Abstract
Preclinical studies have shown synergism between epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors and antifolates in solid tumors. This study is to investigate the efficacy and tolerability of erlotinib plus capecitabine as first-line treatment in older Chinese patients (≥ 65 years) with lung adenocarcinoma.
This is an open-label, single arm, multicenter phase II clinical trial. Sixty- two patients with previously untreated stage IIIB/IV adenocarcinoma and age 65 years or above were enrolled at four tertiary teaching hospitals and 2 provincial hospitals in China; 58 patients fulfilled the study requirements. Erlotinib (150 mg/day) and capecitabine (1000 mg/m2 twice daily on days 1–14) were administered during every 21-day cycle. The primary endpoint was the non-progression rate at 12 weeks. EGFR and K-ras mutation rates were determined using PCR. Tumor expression of different biomarkers was assessed using immunohistochemistry.
In a cohort of 58 patients, 34 patients had no disease progression at 12 weeks following treatment. The objective response rate was 29.3%, and the disease control rate was 75.9%. The objective response rate was significantly higher in patients with EGFR mutations than in those with wild-type EGFR. Patients with thymidine phosphorylase-negative tumors had significantly longer overall survival after one year than patients with thymidine phosphorylase-positive tumors. Forty-four patients had at least one primary adverse events (AEs), including skin rash (n = 30), grade 3 AEs (n = 17), and grade 4 AEs (n = 7).
This is the first phase II clinical trial to assess erlotinib plus capecitabine combination therapy as first-line treatment in older patients with lung adenocarcinoma. Erlotinib/capecitabine chemotherapy was significantly better in patients with EGFR mutations and in those with thymidine phosphorylase-negative tumors. The use of fluorouracil derivatives for the treatment of lung adenocarcinoma warrants further study.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths in China, with non–small-cell lung cancer (NSCLC) accounting for approximately 85% of all lung cancers.1,2 More than 50% of NSCLC patients with advanced disease are over 65 years of age.3 Unfortunately, older patients have more co-morbidities and tend not to tolerate aggressive chemotherapy and radiotherapy as well as younger patients.4 Despite the large burden imposed on the older population by NSCLC, older patients are often excluded from participation in clinical NSCLC drug trials. As a result, they often receive treatments that are untested or inadequately tested for use in their specific population.
Since many drugs are more toxic in older patients,5–7 single-agent chemotherapy has been recommended as the standard first-line treatment for unfit (performance status (PS) ≥2) older patients.8 However, advanced age alone should not preclude appropriate NSCLC treatment.9,10 In patients with PS ≤ 2, the objective response rate (ORR), toxicity, and survival of patients receiving platinum-based treatments are similar to those in younger patients, although patients 70 years old or older have more comorbidities and can expect more leukopenia and neuropsychiatric toxicity. Newer agents that target malignancies more effectively, without toxicity, are thus of interest for the treatment of NSCLC in older patients. Such agents include antifolates and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors.
Pemetrexed is an antifolate agent that has been indicated for use in first- and second-line treatment of NSCLC in non-squamous cell carcinoma.11,12 Oral capecitabine, another antifolate agent, has demonstrated consistent and impressive activity in patients with chemo-naive or pretreated advanced breast cancer13–15 and advanced colorectal cancer.16,17 Capecitabine is relatively well tolerated; as with other antifolate agents, its mechanism of action involves disruption purine and pyrimidine synthesis. Capecitabine is a prodrug that is converted to its active form, 5-fluorouracil (5-FU), by thymidine phosphorylase. Because tumor cells have higher concentrations of thymidine phosphorylase than do normal cells, capecitabine is selectively activated in tumors.18,19 5-FU then acts on cells by inhibiting the activity of thymidylate synthase,20 resulting in the disruption of RNA and DNA synthesis. Other important enzymes involved in the metabolism of 5-FU include dihydropyrimidine dehydrogenase21 and orotate phosphoribosyl transferase (OPRT), a constitutively expressed enzyme that regulates pyrimidine nucleotide biosynthesis. These 4 enzymes (thymidine phosphorylase, thymidylate synthase, dihydropyrimidine dehydrogenase, and OPRT) play an important role in 5-FU metabolism and may affect individual responses to oral fluoropyrimidines, including capecitabine.22
Targeted therapies such as the EGFR tyrosine-kinase–inhibitor erlotinib are generally less toxic than conventional cytotoxic agents, rendering them useful in combination regimens. Preclinical studies have shown that the cytotoxic effects of capecitabine are potentiated by synchronous administration of erlotinib through increased apoptosis in epithelium-derived human tumor models. Erlotinib treatment has been shown to affect thymidine phosphorylase expression in colorectal cancer tumor models.23 In patients with pancreatic cancer, the XELTA study demonstrated that capecitabine/erlotinib combination therapy is well tolerated using the same treatment regimen as in the present study.24
The primary aim of this study (ClinicalTrials.gov Identifier: NCT00816868) was to evaluate the clinical efficacy and toxicity of capecitabine combined with erlotinib in older patients with stage IV or stage IIIB (not suitable for radical radiotherapy) lung adenocarcinoma. Our secondary aim was to evaluate the molecular biomarkers involved in cellular responses to capecitabine and erlotinib to determine whether they are associated with tumor responses and clinical outcomes.
METHODS
Patient Selection
This study was an open-label, single arm, multicentre, phase II trial. Patients over 65 years of age who had histologic or cytologic evidence of measurable metastatic or stage IIIB (not suitable for radical radiotherapy) adenocarcinoma NSCLC were eligible for this study. The eligibility criteria also included the following: naive to lung cancer treatment (including surgery, radiotherapy, systemic chemotherapy, or targeted therapy); Eastern Cooperative Oncology Group performance status ≤2; estimated life expectancy of at least 12 weeks; adequate bone marrow (platelets ≥ 75,000/μL, absolute neutrophil count ≥1500/μL, and hemoglobin ≥10 g/dL); and normal hepatic function (total bilirubin, ≤1.5× the upper limit of normal; aspartate aminotransferase (AST), ≤2.5× normal; and alkaline phosphatase, ≤2.5× normal). Patients who experienced any of the following conditions were excluded: malabsorption syndromes, inability to take oral medication, active peptic ulcer, renal disease, significant ophthalmologic abnormality (especially severe dry eye syndrome, keratoconjunctivitis sicca), newly diagnosed central nervous system metastasis (previously diagnosed and treated central nervous system metastases or spinal cord compression with evidence of stable disease for at least 2 months is permitted), or unstable systemic disease (including active infection, grade 4 hypertension, unstable angina, congestive heart failure, hepatic, and metabolic disease). Criteria for exit from the study or termination of treatment were as follows: (1) Serious adverse clinical events or laboratory tests; in such cases, we stopped the study treatment and took appropriate treatment measures. (2) The need for other medical treatment that might affect the ongoing study treatment; in such cases, we immediately discontinued the study treatment and began the new treatment. (3) Confirmation that a patient disease had progressed. (4) Failure of the subject to meet the inclusion criteria after starting the study. (5) Failure of the subject to obey program instructions.
The protocol was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center at every study site, and all patients were required to provide written informed consent in accordance with the State Food and Drug Administration (SFDA) and institutional guidelines. The completion date of this study is March 2011. After April 2011, we stopped collecting all data. This study has been registered with clinicaltrials.gov (NCT00816868) and with Chinese Thoracic ONcology Group (C-TONG 0807).
Treatment Schedule
All patients received capecitabine (Xeloda; Hoffmann-La Roche, Nutley, NJ) combined with erlotinib (Tarceva; Hoffmann-La Roche). Both drugs in this study were provided free of charge by Shanghai Roche Pharmaceuticals Ltd., Shanghai, P.R.China (ML22206 study). Drug regimens were based on 3-week treatment cycles. Capecitabine (1000 mg/m2) was administered orally twice daily for 14 days followed by 7 days off. Erlotinib (150 mg/day) was administered orally, starting on the same day as capecitabine and continuing for the full 3 weeks of every cycle. The maximum number of cycles was until progression of disease (PD), defined as the emergence of new lesions, an increase in primary lesion > 25%, or the minimum treatment period until the primary lesion increased > 50%.
