Supplemental Digital Content is available in the text
Abstract
Few studies have analyzed the training of endoscopists in the diagnosis of early gastric cancer (EGC). This study assessed whether specific training of endoscopists improves the detection rate of EGC.
The rates of detection of EGC by endoscopists at the Digestive Endoscopy Center of the Affiliated Nanfang Hospital of China Southern Medical University between January 2013 and May 2014 were retrospectively analyzed. Because some endoscopists received training in the diagnosis of EGC, beginning in September 2013, the study was divided into 3 time periods: January to September 2013 (period 1), September 2013 to January 2014 (period 2), and January to May 2014 (period 3). The rates of EGC detection during these 3 periods were analyzed.
From January 2013 to May 2014, a total of 25,314 gastroscopy examinations were performed at our center, with 48 of these examinations (0.2%) detecting EGCs, accounting for 12.1% (48/396) of the total number of gastric cancers detected. The EGC detection rates by trained endoscopists during periods 1, 2, and 3 were 0.3%, 0.6%, and 1.5%, respectively, accounting for 22.0%, 39.0%, and 60.0%, respectively, of the gastric cancers detected during these time periods. In comparison, the EGC detection rates by untrained endoscopists during periods 1, 2, and 3 were 0.05%, 0.08%, and 0.10%, respectively, accounting for 3.1%, 6.0%, and 5.7%, respectively, of the gastric cancers detected during these times. After training, the detection rate by some trained endoscopists markedly increased from 0.2% during period 1 to 2.3% during period 3. Further, the use of magnifying endoscopy with narrow-band imaging (M-NBI) (odds ratio = 3.1, 95% confidence interval 2.4–4.1, P < 0.001) contributed to the diagnosis of EGC.
In conclusion, specific training could improve the endoscopic detection rate of EGC. M-NBI contributed to the diagnosis of EGC.
INTRODUCTION
Gastric cancer is the fourth most common malignant tumor in the world, and the second leading cause of cancer-related deaths.1 Gastric cancer incidence and mortality rates are higher in Japan, China, and South Korea than in other countries.2–6 Medical advances have reduced gastric cancer mortality rates, but in Asia gastric cancer remains the second leading cause of cancer-related deaths.7 Early gastric cancer (EGC) was first defined by the Japan Gastroscopy Association as an adenocarcinoma limited to the mucosa and submucosa, regardless of lymph node metastasis.8 Based on this standard, the 5-year overall survival rate of patients with EGC after surgical treatment has reached 90%.9 Clearly, early detection and treatment of gastric cancer is the best strategy to improve survival. 10 Since 1957, Japanese individuals have been regularly screened for EGC.11–13 Moreover, to improve the detection rate of EGC, some endoscopists undergo intensive training, and new endoscopic techniques have been included in routine examinations in Japan.12,14 In 1985, EGC accounted for 40% of gastric cancers diagnosed in Japan,15 and the National Cancer Center of Japan reported that the percentage of gastric cancer patients diagnosed with EGC increased from 22% in the 1960s to 75% in the 2000s.12 In Western countries, EGC is present in 10% to 20% of patients with resectable gastric cancer,15 and in China EGC accounts for 10% to 20% of gastric cancer. The lower rate of diagnosis of EGC in China than in Japan may be due to a lack of a standardized screening system, as well as to the lack of a screening and follow-up strategy for high-risk populations.
Moreover, as reported in the literature,16 only to popularize gastroscopy is not sufficient to improve the detection rate of EGC, and rates of detection may also be affected by the limited abilities of endoscopists to detect EGC. Rates of detection of colonic adenoma have been associated with the experience of the endoscopist,17 although other studies found no association between polyp detection rate and endoscopist experience.18,19 To date, few studies have examined the association between gastroscopy experience and the detection rate of EGC. Thus, this study investigated the impact of training to improving the experience in the diagnosis of EGC on the EGC detection rate.
PATIENTS AND METHODS
Basic Information About the Endoscopy Center and Endoscopists
The Digestive Endoscopy Center of Guangzhou Nanfang Hospital is one of the largest endoscopy centers in China that meets international standards. Over the past 3 years, approximately 30,000 endoscopies have been performed annually, including approximately 17,800 gastroscopies. The endoscopists at the Center are all skilled in endoscopic diagnosis and treatment, with each endoscopist performing >3000 total gastroscopic examinations since learning gastroscopy.
