Construction of the ALPH-Q Score
Table 3 shows that age, SBP, HE, LC, infection, ascites, Hb, platelet, serum sodium, ALB, alkaline phosphatase, TB, total cholesterol, PT, PTA, INR, QTc, MELD score, and CPS were significantly associated with mortality in the training cohort (all P < 0.05).
TABLE 3.
Univariate Analysis of the Associations Between Mortality and Clinical and Biochemical Characteristics in Patients With Acute-on-Chronic Hepatitis B Liver Failure in Training Cohort
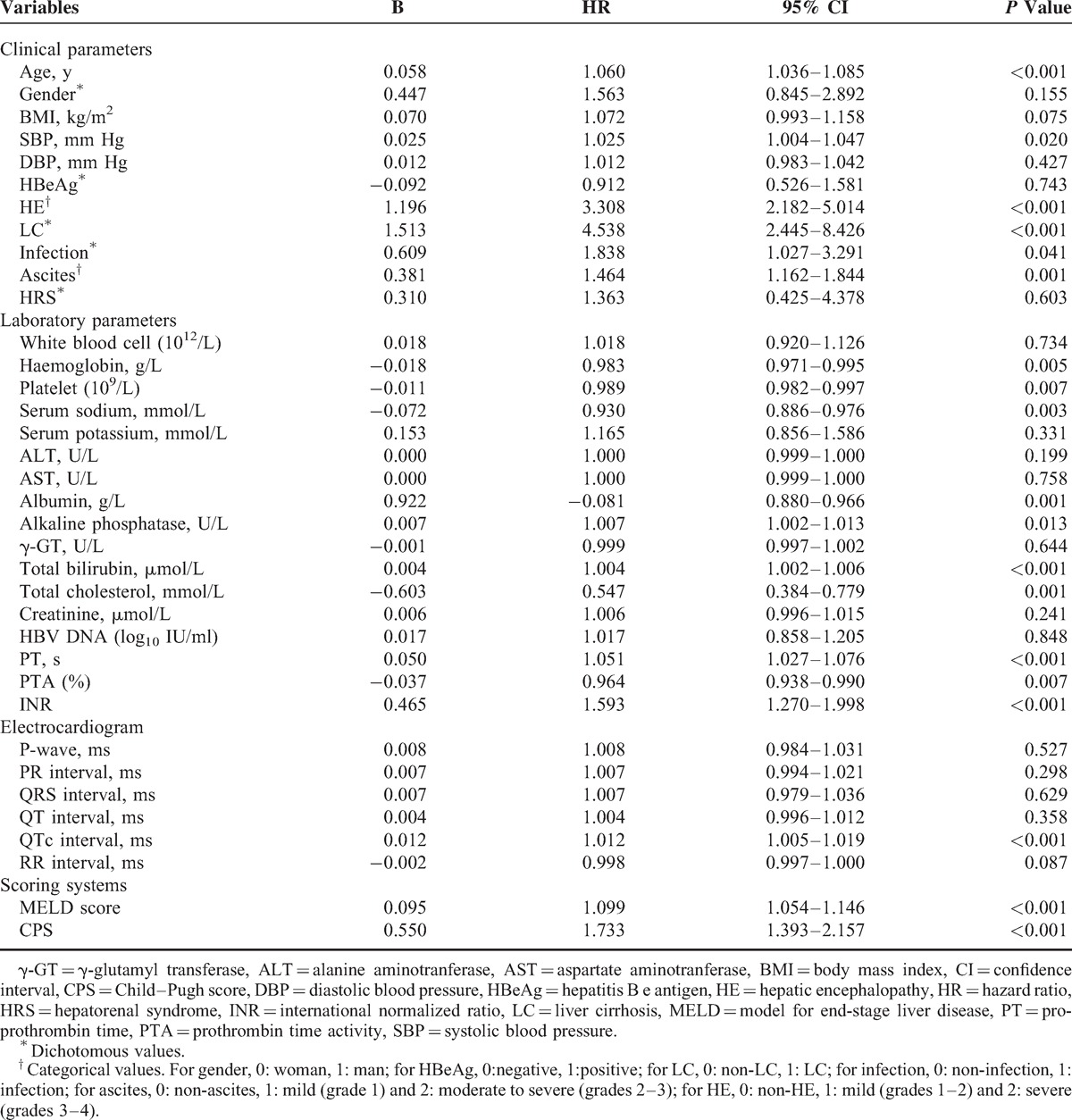
The above variables were entered into the multivariate Cox proportional hazards regression analyses. As Table 4 has presented, age (HR = 1.034, 95% CI: 1.007–1.061, P = 0.012), LC (HR = 2.753, 95% CI: 1.366–5.548, P = 0.005), PT (HR = 1.031, 95% CI: 1.002–1.062, P = 0.038), HE (HR = 2.703, 95% CI: 1.630–4.480, P < 0.001), and QTc (HR = 1.008, 95% CI: 1.001–1.016, P = 0.036) were found to be the independent risk factors. As shown in Figure 2, patients with age ≥45 years or LC or HE or PT ≥ 28 seconds or QTc ≥ 440 milliseconds had a poorer overall survival compared with patients who did not have the characteristics mentioned above.