Patients received both medications until disease progression, unacceptable toxicity, patient refusal, alternate treatment, or the investigator's decision to remove the patient from the study.
Clinical Care of Patients
Complete patient histories and standard laboratory tests including chemistry and hematology were performed at baseline and before the beginning of each treatment cycle. Other safety measures included assessment of physical condition and monitoring of preexisting conditions and adverse events. Patients were assessed before each cycle using the common terminology criteria for adverse events (CTC AE) scale (version 3.0, NCI 2003). Computed tomography (CT), including spiral CT scans, and magnetic resonance imaging (MRI) were performed at baseline and after every 2 cycles of therapy to assess tumor response according to the response evaluation criteria in solid tumors (RECIST).25 Any patient who required a dose reduction continued to receive a reduced dose for the remainder of the study. For any patient who had 2 dose reductions and experienced toxicity that could cause a third dose reduction, follow-up was stopped, and survival was censored at that date. All adverse events occurring after enrolment were followed until the event was resolved or explained. The protocol allowed patients to receive full supportive care therapies concomitantly during the study but no other anticancer therapy, immunotherapy, radiation, surgery for cancer, or experimental medications. Criteria for removal from the study included grade 4 drug-related toxicity requiring a treatment delay ≥2 weeks in duration, progressive disease, withdrawal of consent, non-compliance with study procedures, or a change in the patient's condition requiring other therapy that made further study treatment inappropriate. Once the patient was objectively assessed as having disease progression, follow-up examinations were conducted approximately every 90 days until death or study closure.
The full analysis set (FAS) and per-protocol set (PPS) were further applied if patients had treatment but did not meet both criteria. The FAS is defined as the group of patients who used the test drug and underwent a tumor response assessment at least once after treatment. The PPS is defined as the group of patients in the FAS who also completed treatment without serious breach (such as contraband drug use) and good drug compliance.
Biomarker Studies
Microdissection of Primary Tumors
Sections from formalin-fixed, paraffin-embedded blocks containing 62 NSCLC were obtained from the Cancer Centre of Sun-Yat Sen University, Cancer Hospital of Ha’er bing Medical University, Jiangsu Province Cancer Hospital, Jilin Province Cancer hospital, Zhejiang Province Cancer Hospital, Cancer Hospital of Guangxi Medical University.
EGFR Mutation Analysis
Real-time PCR was performed using 10 ng genomic DNA, which was extracted from specimens, in the presence of 5 μL 10× buffer (160 mM (NH4) 2SO4, 670 mM Tris–HCl (pH 8.8), and 0.1% Tween 20), 10 μL MgCl2 (25 mM), 1 μL of each deoxynucleoside triphosphate (25 mM), 1 μL of primers and probes (50 pM), and 1.0 U Taq enzyme. The PCR cycling conditions were 94 °C for 5 minutes; 15 cycles of 95 °C for 25 seconds, 64 °C for 20 seconds, and 72 °C for 20 seconds; 31 cycles of 93 °C for 25 seconds, 60 °C for 35 seconds, and 72 °C for 20 seconds (fluorescence reading). In all, 29 EGFR mutations were analyzed: 19 deletions between 2235 and 2257 in exon 19 (T790M, L858R, G719A, G719S, G719C, S768I, and L861Q), and 3 insertions in exon 20. Mutations were detected using the AmoyDx EGFR 29 Mutations Detection Kit (Amoy Diagnostics Co., Ltd; Xiamen, Fujian, China). In all, K-ras 7 mutations were analyzed: Gly12Ser, Gly12Arg, Gly12Cys, Gly12Asp, Gly12Ala, Gly12Val, and Gly13Asp. Mutations were detected using the AmoyDx K-ras 7 Mutations Detection Kit (Amoy Diagnositcs Co., Ltd).
Immunohistochemical Staining and Scoring for Thymidylate Synthase, Thymidine Phosphorylase, Dihydropyrimidine Dehydrogenase, and Orotate Phosphoribosyl Transferase Protein Expression
Five unstained, formalin-fixed, paraffin-embedded slides were subjected to haemotoxylin & eosin (H&E) stain and immunohistochemical (IHC) staining for thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase, and OPRT. The slides were subsequently dewaxed and rehydrated using xylene and graded alcohol washes. IHC staining was carried out using the following antibodies: mouse monoclonal thymidylate synthase (Millipore, Temecula, CA), mouse monoclonal dihydropyrimidine dehydrogenase (Abcam, Cambridge, MA), mouse monoclonal thymidine phosphorylase (Abcam) and rabbit polyclonal OPRT (Proteintech, Chicago, IL). The manufacturer's instructions were followed in each case, except that antigen retrieval for all 4 proteins comprised incubation for 20 minutes at 98 °C in a T/T Mega microwave oven (Milestone, Sorisole, Italy).
All of the immunostained slides were reviewed by 2 pathologists who had no knowledge of the clinical status of the patients. In cases of multiple areas of low intensity staining, 5 areas selected at random were scored. In sections where all of the staining appeared to be intense, 1 random field was selected. At least 200 tumor cells were scored per 40× field. All slides were scored in a semi-quantitative manner according to a previously described method, which reflects both the intensity and percentage of cells staining at each intensity (McCarthy et al26). Intensity was classified as 0 (no staining), t1 (weak staining), t2 (moderate staining) and t3 (strong staining). A value designated as the “HSCORE” was obtained for each slide by using the following algorithm: HSCORE = Σ(I × PC), where “I” and “PC” represent the staining intensity and the percentage of cells that stain at each intensity, respectively. The corresponding HSCOREs were calculated separately. A sample was classified as thymidylate synthase-positive and OPRT-positive if the HSCORE was ≥30, as reported previously.27 A sample was classified as dihydropyrimidine dehydrogenase-positive if the HSCORE for OPRT was ≥50, as reported previously.26 A sample was classified as thymidine phosphorylase-positive if the HSCORE for thymidine phosphorylase in that specimen was ≥60, as a cut-off line of 60 showed the most significant survival difference.
Statistical Methods
Primary endpoints were the 12-week non-progression rate of the combination of capecitabine and erlotinib as first-line treatment among elder patients with advanced adenocarcinoma (stage IIIB or stage IV). Second endpoints included objective response, toxicity, progression free survival (PFS), and overall survival (OS). A one-stage Fleming design28 with an exact significance level of P = 0.05 and power of 80% was used to test the hypothesis that the true success rate (non-progression-free rate at 12 weeks and on treatment) was at most 50% versus the alternative hypothesis that the true success rate is at least 66%. A study requires 60 subjects to decide whether the proportion responding, P, is ≤0.500 or ≥0.660. If the number of responses was 37 or more, the hypothesis that P ≤0.500 was rejected with a target error rate of 0.050, and an actual error rate of 0.046. If the number of responses was 36 or less, the hypothesis that P ≥0.660 was rejected with a target error rate of 0.200, and an actual error rate of 0.198. With a sample size of 58 evaluable patients, the regimen would be declared promising if at least 36 successes were observed.
The ORR was defined as the sum of cases with a complete response (CR) plus those with a partial response (PR) that was evaluated at least 6 weeks after the initiation of study drug therapy. Stable disease (SD) was defined as no significant change in the lesion, an increase in lesion size <25%, or a decrease of in lesion size of <50%. The disease control rate (DCR) was defined as the sum of CR + PR + SD. OS time was measured from the date of registration to the date of death from any cause. PFS was measured from the date of registration to the first date of objective progression of the disease or of death from any cause. Patients who had not progressed or had died by the time of the analysis were censored at the date of last contact. OS and PFS were calculated using the Kaplan–Meier method, and the differences between the survival curves were examined by the log-rank test. Comparability between the 2 groups was determined using Chi-square/Fisher's exact test. Results are presented with 95% confidence intervals (CIs) and P values. All statistical assessments were 2 sided and evaluated at the 0.05 level of significance. Statistic analyses were performed using SPSS 15.0 statistics software (SPSS Inc., Chicago, IL).