Endoscopy Training Methods to Improve the Detection of EGC
To improve the abilities of endoscopists to detect EGC, the Center established a voluntary training program in September 2013. The main purposes of the training were as follows. First, strengthen awareness of EGC screening and improve the ability to endoscopically identify EGCs: once a week there was a discussion of cases of EGC, as well as published papers and video studies related to the diagnosis and treatment of EGC. Otherwise, some Japanese endoscopy experts were invited to on-site instruction, and the endoscopists were also encouraged to take an active part in domestic academic conferences regarding EGC. Second, standardize endoscopic examination to improve its quality. The book “Standard Gastroscopy”, edited by the Japanese expert Hosoi Tozo, was used as the reference, and standard gastroscopy was performed in every patient, especially high-risk patients of gastric cancer. Third, learn the use of magnifying endoscopy to aid in the diagnosis of EGC. The criteria for magnifying endoscopic diagnosis of EGC was based on the VS classification system, including an irregular microvascular and/or microsurface pattern together with a clear demarcation line.20 Fourth, specify endoscopic submucosal dissection (ESD) for EGC. All ESD treatments were performed by W.G., who has considerable experience. Finally, good communication between the endoscopist and pathologist is necessary for standardized pathological examinations of ESD specimens. Pathological diagnosis was based on the revised Vienna classification.21 Mucosal high-grade neoplasia (C4), including high-grade adenoma/dysplasia, noninvasive carcinoma (carcinoma in situ), suspicious for invasive carcinoma, and intramucosal carcinoma, were diagnosed as EGC. All histopathological diagnoses were made by experts in gastrointestinal pathology.
Factors emphasized for standard gastroscopy included sufficient preparation, including use of mucus decomposing, antifoaming, and spasmolytic agents to improve the visibility of the gastric mucosa and the use of intravenous anesthesia to facilitate careful examination. A standardized endoscopy procedure was used, including full gas injection during the examination and use of a magnifying endoscopy. The latter can make suspicious lesions more visible based on their surface structure and microvasculature, and facilitate their endoscopic diagnosis.
Study Design
All endoscopy procedures performed from January 1, 2013, to May 1, 2014, were reviewed. Because some endoscopists received training in the diagnosis of EGC, beginning on September 2013, the study was divided into 3 time periods: January 1 to September 1, 2013 (period 1); September 1, 2013, to January 1, 2014 (period 2); and January 1 to May 1, 2014 (period 3). Parameters compared included EGC detection rate by trained and untrained endoscopists and EGC detection rate before and after training. The comparison between before and after training and the analysis of the factors affecting the detection of EGC were assessed in patients who underwent gastroscopy by 2 endoscopists (W.G. and Q.Z.).
This research was approved by the local ethics committee, and before endoscopy, all patients provided written informed consent for the procedures.
Patients and Data Collection
All patient demographic and clinical characteristics were evaluated, including age, gender, status (outpatient/inpatient), and gastrointestinal symptoms such as abdominal pain and vomiting, past medical history mainly including atrophic gastritis, gastric ulcer, and gastric surgery, and the use of magnifying endoscopy with narrow-band imaging (M-NBI, yes/no). Characteristics assessed in the patients with EGC included the site and general morphology of the lesion, surface microstructure, and vascular characteristics. Additionally, endoscopist age and total number of gastroscopy performed since learning gastroscopy were evaluated.
Statistical Analysis
All statistical analyses were performed using SPSS, version 20 (SPSS, Chicago, IL). Normally distributed continuous variables were compared using Student t tests, and normally distributed categorical variables were compared using the χ2 test. Mann–Whitney U test was used for data that are nonnormally distributed. The factors affecting the detection of EGC were assessed by univariate and multivariate analyses. Odds ratio (OR) and 95% confidence intervals (CIs) were determined for variables found significant on multivariate analysis. A 2-sided P value <0.05 was considered statistically significant.
RESULTS
Endoscopist Data
Endoscopists in the EGC-trained group were younger than those in the untrained group (33 ± 5 vs 53 ± 13 years). In contrast, the average number of gastroscopies performed by each endoscopist was lower in trained than in the untrained group (4488 [3609] vs 6000 [5546]) (Table 1).
Table 1.