TABLE 4.
Multivariate Analysis of the Associations Between Mortality and Clinical and Biochemical Characteristics in Patients With Acute-on-Chronic Hepatitis B Liver Failure in Training Cohort
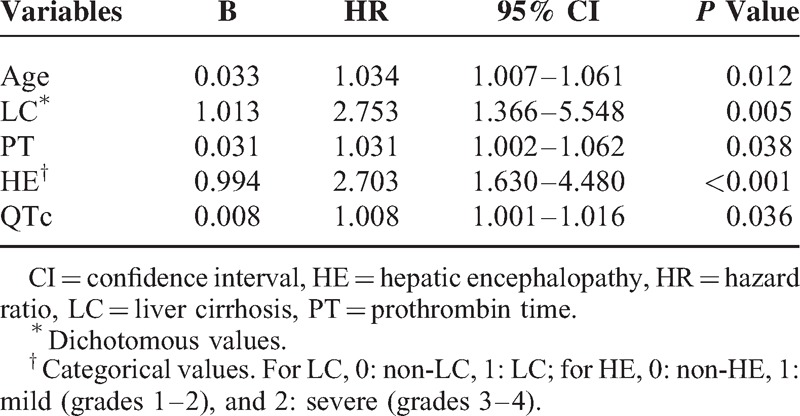
Figure 2.
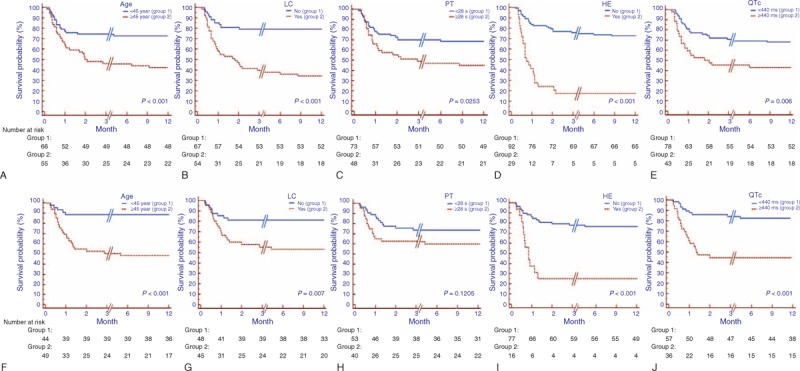
Kaplan–Meier survival curve stratified for patients under different status of age, LC, PT, HE, and QTc. (A) In the training cohort, a shorter overall survival existed in patients with age ≥45 years or liver cirrhosis or hepatic encephalopathy or PT ≥ 28 seconds or QTc ≥ 440 milliseconds (all P < 0.05). (B) In the validation cohort, the overall survival was the same as those in the training cohort, except that of those with PT < 28 seconds and ≥ 28 seconds (P = 0.121). HE = hepatic encephalopathy, LC = liver cirrhosis, PT = prothrombin time.
Finally, ALPH-Q score, a new prognostic score for ACHBLF patients, could be calculated by combining 5 independent risk factors with the regression coefficients. ALPH-Q score = 0.033 ∗ age + 1.013 ∗ LC + 0.031 ∗ PT + 0.994 ∗ HE + 0.008 ∗ QTc.