RESULTS
From February 2009 to September 2009, 62 NSCLC patients were enrolled, 4 of whom could not be assessed (one withdrawal of informed consent and 3 deaths within 30 days after treatment). A flow diagram of patient participation in the study is shown in Figure 1.
FIGURE 1.
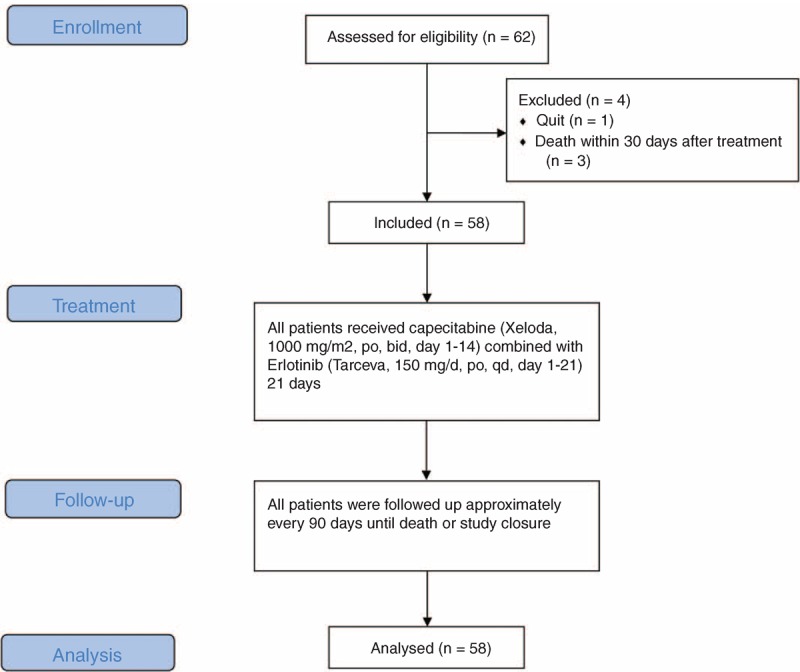
Flow diagram of patient participation.
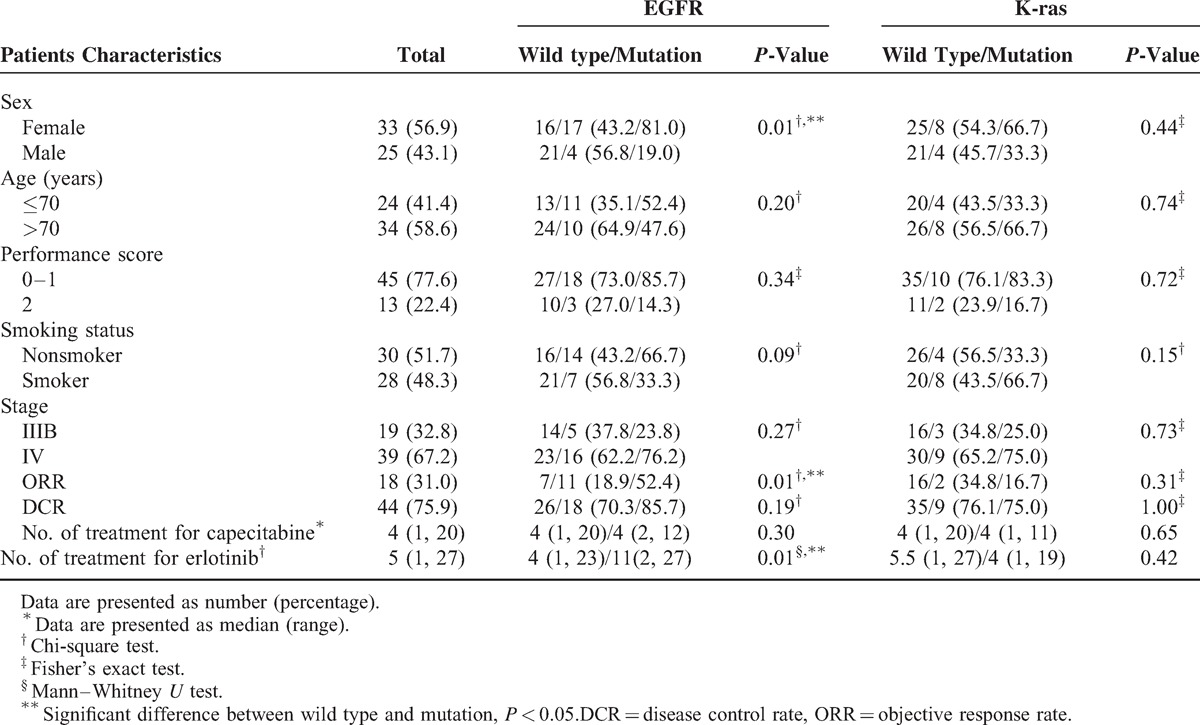
The baseline characteristics of the included 58 patients are listed in Table 1. The median age was 72 years (range, 65–82 years), and 77.6% of the patients had a performance status of 0 or 1 at baseline, 56.9% were female, 48.3 % were smokers, 67.2% had stage IV disease, and all had adenocarcinoma. At the time of this analysis, 86.2 % of patients were off active treatment. The median follow-up time for surviving patients was 16.1 months (range, 1–21.9 months). The median number of treatment cycles received for capecitabine was 4 (range, 1–20 cycles) and for erlotinib was 5 (range, 1–27 cycles). Reductions in the dose of capecitabine and erlotinib were reported in 15.5% and 3.4% of patients, respectively. Forty-three patients (74%) stopped the treatment because of disease progression.
TABLE 1.
Comparison of Baseline Patient Clinical Characteristics for EGFR and K-ras Status (n = 58)
Of the 58 patients, 32 (56%; 95% CI, 42–68%) met the protocol-defined criteria for success (non-progression and receiving treatment at 12 weeks). In addition, 2 patients went off treatment at 12 weeks with a diagnosis of non-progression. Thus, a total of 34 patients were without progression and on treatment at 12 weeks (59%; 95% CI, 46–71%). In the cohort of 58 patients, the ORR was 31% (18 confirmed PRs; 95% CI, 19–43%), the DCR was 75.9% (18 confirmed PRs and 26 patients with SD for ≥6 weeks; 95% CI, 65–87%). The median PFS was 4.13 months (95% CI, 1.9–6.4 months), and over 50% of the patients survived. The secondary endpoints 1-year OS and PFS were 69.5% and 24.5%, respectively (Figure 2A and B).
FIGURE 2.