Comparison of Basic Information and the Detection Rate of EGC Between the Training and Nontraining Groups
Rates of EGC Detection
From January 1, 2013 to May 1, 2014, a total of 25,314 gastroscopy examinations were performed by 14 endoscopists, with 48 (0.2%) positive for EGC. These 48 patients accounted for 12.1% of the 396 patients diagnosed with gastric cancer during this time period. Seven endoscopists underwent the training in the diagnosis of EGC and seven other endoscopists did not. The EGC detection rates by trained endoscopists during periods 1, 2, and 3, were 0.3%, 0.6%, and 1.5%, respectively, accounting for 22.0%, 39.0%, and 60.0%, respectively, of the gastric cancers diagnosed during those time periods. In contrast, the EGC detection rates by untrained endoscopists during periods 1, 2, and 3 were 0.05%, 0.08%, and 0.10%, respectively, accounting for 3.1%, 6.0%, and 5.7%, respectively, of the gastric cancers diagnosed (Table 1).
Impact of Training on the Detection Rate of EGC and Characteristics of EGC
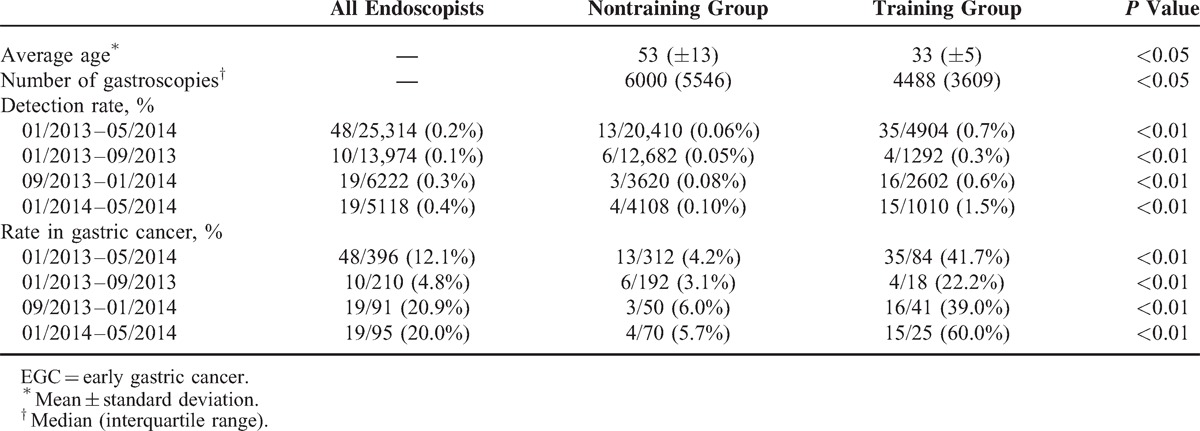
From January 1, 2013, to May 1, 2014, W.G. and Q.Z. performed a total of 3153 gastroscopies, with 1226, 1318, and 609 gastroscopies performed during periods 1, 2, and 3, respectively. The rates of detection of EGC during these 3 time periods were 0.2%, 1.1%, and 2.3%, respectively, accounting for 20.0%, 66.7%, and 66.7%, of the gastric cancers diagnosed (Figure 1).
Figure 1.
Rates of detection of EGC by W.G. and Q.Z. before and after training on early gastric cancer diagnosis. EGC = early gastric cancer, HGN = high-grade neoplasia, LGN = low-grade neoplasia.
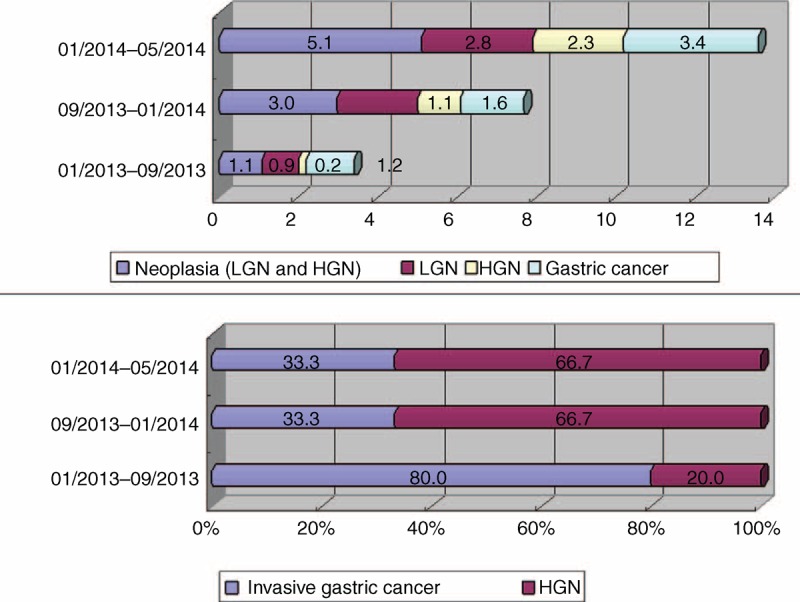
After undergoing training for EGC diagnosis, W.G. and Q.Z. performed 1927 gastroscopies from September 2013 to May 2014 (periods 2 and 3). During this time, these 2 endoscopists detected EGCs in 28 patients, and these patients underwent complete ESD resection. Of these 1927 gastroscopies, 303 were performed under M-NBI, with 23 of the latter having endoscopic features typical of EGC (VS classification). Tissue biopsies were obtained from the 23 patients. Pathological examination showed that 2 patients had high-grade neoplasias, 13 had low-grade neoplasias, and 8 were negative for neoplasia. Based on these findings, 20 of the 23 patients underwent resection with ESD. Final pathological examination of the resected tissue specimens showed that 18 patients had high-grade neoplasias and 2 had low-grade neoplasias (Figure 2).