Performance of ALPH-Q Score in the Training Cohort
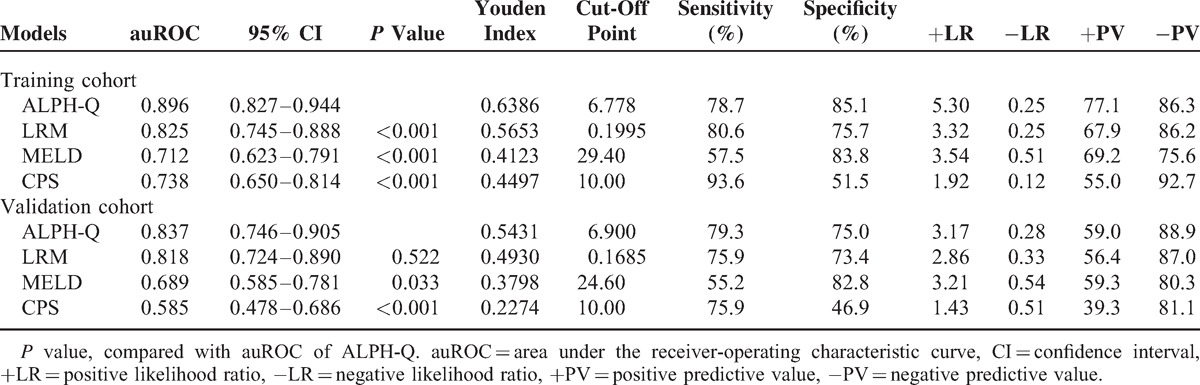
Figure 3A shows the ability of the ALPH-Q score to predict 3-months mortality risk in patients with ACBHLF. The performance of the new score was high, with an auROC of 0.896 (95% CI: 0.827–0.944). In the same dataset, LRM had an auROC of 0.825 (95% CI: 0.735–0.888), an MELD score of 0.712 (95% CI: 0.623–0.791), a CPS of 0.738 (95% CI: 0.650–0.814), significantly lower than that of the ALPH-Q score (all P < 0.001). When using a best cut-off value of 6.778 for the ALPH-Q score, the sensitivity was 78.7%, the specificity was 85.1%, the positive likelihood ratio was 5.3, the negative likelihood ratio was 0.25, and the positive predictive and negative predictive values were 77.1 and 86.3, respectively (Table 5).
Figure 3.
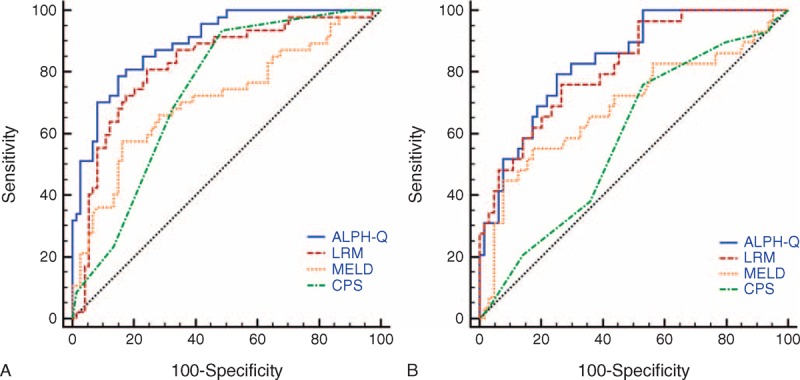
ROC analysis of the predictive accuracy of ALPH-Q score and other models to predict 3-mo mortality of acute-on-chronic hepatitis B liver failure in training cohort (A) and validation cohort (B).
TABLE 5.
Predictive Value of 3-Mo Mortality of the ALPH-Q Score and Other Models in the Training and Validation Cohorts
Performance of ALPH-Q Score in the Prospective Validation Cohort
As shown in Figure 3B, the auROC analysis was performed to test the robustness of the ALPH-Q score in the validation cohort. The auROC was 0.837 (95% CI: 0.746–0.905), which was greater than 0.7 and suggested that the new model had its utility. The auROC of LRM, MELD, and CPS scoring systems was 0.818 (95% CI: 0.724–0.890), 0.689 (95% CI: 0.585–0.781), and 0.585 (95% CI: 0.478–0.686), respectively. The performance of the ALPH-Q score was significantly better than that of MELD and CPS (P = 0.033, P < 0.001, respectively). No statistical difference was obtained between the ALPH-Q score and the LRM (P = 0.522); however, the ALPH-Q score tended to be better than those of LRM (Table 5).