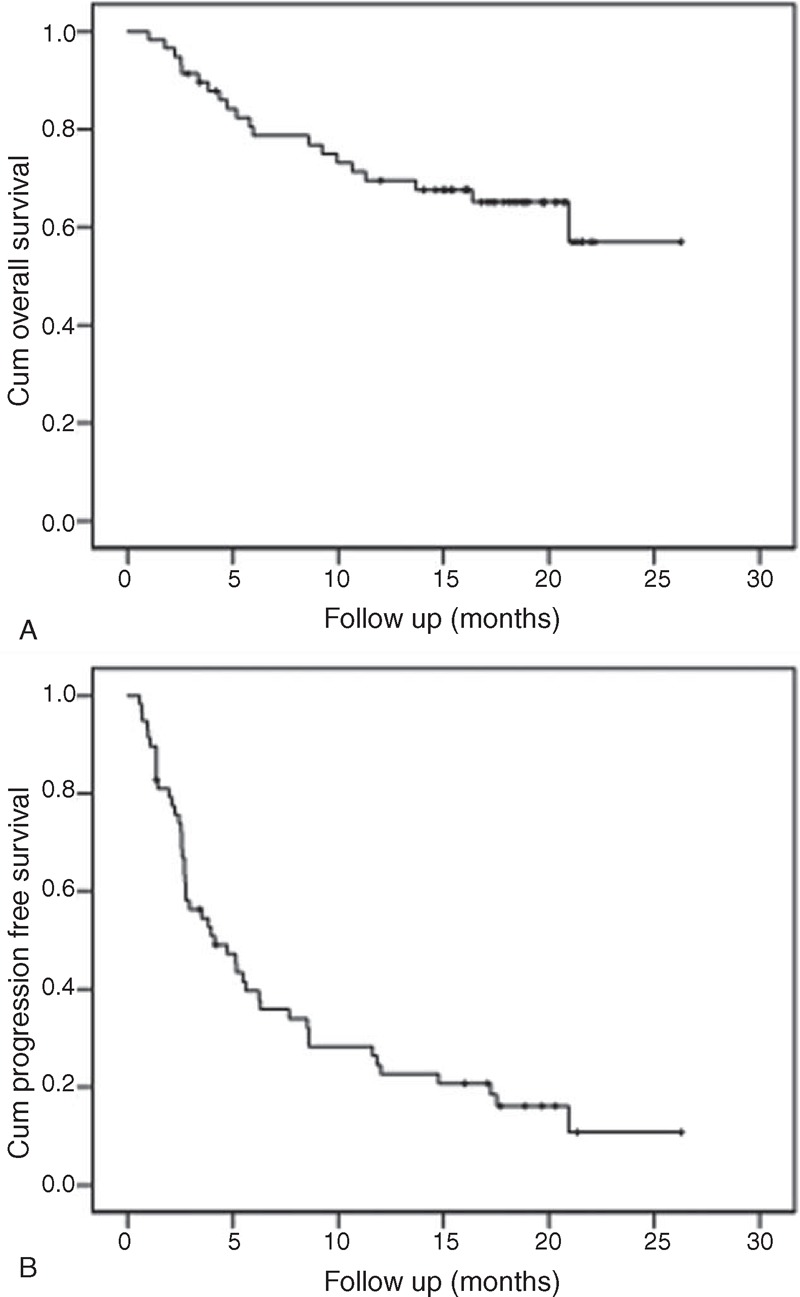
The secondary endpoints OS (A) and PFS (B).
It is noteworthy that 18 patients in our cohort had a PR, with a median PFS of 17.5 months (8.3–26.8 months) and a 1-year survival rate of 89% (data not shown).
The cohort EGFR mutation rate was 36.2% (21/58), and the K-ras mutation rate was 19.4% (12/58). EGFR mutation rates were significantly associated with gender (mutation rates in female vs male, 51% vs 16%; P = 0.005), while K-ras mutation rates were not associated with any clinical characteristics (Table 1). Patients with EGFR mutations had significantly higher ORRs than patients with wild-type EGFR (52.4%% vs 18.9%; P = 0.008); however, the ORR and DCR were not significantly associated with K-ras mutations (Table 1). In addition, patients with EGFR mutations had a significantly longer PFS than those with wild-type EGFR (Figure 2A).
Thymidylate synthase protein expression was observed in 30 out of 52 patients (57.7%), dihydropyrimidine dehydrogenase in 20 out of 47 patients (42.5%), thymidine phosphorylase in 25 out of 48 patients (52.1%), and OPRT in 12 out of 25 patients (48%). The rate of positive thymidine phosphorylase expression was significantly higher in patients with an Eastern Cooperative Oncology Group (ECOG) PS of 0 to 1 than in those with PS = 2 (thymidine phosphorylase, 92% vs 8%; P = 0.033) (Table 2). Immunohistochemistry staining of thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyl transferase expression (OPRT) is shown in Figure 3.
TABLE 2.
Comparison of Baseline Patient Clinical Characteristics for TS, DPD, TP, OPRT Expression
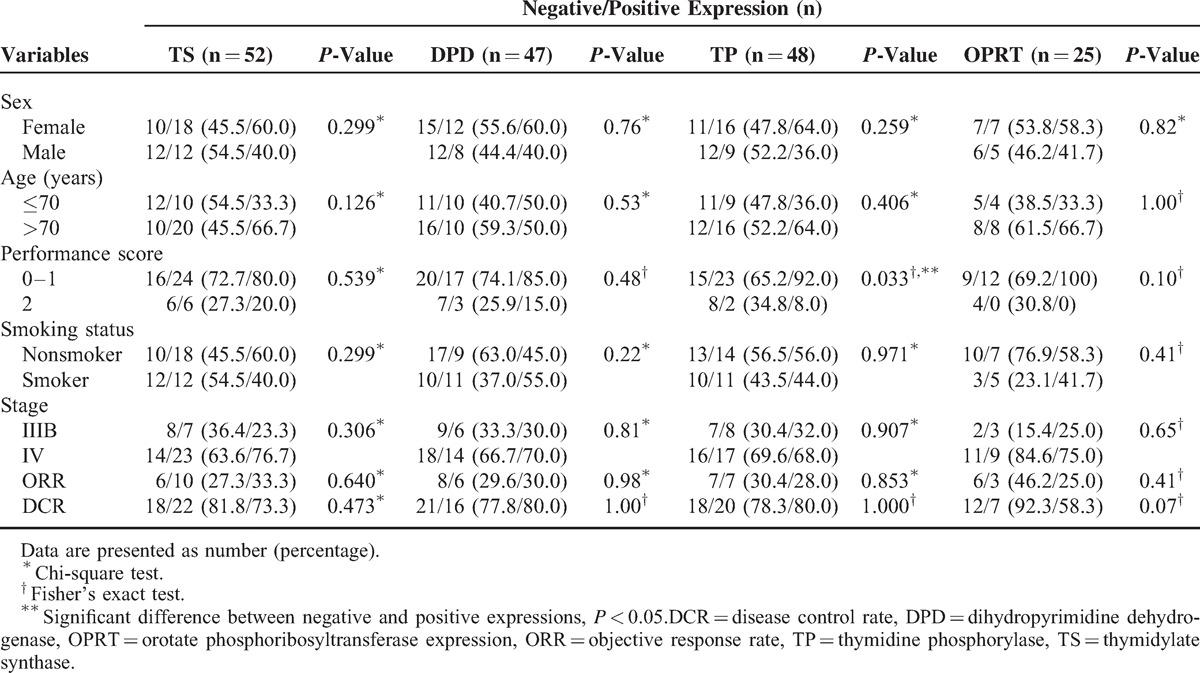
FIGURE 3.
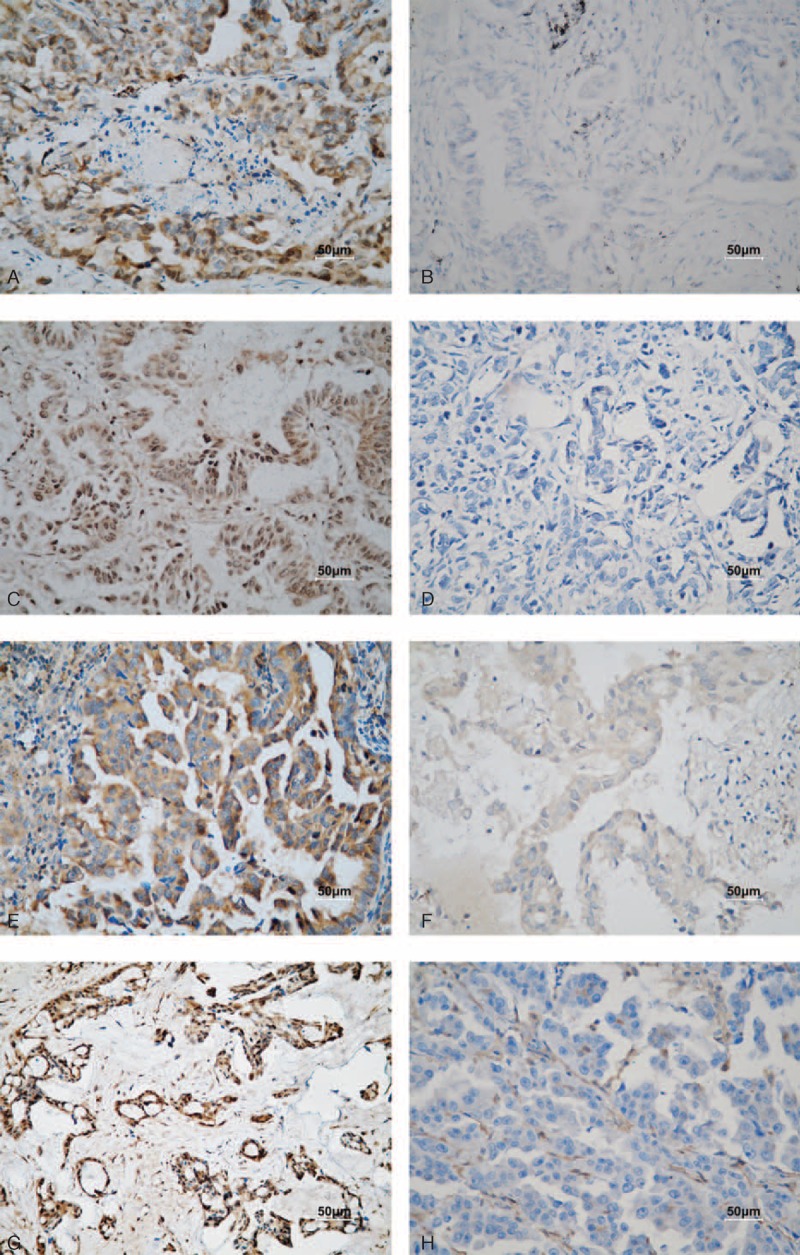
Immunohistochemical staining of human NSCLC tissues (200× magnification). (A and B) Carcinoma with positive and negative thymidylate synthase expression. (C and D) Carcinoma with positive and negative dihydropyrimidine dehydrogenase expression. (E and F) Carcinoma with positive and negative orotate phosphoribosyltransferase expression. (H and I) Carcinoma with positive and negative thymidine phosphorylase expression. Scale bar, 50 μm.