Figure 2.
Flow chart of 1927 gastroscopies performed by W.G. and Q.Z. “VS feature” = an irregular microvascular and/or microsurface pattern together with a clear demarcation line, LGN = low-grade neoplasia, HGN = high-grade neoplasia, ESD = endoscopic submucosal dissection, M-NBI = magnifying endoscopy with narrow-band imaging.
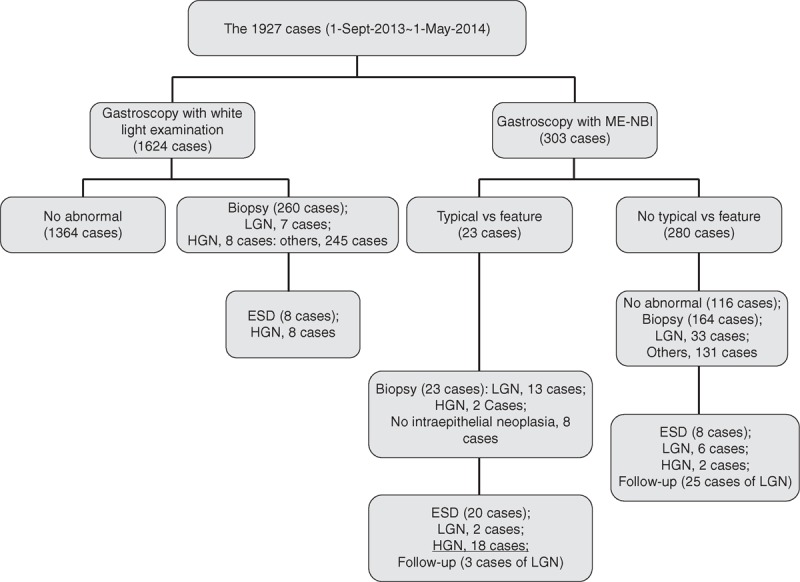
As shown in Table 2, one of the 28 EGCs was located at the gastric fundus, 8 at the lesser curvature of the gastric corpus, 1 at the greater curvature of gastric corpus, 2 at the gastric angle, and 16 at the gastric antrum. Six were surface protruding type (0–IIa), 14 were surface depressed type (0–IIc), 2 were flat type (0–IIb), and 6 was mixed type (0–IIa+IIc). In general, EGCs have various morphological characteristics and the lesions may be very subtle. Of the 28 EGCs, 24 cases were shown in Figure 3, showing the general morphology of the lesion under the white-light gastroscopy of these EGCs, and their corresponding M-NBI images and pathological results were provided in the supplemental data file (http://links.lww.com/MD/A149).
Table 2.
Sites and the General Morphologies of EGCs Detected by W.G. and Q.Z. After Training
Figure 3.