DISCUSSION
Recently, several models have been constructed for the selection of patients for liver transplantation, of which the CPS and MELD are the most widely known. However, all these models were established among European and American populations, and not using data from patients with ACLF. The etiology of ACLF differs by geographic location. The causes of ACLF among European and American patients are typically alcohol consumption, hepatitis C, and cholestasis, whereas, with high HBV prevalence, ACHBLF accounts for the majority of ACLF in China. Thus, a new logistic regression model (LRM), specific to ACHBLF, was established by our previous data and shown to have a better performance than CPS and MELD scores. However, the LRM merely considered liver-relative parameters. Furthermore, as a logistic analysis, the LRM may lose the information of the survival time inescapability and may have an effect on the predicting prognosis of ACHBLF.
In this study, we established and validated an ALPH-Q score to predict the short-term prognosis of ACHBLF patients. To our knowledge, this is the first prognostic scoring system that has integrated a QTc parameter for the prediction of mortality in ACHBLF patients. On the basis of Cox proportional hazards regression analysis, the ALPH-Q score performed better than CPS, MELD, and LRM. In a prospective validation cohort, the improvement over CPS and MELD was 25.2% and 14.8%, respectively. No statistical difference was obtained between the ALPH-Q score and the LRM (P = 0.522); however, the ALPH-Q score tended to be better than those of LRM.
Among the ALPH-Q scores, five independent factors were found to be associated with survival among patients with ACHBLF, namely, age, liver cirrhosis, prothrombin time, hepatic encephalopathy, and QTc. Age was associated with the risk of mortality, with older patients having worse survival rates. This result was similar in previous reports.9,10 In this study, we found liver cirrhosis to be an independent risk factor. Cirrhosis represents the end stage of chronic liver disease, and although the definition of ACHBLF has no direct relation with liver cirrhosis, patients with underlying liver cirrhosis may have a increased morbidity, together with a poor life quality.29,30 Our new score showed that PT is a useful risk factor. The extent of PT is based on the levels of blood coagulation factors that are synthesized in the liver, and a prolonged PT may reflect the decline in liver synthetic capacity.31 Therefore, PT may become a common monitorable factor in patients with liver dysfunction. HE was a major clinical event in the natural history of cirrhosis that affects survival of patients. Some studies have indicated that HE correlates significantly with mortality in ACHBLF, particularly among patients with low MELD scores.9,10,32 Thus, this clinical event should be considered. In our new prognostic score, HE played an important role and was the only subjective indicator.
The mutual interaction between the liver and the heart has been established for some time. A large number of studies have proved that the QTc may play an important role in the prognosis of patients with liver diseases and could be a useful indicator for patients who require liver transplantation.12,17,33 On the basis of this study, we reconfirmed that QTc was a major predictive indicator on the prognosis of ACHBLF. Figure 3 shows that mortality among patients with prolongation of QTc interval was significantly higher than that of patients with normal QTc interval in the two cohorts (both P < 0.01). Our analysis was in accordance with a previous study34 that QTc prolongation in cirrhosis patient was correlated with the CPS.
Sudden deaths and ventricular arrhythmias had been reported to be related to the high-mortality risk with the prolongation of QTc;12,14 however, the pathophysiology of QTc prolongation in ACHBLF remained unclear. Previous studies showed that the increased plasma norepinephrine concentration, an activator of the sympathetic nervous system (SNS), might play an important role in a prolong QTc.14,34,35 However, other studies showed that there was no relation between QTc and SNS in these patients.36 Portal hypertension and porto-systemic shunts had to be present to be responsible,37,38 but this remains inconclusive.
There are, however, some limitations of this study. First, the ALPH-Q score was built on a single-center cohort and tested on another single-center cohort. It could be argued that data originating from other centers might lead to different conclusions. Thus, multicenter, prospective studies of larger populations with longer-term followup are needed. Furthermore, to exclude the effect of cardiovascular factors, patients with confirmed cardiovascular diseases or taking any agents affecting QTc interval before the establishment of ACHBLF were excluded, such as β-blockers, even if it is recommended in patients with portal hypertension associated with chronic liver diseases. A larger population and subgroup analysis for cardiovascular diseases should be needed in the further study. Finally, a dynamic analysis of ACHBLF in different stages is more important and meaningful than a single-point measurement.