Kaplan–Meier curves with log-rank tests for OS showed a significant difference between patients with positive and negative expression of thymidine phosphorylase protein in tumors (P = 0.042). Patients who had tumors that were negative for thymidine phosphorylase protein expression had significantly longer OS compared to thymidine phosphorylase-positive patients (1 year OS, 77% vs 58%) (Figure 4E) Figure 4; however, the difference in median PFS time between these 2 groups was not statistically significant (5.13 months for positive patients vs 5.47 months for negative patients; P = 0.499) (Figure 5E).
FIGURE 4.
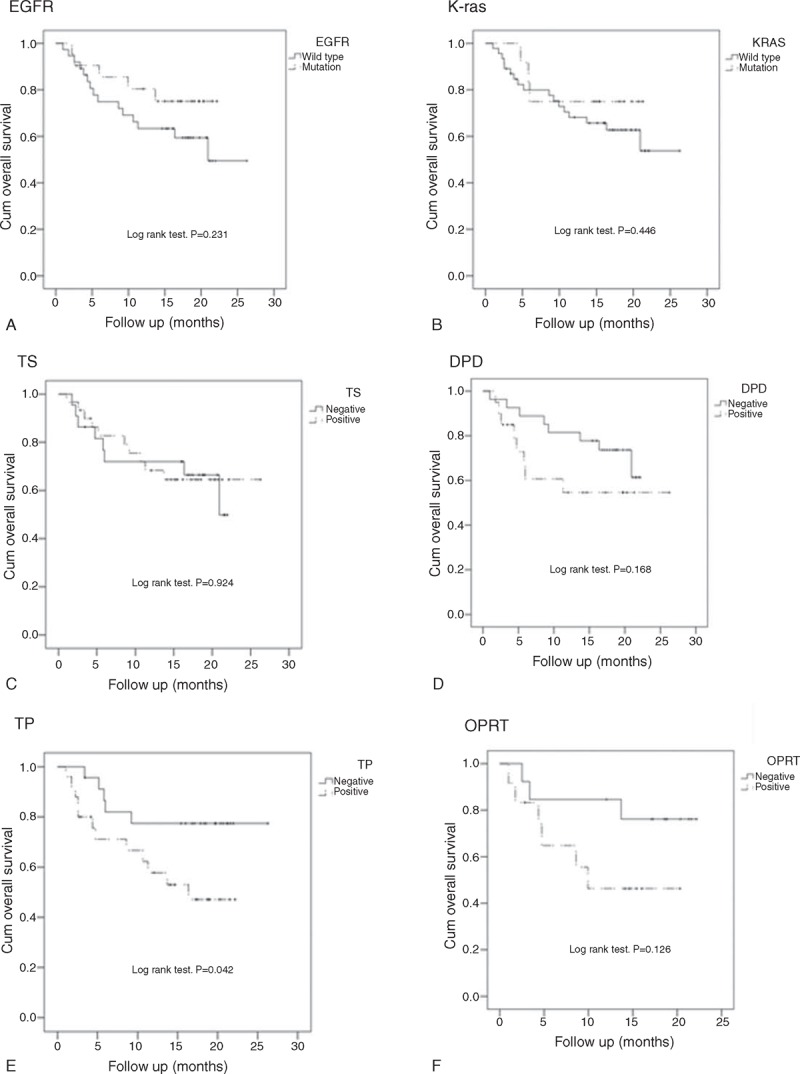
Survival analysis for OS by EGFR (A) and K-ras (B) mutation status, and thymidylate synthase (TS) (C), dihydropyrimidine dehydrogenase (DPD) (D), thymidine phosphorylase (TP) (E), and orotate phosphoribosyltransferase (OPRT) (F) expression status.
FIGURE 5.
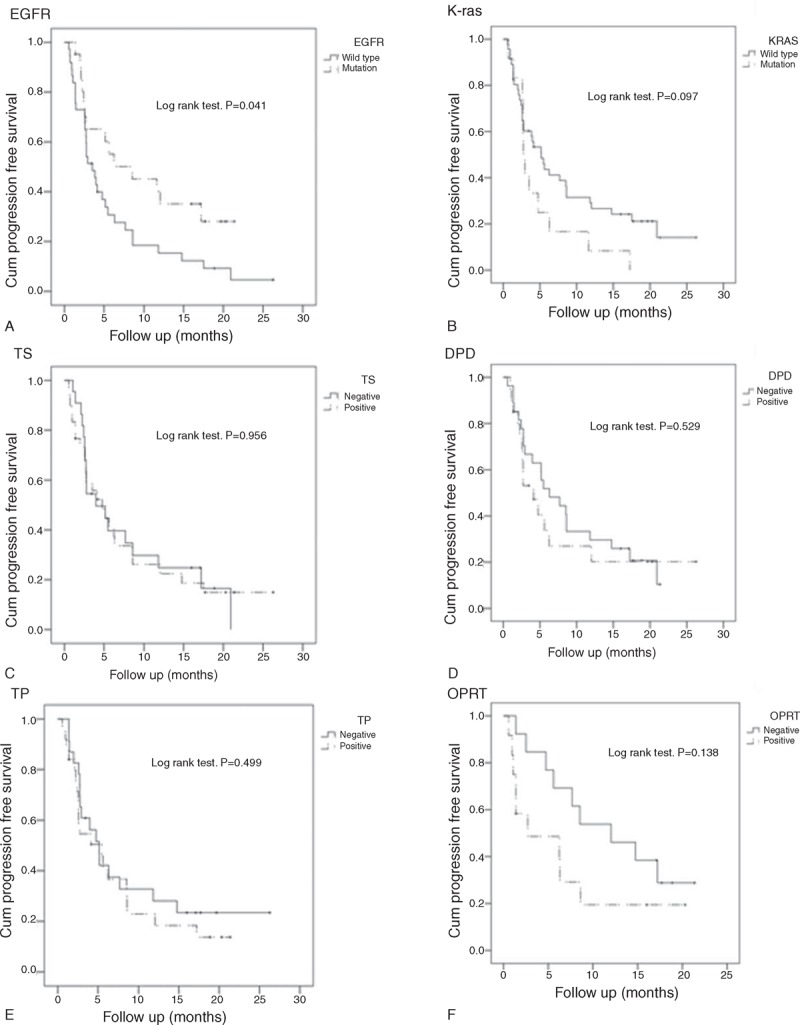
Survival analysis for PFS by EGFR (A) and K-ras (B) mutation status, and thymidylate synthase (TS) (C), dihydropyrimidine dehydrogenase (DPD) (D), thymidine phosphorylase (TP) (E), and orotate phosphoribosyltransferase (OPRT) (F) expression status.