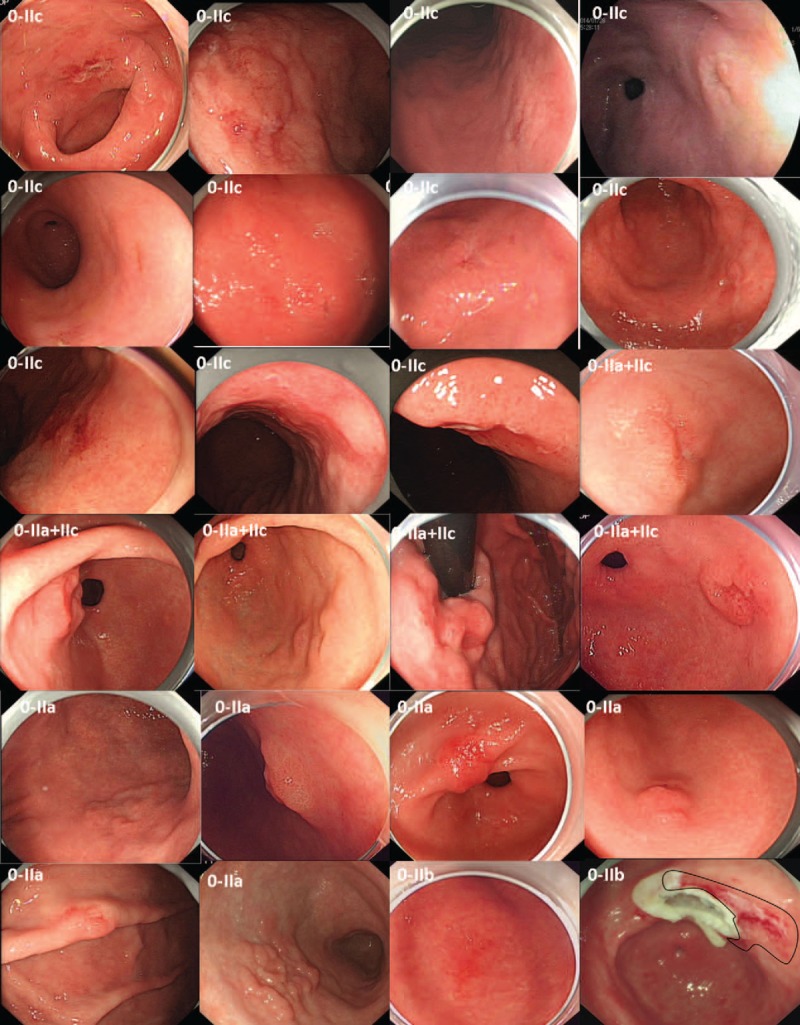
EGC lesions detected after training; the M-NBI images and pathological results are shown in a supplemental data file (http://links.lww.com/MD/A149). Under white-light gastroscopy, these EGCs exhibited different morphologic characteristics. EGC = early gastric cancer, M-NBI = magnifying endoscopy with narrow-band imaging.
Examples of EGC
Figure 4 shows 4 typical EGCs, classified as 0–IIc, 0–IIb, 0–IIa, and 0–IIc lesions, respectively. All 4 were located at the gastric antrum, and all had typical appearance on M-NBI, including an irregular microvascular and an irregular microsurface pattern with a demarcation line. Pathological examination of ESD-resected specimens showed that all 4 were high-grade neoplasias.
Figure 4.
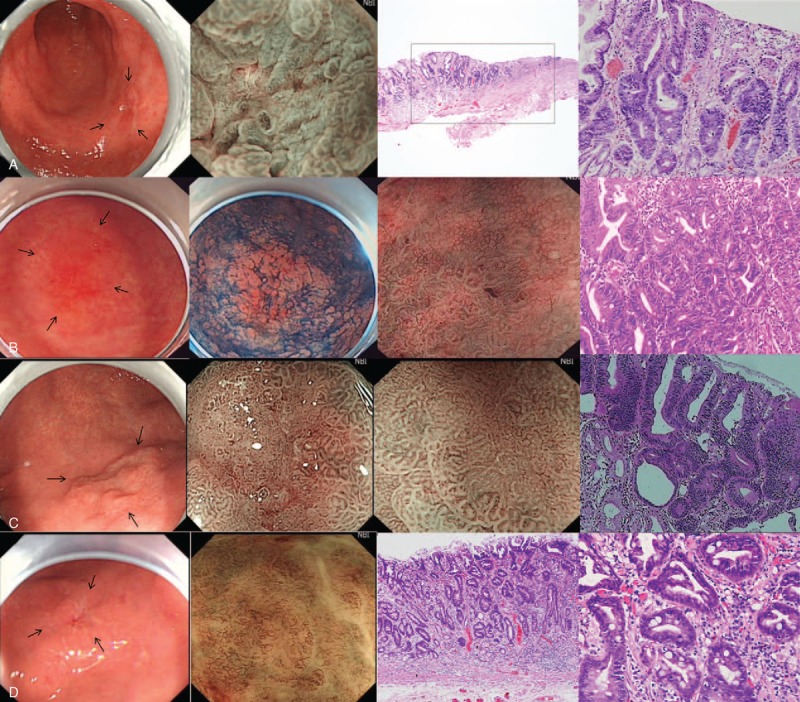
Examples of EGC categorized as types 0–IIc (A), 0–IIb (B), 0–IIa (C), and 0–IIc (D), respectively. They all had typical VS features of EGC. All of these lesions were diagnosed as high-grade neoplasias. EGC = early gastric cancer.
