Abstract
Hepatic angiosarcoma (HAS) is rare but often fatal. A review of literature in 1979 found that only 3% of the 70 patients lived for more than 2 years, but the survival might have been improved over the years. We conducted a retrospective study and reviewed the medical records of patients who visited a teaching hospital in Taiwan from January 2000 to August 2010 and had pathological proof of HAS. In addition, we conducted a review of literature and compared those who survived for 2 years or more to those who did not. Of the 3503 patients with primary liver cancer we identified, 9 had HAS, of whom 3 (33.3%) survived for 2 years or more. One survived for 24 months without surgical resection, and the other two received surgery with postoperative chemotherapy and were still alive 32 and 37 months later, respectively. Through reviewing literature, we identified 3 more patients in Taiwan who had survived for 2 years or more. One survived for 42 months without surgical resection, the other two received segmentectomy with postoperative chemotherapy or radiotherapy. We also identified 8 such cases outside Taiwan, including 1 who received chemotherapy without surgery and survived for 53 months. None of the differences in the clinical characteristics between those who had and had not survived for 2 years or more reached statistical significance. In conclusion, we believe the combination of surgery and adjuvant chemotherapy may be able to achieve long-term survival in some HAS patients nowadays, and it is even possible to achieve fair survival using chemotherapy alone.
INTRODUCTION
Hepatic angiosarcoma (HAS) is a rare type of liver cancer, accounting for <1% of all sarcomas1 and only about 2% of hepatic malignancy.2,3 It is related to environmental toxins such as arsenic, vinyl chloride monomer, thorium dioxide (Thorotrast), and radium;2,4,5 however, many patients have no known exposures to these carcinogens.2,6 The most common initial complaints are nonspecific symptoms such as right upper quarterant pain, fatigue, weakness, and weight loss. Many patients are diagnosed accidentally without symptoms, making it rarely diagnosed antemortem.2 Surgical resection is the most effective treatment2,7,8 but usually fails due to advanced tumors and rapid recurrence with consequent unresectability.7,9 In the past, delayed diagnosis and rapid progression of disease lead to death in most patients within 6 months after disease onset,2,7,8 mainly due to tumor rupture, disseminated intravascular coagulation, and hepatic failure.2 In 1979, a review of the literature found that only 3% of the 70 patients had lived for more than 2 years.2 With the advancement of medical care since then, however, some studies found that patients could survive for a long period after hepatic resection.7–10 Recent studies also found that chemotherapy could prolong the survival of patients with advanced HAS.6,11 Therefore, we conducted a study to evaluate the improvement in the survival of HAS patients over the years and to identify treatment that may lead to long-term survival, which is defined as survival for 2 years or more on the basis of the previous review in 1979.2
METHODS
Patients
We conducted a study at Kaohsiung Veterans General Hospital in Kaohsiung, which is a 1,330-bed teaching hospital adjacent to the major PVC production area and near an endemic area of arsenic exposure from drinking water—generally known as the “blackfoot diseases endemic area.”4 We reviewed the medical records of patients older than 20 years with primary liver cancers and identified those who were diagnosed as having HAS through pathological examination of specimens obtained by fine-needle aspiration or surgical procedures from January 2000 to August 2010. We identified HAS patients who had long-term survival for further study. The research was performed in accordance with the principles of the Declaration of Helsinki and had been approved by the Institutional Review Board at the Kaohsiung Veterans General Hospital.
Clinical Evaluation of Patients
The onset of disease was defined as the time of appearance of clinical symptoms and signs that were ascribed or related to the disease, or the time of accidental finding of tumor by image studies. On each patient, we collected data on demographic characteristics (age, sex, occupational history, and exposures to related carcinogens), Eastern Cooperative Oncology Group (ECOG) performance status,12 medical history, laboratory tests (hemogram and biochemistry), markers of hepatitis B virus (hepatitis B surface antigen) and hepatitis C virus (anti-hepatitis C antibody) infections, markers of hepatic tumor (alpha-fetoprotein, carbohydrate antigen19–9, and carcinoembryonic antigen), 15-minute retention rate of indocyanine green test,13 clinical course, and treatment modalities. Image studies, including sonography, magnetic resonance imaging (MRI), and computed tomography (CT), were reviewed. Characteristics of HAS, including the size, location, pathological pattern, tumor number, existence of liver rupture, and metastatic sites were also recorded. Evaluation of response to therapies was carried out using the Response Criteria in Solid Tumors as reference.12,14 Tumor stages before therapy were determined by the TNM stage of the American Joint Committee on Cancer 7th Edition.15 The occupational history and environmental exposures to toxic agents including underground water drinking were studied. The survival periods were recorded from disease onset.
Identification of Cases in the Literature
To confirm our findings, we conducted a thorough literature search in the PubMed database using “angiosracoma” as the key word and reviewed the retrieved articles to identify cases of HAS, with a focus on those reported in Taiwan. Further search of the literature was conducted through reviewing the references listed in retrieved articles.16–23
Statistical Analysis
To identify factors affecting the survival, we compared patients with and without long-term survival. Differences between the 2 groups were evaluated using the Mann–Whitney U test for continuous variables and Fisher exact test for categorical variables. We performed statistical analyses using SPSS software for Windows (Version 17; SPSS Inc., Chicago, IL, USA) and considered p values <0.05 as statistically significant.