In conclusion, we are the first to establish the new prognosis score that integrated QTc parameter, named the ALPH-Q score. The ALPH-Q score was superior to CPS, MELD, and LRM scoring systems in predicting short-term mortality risk in patients with ACHBLF and could be an ideal prognostic score to determine the order of these patients undergoing liver transplantation.
Footnotes
Abbreviations: ACHBLF = acute-on-chronic hepatitis B liver failure, ACLF = acute-on-chronic liver failure, ALT = alanine aminotranferase, AST = aspartate aminotranferase, auROC = area under the receiver-operating characteristic curve, BMI = body mass index, CI = confidence interval, CPS Child–Pugh = score, DBP = diastolic blood pressure, ECG = electrocardiogram, HbeAg = hepatitis B e antigen, HBV = hepatitis B virus, HE = hepatic encephalopathy, HR = hazard ratio, HRS = hepatorenal syndrome, INR = international normalized ratio, LC = liver cirrhosis, LRM = logistic regression model, MELD = model of end-stage liver disease, PT = prothrombin time, PTA = prothrombin time activity, QTc = QT interval corrected for the heart rate, γ-GT = γ-glutamyl transferase, SBP = systolic blood pressure, SD = standard deviation, +PV = positive predictive value, −PV = negative predictive value, +LR = positive likelihood ratio, −LR = negative likelihood ratio.
S-JW and H-DY are co-first authors.
S-JW, H-DY, Z-XZ, Y-YX, W-JH, and Y-PC contributed to data collection. S-JW, F-LW, B-ZY, and W-JH contributed to data analysis. S-JW and H-DY drafted the manuscript. K-QS, M-HZ, F-LW, and Y-CF contributed to the study design. K-QS, W-JH, and M-HZ helped to draft the manuscript. Y-CF contributed to statistical analysis. Y-PC and M-HZ conceived the study. M-HZ was responsible for study supervision and obtaining funding.
This work was supported by grants from the Scientific Research Foundation of Wenzhou, Zhejiang Province, China (H20090014, Y20090269), Health Bureau of Zhejiang Province (2010KYB070), Research Foundation of Education Bureau of Zhejiang Province (Y201009942), Fresh Talent Program for Science and Technology, Department of Zhejiang Province (2013R413018, 2013R413035, and 2013R413015), Research Funds for Tian Qing Liver Diseases (TQGB20120057), and Project of New Century 551 Talent Nurturing in Wenzhou.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vizzutti F, Arena U, Laffi G, et al. Acute on chronic liver failure: from pathophysiology to clinical management. Trends Anaesth Crit Care 2013; 3:122–129. [Google Scholar]
- 3.Liu J, Fan D. Hepatitis B in China. Lancet 2007; 369:1582–1583. [DOI] [PubMed] [Google Scholar]
- 4.Seto W-K, Lai C-L, Yuen M-F. Acute-on-chronic liver failure in chronic hepatitis B. J Gastroenterol Hepatol 2012; 27:662–669. [DOI] [PubMed] [Google Scholar]
- 5.Aguirre Valadez J, Torre Delgadillo A, Chavez Tapia N, et al. Acute-on-chronic liver failure: a review. Therapeutics Clin Risk Manage 2014; 10:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohamed R, Forsey PR, Davies MK, et al. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology 1996; 23:1128–1134. [DOI] [PubMed] [Google Scholar]
- 7.Chan AC, Fan ST, Lo CM, et al. Liver transplantation for acute-on-chronic liver failure. Hepatol Int 2009; 3:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu FL, Shi KQ, Chen YP, et al. Scoring systems predict the prognosis of acute-on-chronic hepatitis B liver failure: an evidence-based review. Expert Rev Gastroenterol Hepatol 2014; 8:623–632. [DOI] [PubMed] [Google Scholar]
- 9.Xia Q, Dai X, Zhang Y, et al. A modified MELD model for Chinese pre-ACLF and ACLF patients and it reveals poor prognosis in pre-ACLF patients. PLoS ONE 2013; 8:e96437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng MH, Shi KQ, Fan YC, et al. A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol 2011; 9:351–356. [DOI] [PubMed] [Google Scholar]
- 11.Yang WB, Chen EQ, Bi HX, et al. Different models in predicting the short-term prognosis of patients with hepatitis B virus-related acute-on-chronic liver failure. Ann Hepatol 2012; 11:311–319. [PubMed] [Google Scholar]