Factors Affecting EGC Detection
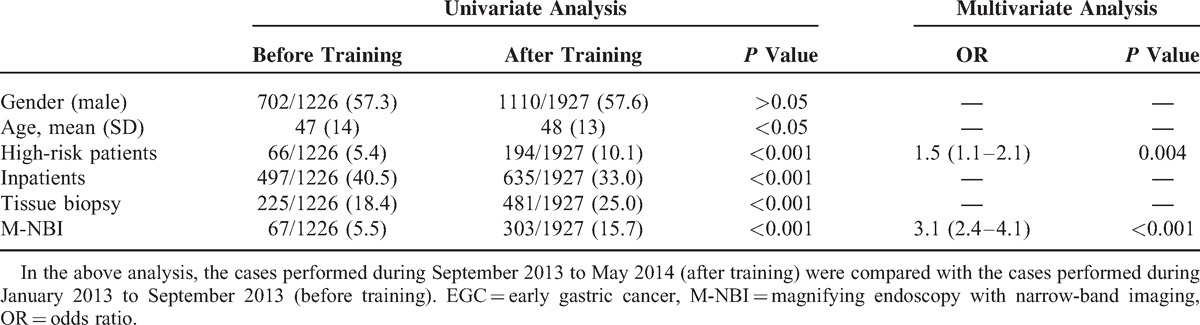
The rate of detection of EGC by W.G. and Q.Z. was very high after training, from September 2013 to May 2014, and greatly increased compared with before training, from January to September 2013. Univariate and multivariate analyses showed that screening of high-risk patients (ie, those with atrophic gastritis, gastric ulcer, or stomach surgery history) (OR = 1.5, 95% CI 1.1–2.1, P = 0.004) and the use of M-NBI (OR = 3.1, 95% CI 2.4–4.1, P < 0.001) were independently associated with the detection of EGC. Moreover, 15.7% of 1927 gastroscopies were performed under M-NBI after training, compared with 5.5%of 1226 gastroscopies before training. Gender, age, and patient status (outpatient/inpatient) had no significant differences between before and after training. Additionally, the number of cases having tissue biopsies performed were similar before and after training (Table 3).
Table 3.
Analysis of the Related Influencing Factors on the Detection of EGC
DISCUSSION
The current study showed that training for EGC diagnosis plays an important role in improving the detection rate of EGC. Improvements in detection rate were not due solely to the number of gastroscopies performed, but require a standardized gastroscopy technique, a strong awareness of EGC screening, and a good ability to identify EGC by endoscopy. Training in these aspects effectively improved the ability of endoscopists to detect EGC.
Between January 1, 2013, and May 1, 2014, a total of 25,314 gastroscopy examinations were performed at our center, with a detection rate of EGC of only 0.2%. Senior endoscopists performed a large number of gastroscopy examinations, but their EGC detection rate was only 0.06%, whereas younger endoscopists had a higher detection rate of 0.7%. In particular, training significantly increased the EGC detection rate to as high as 1.5%. In general, clinical diagnosis of EGC is difficult because most patients are asymptomatic and lesions may be missed during gastroscopy or may be mistakenly considered nonneoplastic. Additionally, the biopsy sample may be insufficient for diagnosis. Thus, unlike ordinary gastroscopy, endoscopic diagnosis for EGC places higher demands on endoscopists.
Younger endoscopists have more time, willingness, and enthusiasm to undergo training on EGC diagnosis. Indeed, extensive training in the diagnosis of EGC has been found to improve the lesion detection rate.22 In that study, the training duration was 2 years, whereas in the current study, the training period was much shorter. However, even a short training period significantly improved the detection rate of EGC, from 0.2% before to 2.3% after training. Our endoscopy center performs approximately 17,800 gastroscopies annually, allowing young endoscopists to gain considerable experience during a short period of time. The incidence of gastric cancer is high in China, making the ability of endoscopists to detect EGC of utmost importance.