To understand the effect of tumor EGFR mutation status and fluorouracil-related enzyme expression on the prognosis of combination therapy, combination analysis was applied to evaluate the predicted values of these 4 markers (EGFR mutations, thymidine phosphorylase, thymidylate synthase, and dihydropyrimidine dehydrogenase). The patients were divided into groups based on marker expression; a significant difference in OS between the groups was observed (P = 0.010) (Figure 6, A2). The OS was highest in patients who had EGFR mutations and were thymidine phosphorylase-negative (86%) and thymidine phosphorylase-positive (83%), followed by patients whose tumors expressed wild-type EGFR and were thymidine phosphorylase-negative (74%) and thymidine phosphorylase-positive (34%). However, there was no statistically significant difference in PFS between 3 of the combination groups (Figure 6B).
FIGURE 6.
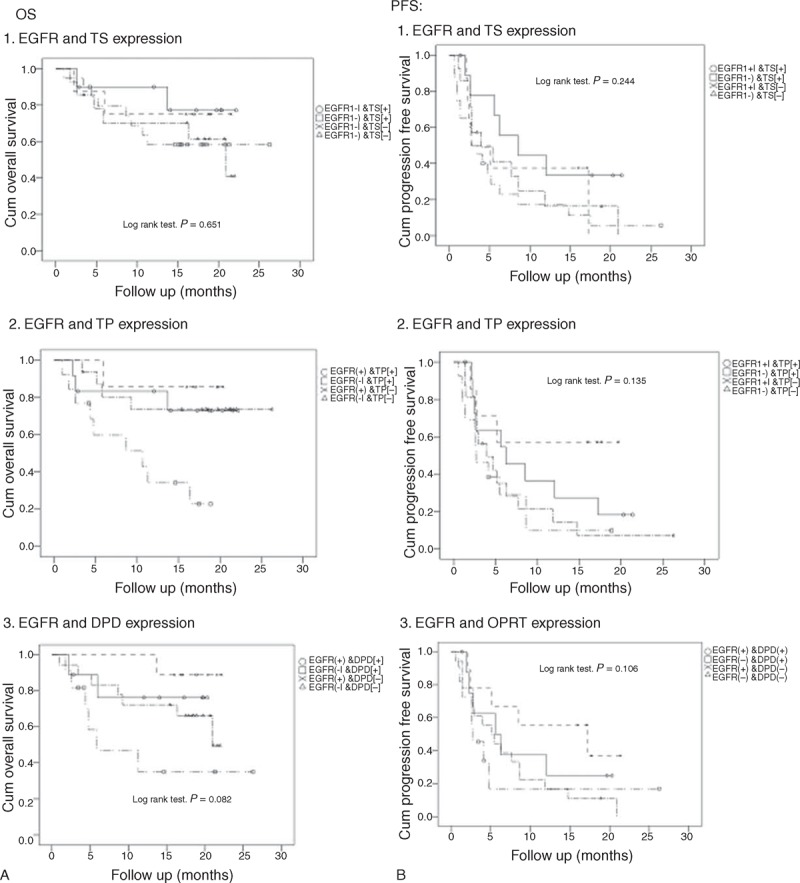
Survival analysis for OS and PFS according to EGFR mutation status and expression of thymidylate synthase (TS) (1), thymidine phosphorylase (TP) (2), and dihydropyrimidine dehydrogenase (DPD) (3).
The group of patients with tumors expressing wild-type EGFR was further investigated to determine the association between EGFR mutation status and 5-FU–related enzymes. The results demonstrated that patients with thymidine phosphorylase-negative tumors had a significantly longer OS than those with thymidine phosphorylase-positive tumors (P = 0.013, Figure 7B).
FIGURE 7.
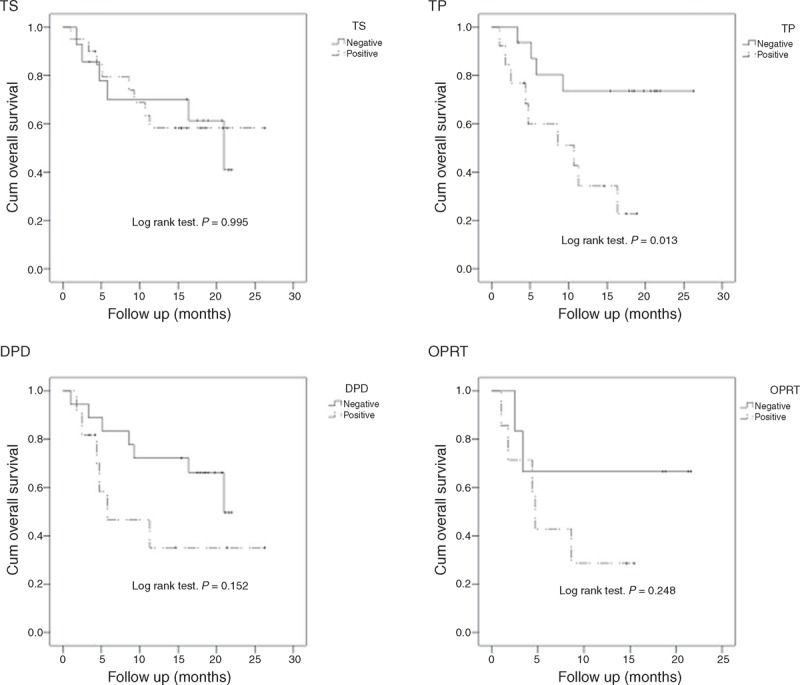
Survival analysis for OS by thymidylate synthase (TS), dihydropyrimidine dehydrogenase (DPD), thymidine phosphorylase (TP), and orotate phosphoribosyltransferase expression (OPRT) in 37 patients with wild-type EGFR.
Patients with primary adverse events (AEs) were summarized in Table 3. Forty-four patients (75.9%) had at least 1 of AEs, including 30 patients (51.7%) had skin rash. Seventeen patients (29.3%) had grade 3 AEs; 7 patients (12.1%) had grade 4 AEs. Grade 3 AEs comprised mainly skin rash, diarrhea, hand-foot syndrome, and hyperbilirubinemia.
TABLE 3.
Summary of Patients With Primary Adverse Events (AE) (N = 58)
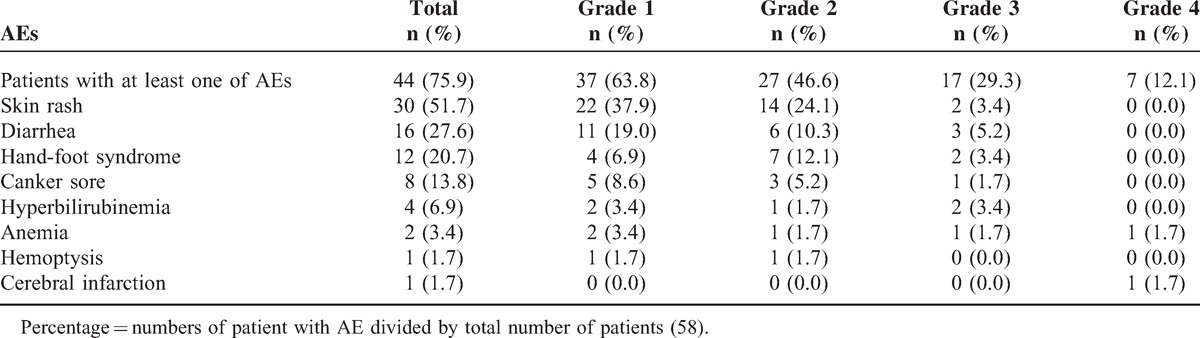
The most common AE was skin rash. Grade III and IV AEs included skin rash (2), diarrhea (3), hand-foot syndrome (2), canker sore (1), hyper-bilirubinemia (2), anemia (2), and cerebral infraction (1). In addition, three grade 5 events (none deemed treatment-related) were observed (disease progression, n = 2; acute gastrointestinal hemorrhage, n = 1).