- 12.Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J 2013; 34:2804–2811. [DOI] [PubMed] [Google Scholar]
- 13.Reddy KR. Pere G, Kamath P S. Chronic liver failure: mechanisms management. Vicente Arroyo, vol. 142. Totowa, NJ: Humana Press; 2011. 183.ISBN: 978-1-60761-865-2. Gastroenterology. 2012. [Google Scholar]
- 14.Zambruni A, Trevisani F, Caraceni P, et al. Cardiac electrophysiological abnormalities in patients with cirrhosis. J Hepatology 2006; 44:994–1002. [DOI] [PubMed] [Google Scholar]
- 15.Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol 1997; 2:177–194. [Google Scholar]
- 16.Finucci G, Lunardi F, Sacerdoti D, et al. Q-T interval prolongation in liver cirrhosis reversibility after orthotopic liver transplantation. Jpn Heart J 1998; 39:321–329. [DOI] [PubMed] [Google Scholar]
- 17.Kosar F, Ates F, Sahin I, et al. QT interval analysis in patients with chronic liver disease: a prospective study. Angiology 2007; 58:218–224. [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003; 326:41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy: definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002; 35:716–721. [DOI] [PubMed] [Google Scholar]
- 20.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38:518–526. [DOI] [PubMed] [Google Scholar]
- 21.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Int Ascites Club Hepatol 1996; 23:164–176. [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatology 2010; 53:397–417. [DOI] [PubMed] [Google Scholar]
- 23.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31:864–871. [DOI] [PubMed] [Google Scholar]
- 24.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 25.Schlichting P, Christensen E, Andersen PK, et al. Prognostic factors in cirrhosis identified by Cox's regression model. Hepatology 1983; 3:889–895. [DOI] [PubMed] [Google Scholar]
- 26.Wang FS, Zhang Z. Liver: how can acute-on-chronic liver failure be accurately identified? Nature reviews. Gastroenterol Hepatology 2013; 10:390–391. [DOI] [PubMed] [Google Scholar]
- 27.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–1437. [DOI] [PubMed] [Google Scholar]
- 28.Kribben A, Gerken G, Haag S, et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012; 142:782–789. [DOI] [PubMed] [Google Scholar]
- 29.Sun QF, Ding JG, Xu DZ, et al. Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat 2009; 16:464–470. [DOI] [PubMed] [Google Scholar]
- 30.Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol 2013; 19:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripodi A, Caldwell SH, Hoffman M, et al. Review article: the prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Alimentary Pharmacol Therapeutics 2007; 26:141–148. [DOI] [PubMed] [Google Scholar]
- 32.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004; 40:802–810. [DOI] [PubMed] [Google Scholar]
- 33.Zahmatkeshan M, Fallahzadeh E, Najib SS, et al. The relationship between QT interval dispersion and end-stage liver disease score in the patients undergoing orthotopic liver transplantation. Int Cardiovasc Res J 2013; 7:135–140. [PMC free article] [PubMed] [Google Scholar]
- 34.Bernardi M, Calandra S, Colantoni A, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998; 27:28–34. [DOI] [PubMed] [Google Scholar]
- 35.Thuluvath PJ, Triger DR. Autonomic neuropathy and chronic liver disease. Q J Med 1989; 72:737–747. [PubMed] [Google Scholar]
- 36.Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomic cardiovascular reflexes in patients with cirrhosis. J Hepatol 2001; 35:733–738. [DOI] [PubMed] [Google Scholar]
- 37.Trevisani F, Merli M, Savelli F, et al. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol 2003; 38:461–467. [DOI] [PubMed] [Google Scholar]
- 38.Ytting H, Henriksen JH, Fuglsang S, et al. Prolonged Q-T(c) interval in mild portal hypertensive cirrhosis. J Hepatol 2005; 43:637–644. [DOI] [PubMed] [Google Scholar]