From September 2013 to May 2014, W.G. and Q.Z. performed a total of 1927 gastroscopies on 1292 outpatients and 635 inpatients. Most patients had gastrointestinal symptoms, and 194 had a previous medical history of atrophic gastritis, gastric ulcer, or gastric surgery, putting them at high-risk for gastric cancer. Multivariate analysis showed that screening of high-risk populations can aid the detection of EGC (OR = 1.6). Gastric atrophy is a risk factor for gastric cancer, with tumorigenesis occurring through a gastritis–atrophy–metaplasia–cancer sequence.23,24 The current study did not analyze Helicobacter pylori (HP) infection in these patients. However, HP infection is a risk factor for gastric cancer, with the risk of gastric cancer being 2- to 3-fold higher in patients with than without chronic HP infection.25,26 The ABC classification, including concomitant measurement of serum pepsinogen and serum anti-HP antibodies, is used in population-based screening of gastric cancer in Japan and is a good method of identifying patients at high-risk for gastric cancer.23,27 Additionally, studies have shown that the risk of cancer remains high 15 years after gastric surgery, particularly gastric ulcer surgery.28–31 Thus, screening for EGC in high-risk patients of gastric cancer is very important, and gastroscopy should be performed by trained endoscopists having plentiful experience in EGC diagnosis, when these patients receive gastroscopies.
The ability to identify suspicious lesions under ordinary white-light imaging (WLI) endoscopy is very crucial. In the present study, 2 patients showed changes in the color of the gastric mucosa (0–IIb). Congested and whitish mucosa may be endoscopic features of EGC, with whitish mucosa suggesting gastric signet-ring cell carcinoma and diffuse gastric cancer.32 Most of the EGCs in our study were the superficial depressed type (0–IIc), which is the most frequent morphological type of EGC.33 Other types of EGC had the protruding (0–I), superficial elevated (0–IIa), superficial flat (0–IIb), and mixed type lesions.33 Of the 1927 patients, 303 underwent M-NBI, with multivariate analysis showing that the use of M-NBI played a role in detecting EGC. Combining conventional WLI endoscopy and M-NBI was shown to have high diagnostic accuracy for EGC, with a sensitivity of 95% and a specificity of 97%.34
Although suspicious lesions are usually biopsied, the size of an EGC may be small or the lesion may not be obvious under conventional white-light endoscopy. Thus, biopsy may be inaccurate or of insufficient depth, resulting in false-negative pathological results. In our study, only 10 of the 28 EGCs, which were detected by W.G. and Q.Z. from September 2013 to May 2014, were preliminarily diagnosed as high-grade neoplasias by tissue biopsy. The other 18 patients were not diagnosed by tissue biopsy, but the lesions of 16 patients exhibited features typical of EGC under M-NBI. These lesions were removed by ESD, with pathological examination showing that all were high-grade neoplasias. M-NBI can be used as an optical biopsy tool.35 Moreover, endoscopists should be aware that the diagnosis of EGC should not rely solely on tissue biopsy findings.
Our study was a single-center study, and a summary of the experience of a single endoscopy center. Nevertheless, the study included a large sample size, and the research result may be applicable to other endoscopy centers, especially to those in which the rate of detection of EGC by endoscopists is not satisfactory.
CONCLUSIONS
In summary, training on EGC diagnosis can improve the rate of detection of EGC by endoscopists. M-NBI contributed to the diagnosis of EGC.
Footnotes
Abbreviations: ESD = endoscopic submucosal dissection, M-NBI = magnifying endoscopy with narrow-band imaging, OR = odds ratio, CI = confidence interval, HP = Helicobacter pylori.
This study was supported by the grant from the National Natural Science Foundation of China (No. 81101610), Scientific Research Projects of Guangdong Province (No. 2013B021800311) and Medical Research Foundation of Guangdong Province (No. WSTJJ2011110142092192119760913465X)
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014; 20:4483–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol 2006; 12:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2007: a study of 21 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. J Clin Oncol 2013; 43:328–336. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Lee HS, Kim N, et al. Prevalence and clinicopathologic characteristics of gastric cardia cancer in South Korea. Helicobacter 2012; 17:358–368. [DOI] [PubMed] [Google Scholar]
- 6.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010; 25:479–486. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. http://globocan.iarc.fr Accessed September 20, 2013. [Google Scholar]