DISCUSSION
In our cohort of 58 patients, 59% met the protocol-defined criteria for success. The ORR was 31%, and the DCR was 75.9%. The 1-year OS was 69.5%, and the PFS was 24.5%. Analysis of AEs revealed that 29.3% of the patients experienced AEs of grade 3, while 12.1% experienced those of grade 4. Patients with EGFR mutations had a significantly higher ORR and longer PFS than those with wild-type EGFR. Thymidine phosphorylase expression was significantly and negatively associated with OS.
The overall response rate of 31% observed in this study was more favorable than that reported for third-generation platinum-based chemotherapy in a subgroup of older patients in ECOG studies 1549 and 5592 (24.5% and 23%, respectively).9,10 In addition, the OS of 26 months in our study is superior to that of other clinical trials of non-platinum doublet regimens or monotherapy as first-line treatment, in which the median OS was only 5.1 to 12.6 months.8,29,30 Although we failed to reach our primary endpoint of the study (36 successful cases without progressive disease at 12 weeks), the treatment results were still good, particularly in the patients with a PR, considering that these patients were older and had advanced adenocarcinoma.
We found that the combination of erlotinib and capecitabine was well tolerated in older patients (median age, 72 years), with an incidence of 12.1% for AEs of grade 4 and 29.3% for AEs of grade 3, regardless of investigator-attributed causality. Drug-related AEs included diarrhea, hand-foot syndrome, canker sores, rash, hyperbilirubinemia, and drowsiness. These results are consistent with those of Kulke et al31 who found that the combination of capecitabine and erlotinib for the treatment of advanced pancreatic cancer could be administered safely in a group of patients with a median age of 60. Similarly, Lopez et al24 concluded that the combination of capecitabine with erlotinib has a favorable safety profile in patients with metastatic pancreatic cancer (median age, 64). A phase III trial of combined capecitabine/erlotinib treatment for pancreatic cancer also found this treatment to be safe (median age, 65).32 To our knowledge, no other studies have provided data regarding the safety of this drug combination in patients with lung cancer, and questions thus still remain. In our study, 3 patients died within 1 month after treatment. We did not include those 3 patients who died because they did not complete the first-line treatment or evaluation program required for meeting the FAS and PPS criteria, and the main endpoint of our study was efficacy. While the lack of clear safety data is a limitation of our study, the adverse events profile is nevertheless promising and thus suggests that the combination of capecitabine and erlotinib for treating older patients with NSCLC is worthy of further evaluation.
Activating mutations in the EGFR tyrosine kinase are present in 10% to 15% of NSCLCs; these mutations confer hypersensitivity to the oral tyrosine kinase inhibitors gefitinib and erlotinib and are predictive of tumor responsiveness to these 2 agents.33 Our results indicate that both the ORR and PFS were significantly better in patients with EGFR mutations than in those with wild-type EGFR, which is consistent with reports of previous studies.34,35 In addition, patients with wild-type EGFR (the prognosis of whom is thought to be poor) appeared to benefit from this drug regimen as well. The 1-year survival of these patients was greater in those with thymidine phosphorylase-negative tumors compared to those with thymidine phosphorylase-positive tumors (74% vs 34%, respectively; P = 0.013).
It is intriguing that erlotinib plus capecitabine treatment was beneficial in some of the patients with wild-type EGFR. Two particularly interesting possibilities may explain this observation. First, the angiogenic and anti-apoptotic activities of thymidine phosphorylase might have affected the prognosis of the patients with wild-type EGFR.36 Second, the effects of combination treatment with erlotinib and capecitabine may differ depending on EGFR mutation status. In patients with EGFR mutations, the action of erlotinib may be enhanced by weak synergy with capecitabine. In patients with wild-type EGFR, erlotinib might upregulate the expression of thymidine phosphorylase in tumor tissue of thymidine phosphorylase-negative patients, thereby enhancing the anti-tumor effects of capecitabine. For these patients, capecitabine would play a more important role.
Biomarker analysis in this study demonstrated that thymidine phosphorylase expression status was predictive of OS in our patient cohort, with significantly higher survival in patients with thymidine-phosphorylase–negative tumors (P = 0.013). Similarly, a previous study showed that high thymidine phosphorylase expression was related to extensive angiogenesis and poor prognosis in colorectal cancer patients.37 The relationship between high thymidine phosphorylase expression and poor outcomes has also been reported for NSCLC38 and in studies of colorectal,39 renal,40 and breast cancers.41 However, the nature of the relationship between thymidine phosphorylase expression and outcome varies with tumor type.39 This observation most likely reflects the numerous roles played by thymidine phosphorylase in tumor progression. Thymidine phosphorylase is not only involved in nucleotide metabolism, but also prevents apoptosis and induces angiogenesis. All of these activities promote tumor growth and metastasis. Ironically, thymidine phosphorylase activity is also required for the activation of capecitabine. This duality illustrates the complexity of the role of thymidine phosphorylase in tumor progression and in the clinical response to fluoropyrimidine-based chemotherapy.36
We observed that patients whose tumors were negative for dihydropyrimidine dehydrogenase or OPRT expression tended to have longer OS, but these differences were not significant. Additionally, thymidylate synthase protein expression was not related to prognosis with this treatment regimen. Other studies have reported results that differ from ours, demonstrating significant correlations between 5-F-U biomarkers and prognosis.22,42–45 Most of these studies were retrospective in nature, while ours was prospective.
Limitations in our study include the single arm design and the small number of patients enrolled with variant background in these limited cases. Thus, further large-scale analysis is still necessary. In addition, uncertainty regarding the cause of 3 patient deaths during this study will require further safety studies on this drug regimen. This preliminary phase II trial has provided data from which we can tailor our design for the phase III trial.
The present study is the first phase II clinical trial to evaluate capecitabine combined with erlotinib as first-line treatment in older Chinese patients with lung adenocarcinoma. The outcome of erlotinib/capecitabine chemotherapy was significantly better in patients with EGFR mutations and in those with thymidine phosphorylase-negative tumors. This pilot study was also the first to evaluate the predictive value of thymidylate synthase/thymidine phosphorylase/dihydropyrimidine dehydrogenase/OPRT expression in this patient population. Further studies to investigate the potential therapeutic capacity and prognostic value of these biomarkers are thus warranted.
Acknowledgments
Support for third-party language editing for this manuscript was provided by Shanghai Roche Pharmaceuticals Ltd. All decisions regarding the final content were made by the authors. As such, the authors take full responsibility for the content and expression of the submitted manuscript.
Disclose any potential conflicts of interest: All capecitabine and erlotinib used in this study were gifts from Shanghai Roche Pharmaceuticals Ltd.
Footnotes
Abbreviations: AE = adverse events, CIs = confidence intervals, CR = Complete response, CRC = Colorectal cancer, CT = computed tomography, CTC AE = common terminology criteria for adverse events, DCR = disease control rates, DPD = dihydropyrimidine dehydrogenase, ECOG = Eastern Cooperative Oncology Group, EGFR = epidermal growth factor receptor, FAS = full analysis set, H&E = haemotoxylin & eosin, IHC = immunohistochemistry, MRI = magnetic resonance imaging, NSCLC = non–small-cell lung cancer, OPRT = orotate phosphoribosyl transferase, ORR = objective response rate, OS = overall survival, PD = progression of disease, PFS = progression-free survival, PPS = per-protocol set, PR = partial response, PS = performance status, RECIST = response evaluation criteria in solid tumors, SD = stable disease, SFDA = state food and drug administration, Tarceva = erlotinib, TP = thymidine phosphorylase, TS = thymidylate synthase, Xeloda = capecitabine, 5-FU = -fluorouracil.
Hong-Yun Zhao and Gong-Yan Chen contributed equally to this work.
Clinical Trial No.: ClinicalTrials.gov Identifier: NCT00816868.
Trial registration: Clinical Trials NCT00816868.
National Natural Science Foundation of China (Grant No. 81201718).