- 8.Murakami T. Pathomorphological diagnosis. Definition and gross classification of early gastric cancer. Gann Monogr Cancer Res 1971; 11:53–55. [Google Scholar]
- 9.Marrero JM, Savalgi RS, McCormick C, et al. Progression of gastric mucosal dysplasia of the postgastrectomy stomach. Surg Technol Int 2000; 9:333–337. [PubMed] [Google Scholar]
- 10.Yeoh KG. How do we improve outcomes for gastric cancer? J Gastroenterol Hepatol 2007; 22:970–972. [DOI] [PubMed] [Google Scholar]
- 11.Everett SM, Axon AT. Early gastric cancer in Europe. Gut 1997; 41:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada M, Oda I, Taniguchi H, et al. Chronological trend in clinicopathological characteristics of gastric cancer. Nihon Rinsho 2012; 70:1681–1685. [PubMed] [Google Scholar]
- 13.Kitagawa M. Current situation and future of gastric cancer screening examinations: from the viewpoint of gastric X-ray screening. Nihon Hoshasen Gijutsu Gakkai Zasshi 2005; 61:881–886. [DOI] [PubMed] [Google Scholar]
- 14.Sugano K. Gastric cancer: pathogenesis, screening, and treatment. Gastrointest Endosc Clin N Am 2008; 18:513–522. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Tada M, Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy 1995; 27:27–31. [DOI] [PubMed] [Google Scholar]
- 16.Suvakovic Z, Bramble MG, Jones R, et al. Improving the detection rate of early gastric cancer requires more than open access gastroscopy: a five year study. Gut 1997; 41:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters SL, Hasan AG, Jacobson NB, et al. Level of fellowship training increases adenoma detection rates. Clin Gastroenterol Hepatol 2010; 8:439–442. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Chung IK, KIM SJ, et al. An adequate level of training for technical competence in screening and diagnostic colonoscopy: a prospective multicenter evaluation of the learning curve. Gastrointest Endosc 2008; 67:683–689. [DOI] [PubMed] [Google Scholar]
- 19.Spier BJ, Benson M, Pfau PR, et al. Colonoscopy training in gastroenterology fellowships: determining competence. Gastrointest Endosc 2010; 71:319–324. [DOI] [PubMed] [Google Scholar]
- 20.Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy 2009; 41:462–467. [DOI] [PubMed] [Google Scholar]
- 21.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002; 51:130–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazato T, Oyama T, Yoshida T, et al. Two years’ intensive training in endoscopic diagnosis facilitates detection of early gastric cancer. Intern Med 2012; 51:1461–1465. [DOI] [PubMed] [Google Scholar]
- 23.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut 2005; 54:764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer 2014; 134:1445–1457. [DOI] [PubMed] [Google Scholar]
- 25.Danesh J. Helicobacter pylori and gastric cancer: systematic review of epidemiological studies. Aliment Pharmacol Ther 1999; 13:851–856. [DOI] [PubMed] [Google Scholar]
- 26.Eslick GD, Lim LL, Byles JE, et al. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol 1999; 94:2373–2379. [DOI] [PubMed] [Google Scholar]
- 27.Inoue K. Gastric cancer screening using ABC classification. Nihon Rinsho 2012; 70:1790–1794. [PubMed] [Google Scholar]
- 28.Tersmette AC, Giardiello FM, Tytgat GN, et al. Carcinogenesis after remote peptic ulcer surgery: the long-term prognosis of partial gastrectomy. Scand J Gastroenterol 1995; 212 (suppl):S96–S99. [DOI] [PubMed] [Google Scholar]
- 29.Fisher SG, Davis F, Nelson R, et al. A cohort study of stomach cancer risk in men after gastric surgery for benign disease. J Natl Cancer Inst 1993; 85:1303–1310. [DOI] [PubMed] [Google Scholar]
- 30.Tersmette AC, Offerhaus GJ, Tersmette KW, et al. Meta-analysis of the risk of gastric stump cancer: detection of high risk patient subsets for stomach cancer after remote partial gastrectomy for benign condition. Cancer Res 1990; 50:6486–6489. [PubMed] [Google Scholar]
- 31.Molloy RM, Sonnenberg A. Relation between gastric cancer and. previous peptic ulcer disease. Gut 1997; 40:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HH, Lee SY, Yoon HY, et al. Change of mucosal color in early gastric cancer. J Dig Dis 2012; 13:510–516. [DOI] [PubMed] [Google Scholar]
- 33.Schlemper RJ, Hirata I, Dixon MF. The macroscopic classification of early neoplasia of the digestive tract. Endoscopy 2002; 34:163–168. [DOI] [PubMed] [Google Scholar]
- 34.Ezoe Y, Muto M, Uedo N, et al. Magnifying narrowband imaging is more accurate than conventional white-light imaging in diagnosis of gastric mucosal cancer. Gastroenterology 2011; 141:2017–2025. [DOI] [PubMed] [Google Scholar]
- 35.Yao K, Doyama H, Gotoda T, et al. Diagnostic performance and limitations of magnifying narrow-band imaging in screening endoscopy of early gastric cancer: a prospective multicenter feasibility study. Gastric Cancer 2014; 17:669–679. [DOI] [PubMed] [Google Scholar]