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest 2005; 128:452–462. [DOI] [PubMed] [Google Scholar]
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24:4539–4454. [DOI] [PubMed] [Google Scholar]
- 3.Havlik RJ, Yancik R, Long S, et al. The National Institute on Aging and the National Cancer Institute SEER collaborative study on comorbidity and early diagnosis of cancer in the elderly. Cancer 1994; 74:2101–6. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol 2001; 2:533–543. [DOI] [PubMed] [Google Scholar]
- 5.Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003; 95:362–372. [DOI] [PubMed] [Google Scholar]
- 6.Gridelli C. Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 1999; 91:66–72. [DOI] [PubMed] [Google Scholar]
- 7.Hesketh PJ, Chansky K, Lau DH, et al. Sequential vinorelbine and docetaxel in advanced non-small cell lung cancer patients age 70 and older and/or with a performance status of 2: a phase II trial of the Southwest Oncology Group (S0027). J Thorac Oncol 2006; 1:537–544. [PubMed] [Google Scholar]
- 8.Gridelli C, Maione P, Rossi A, et al. Management of unfit older patients with advanced NSCLC. Cancer Treat Rev 2009; 35:517–521. [DOI] [PubMed] [Google Scholar]
- 9.Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 2002; 94:173–181. [DOI] [PubMed] [Google Scholar]
- 10.Langer CJ, Vangel M, Schiller J, et al. Age-specific subanalysis of ECOG 1594: Fit elderly patients (70–80 yrs) with NSCLC do as well as younger pts (<70). Proc Am Soc Clin Oncol 2003; 22:639. [Google Scholar]
- 11.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26:3543–3551. [DOI] [PubMed] [Google Scholar]
- 13.Reichardt P, Von Minckwitz G, Thuss-Patience PC, et al. Multicenter phase II study of oral capecitabine (Xeloda(")) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol 2003; 14:1227–1233. [DOI] [PubMed] [Google Scholar]
- 14.Blum JL, Dieras V, Lo Russo PM, et al. Multicenter, Phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 2001; 92:1759–1768. [DOI] [PubMed] [Google Scholar]
- 15.Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 1999; 17:485–493. [DOI] [PubMed] [Google Scholar]
- 16.Cassidy J, Twelves C, Van Cutsem E, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 2002; 13:566–575. [DOI] [PubMed] [Google Scholar]
- 17.Twelves C. Xeloda Colorectal Cancer G. Capecitabine as first-line treatment in colorectal cancer. Pooled data from two large, phase III trials. Eur J Cancer 2002; 38:15–20. [DOI] [PubMed] [Google Scholar]
- 18.Schuller J, Cassidy J, Dumont E, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol 2000; 45:291–297. [DOI] [PubMed] [Google Scholar]
- 19.Miwa M, Ura M, Nishida M, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998; 34:1274–1281. [DOI] [PubMed] [Google Scholar]
- 20.Berger SH, Hakala MT. Relationship of dUMP and free FdUMP pools to inhibition of thymidylate synthase by 5-fluorouracil. Mol Pharmacol 1984; 25:303–309. [PubMed] [Google Scholar]
- 21.Nishimura G, Terada I, Kobayashi T, et al. Thymidine phosphorylase and dihydropyrimidine dehydrogenase levels in primary colorectal cancer show a relationship to clinical effects of 5’-deoxy-5-fluorouridine as adjuvant chemotherapy. Oncol Rep 2002; 9:479–482. [PubMed] [Google Scholar]
- 22.Oguri T, Achiwa H, Bessho Y, et al. The role of thymidylate synthase and dihydropyrimidine dehydrogenase in resistance to 5-fluorouracil in human lung cancer cells. Lung Cancer 2005; 49:345–351. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi KF, Yanagisawa M, Sekiguchi F, Tanaka Y. Antitumor activity of erlotinib in combination with capecitabine in human tumor xenograft models. Cancer Chemother Pharmacol 2006; 57:693–702. [DOI] [PubMed] [Google Scholar]
- 24.Lopez R, Mendez CM, Fernandez MJ, et al. Phase II trial of erlotinib plus capecitabine as first-line treatment for metastatic pancreatic cancer (XELTA study). Anticancer Res 2013; 33:717–723. [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy KD, Prime J, Harmon T, Pollenz R. Receptor-mediated phosphorylation of astroglial intermediate filament proteins in cultured astroglia. J Neurochem 1985; 44:723–730. [DOI] [PubMed] [Google Scholar]
- 27.Huang CL, Yokomise H, Kobayashi S, et al. Intratumoral expression of thymidylate synthase and dihydropyrimidine dehydrogenase in non-small cell lung cancer patients treated with 5-FU-based chemotherapy. Int J Oncol 2000; 17:47–54. [PubMed] [Google Scholar]
- 28.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics 1982; 38:143–151. [PubMed] [Google Scholar]
- 29.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc 2009; 6:233–241. [DOI] [PubMed] [Google Scholar]
- 30.Avery EJ, Kessinger A, Ganti AK. Therapeutic options for elderly patients with advanced non-small cell lung cancer. Cancer Treat Rev 2009; 35:340–344. [DOI] [PubMed] [Google Scholar]
- 31.Kulke MH, BL, Ryan DP, Clark JW, et al. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol 2007; 25:4787–4792. [DOI] [PubMed] [Google Scholar]
- 32.Heinemann V, Vehling-Kaiser U, Waldschmidt D, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the ’Arbeitsgemeinschaft Internistische Onkologie’ (AIO-PK0104). Gut 2013; 62:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schettino C, Bareschino MA, Sacco PC, et al. New molecular targets in the treatment of NSCLC. Curr Pharm Des 2013; 19:5333–5343. [DOI] [PubMed] [Google Scholar]
- 34.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 35.Dahabreh IJ, Linardou H, Siannis F, et al. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res 2010; 16:291–303. [DOI] [PubMed] [Google Scholar]
- 36.Bronckaers A, Gago F, Balzarini J, Liekens S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med Res Rev 2009; 29:903–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takebayashi Y, Akiyama S, Akiba S, et al. Clinicopathologic and prognostic significance of an angiogenic factor, thymidine phosphorylase, in human colorectal carcinoma. J Natl Cancer Inst 1996; 88:1110–1117. [DOI] [PubMed] [Google Scholar]
- 38.Koukourakis MI, Giatromanolaki A, O’Byrne KJ, et al. Platelet-derived endothelial cell growth factor expression correlates with tumour angiogenesis and prognosis in non-small-cell lung cancer. Br J Cancer 1997; 75:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Triest B, Pinedo HM, Blaauwgeers JL, et al. Prognostic role of thymidylate synthase, thymidine phosphorylase/platelet-derived endothelial cell growth factor, and proliferation markers in colorectal cancer. Clin Cancer Res 2000; 6:1063–1072. [PubMed] [Google Scholar]
- 40.Imazono Y, Takebayashi Y, Nishiyama K, et al. Correlation between thymidine phosphorylase expression and prognosis in human renal cell carcinoma. J Clin Oncol 1997; 15:2570–2578. [DOI] [PubMed] [Google Scholar]
- 41.Fox SB, Moghaddam A, Comley M, et al. The angiogenic factor platelet-derived endothelial cell growth factor/thymidine phosphorylase is up-regulated in breast cancer epithelium and endothelium. Br J Cancer 1996; 73:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakano J, Huang C, Liu D, et al. Evaluations of biomarkers associated with 5-FU sensitivity for non-small-cell lung cancer patients postoperatively treated with UFT. Br J Cancer 2006; 95:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyoshi T, Kondo K, Toba H, et al. Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase expression in tumor tissue, regarding the efficacy of postoperatively administered UFT (tegafur+uracil) in patients with non-small cell lung cancer. Anticancer Res 2007; 27:2641–2648. [PubMed] [Google Scholar]
- 44.Wu M-F, Hsiao Y-M, Huang C-F, et al. Genetic determinants of pemetrexed responsiveness and nonresponsiveness in non-small cell lung cancer cells. J Thorac Oncol 2010; 5:1143–1151. [DOI] [PubMed] [Google Scholar]
- 45.Giovannetti E, Lemos C, Tekle C, et al. Molecular mechanisms underlying the synergistic interaction of erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor, with the multitargeted antifolate pemetrexed in non-small-cell lung cancer cells. Mol Pharmacol 2008; 73:1290–1300. [DOI] [PubMed] [Google Scholar]