RESULTS
Cases Identified at the Study Hospital
Of the 3503 patients with primary liver cancer, including 2563 (73.2%) men and 940 (26.8%) women, who visited the hospital during the study period, 9 had HAS. Three (33.3%) of the HAS patients had long-term survival (Tables 1 and 2), and we summarized the clinical courses in Figure 1.
TABLE 1.
Initial Clinical Features of Patients of Hepatic Angiosarcoma with Survival for 2 Years or More
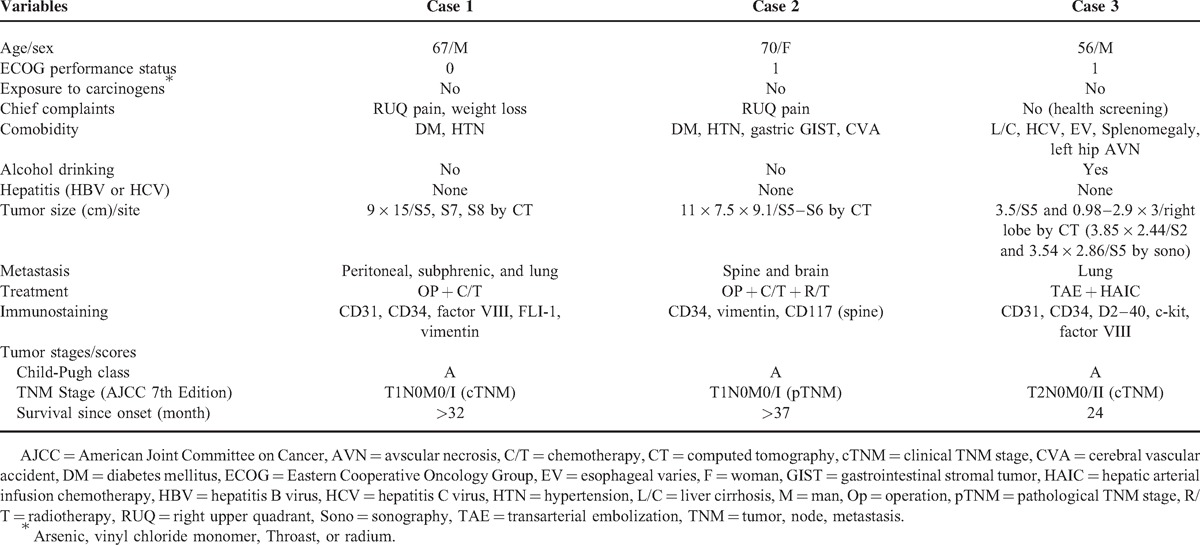
TABLE 2.
Cases of Hepatic Angiosarcoma with Survival for 2 Years or More in the Current Study and the Literature
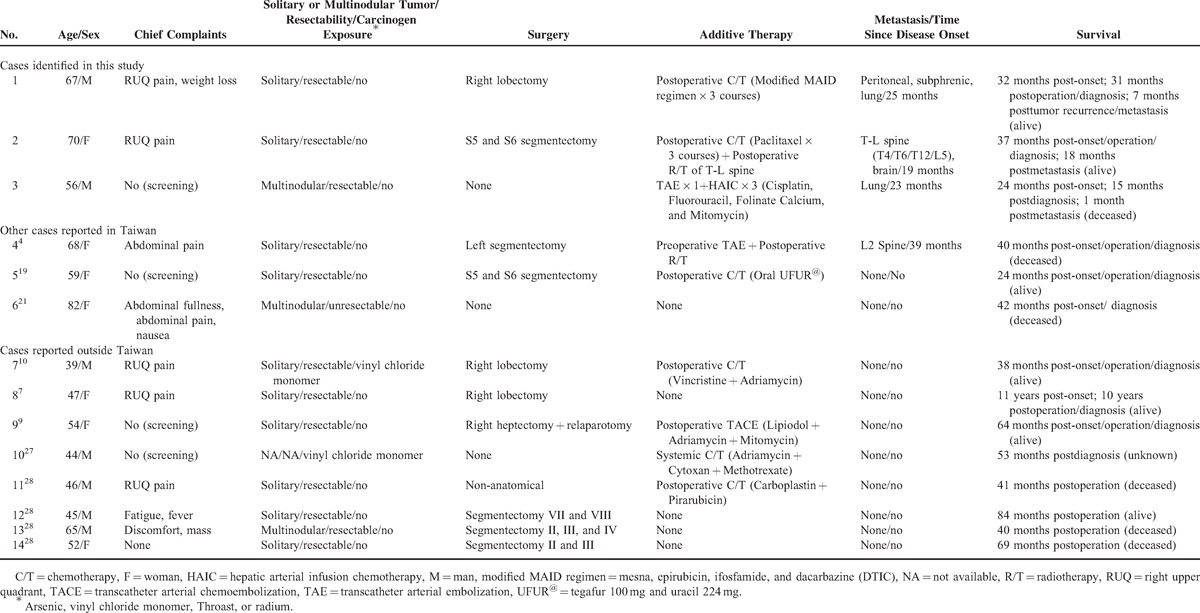
FIGURE 1.
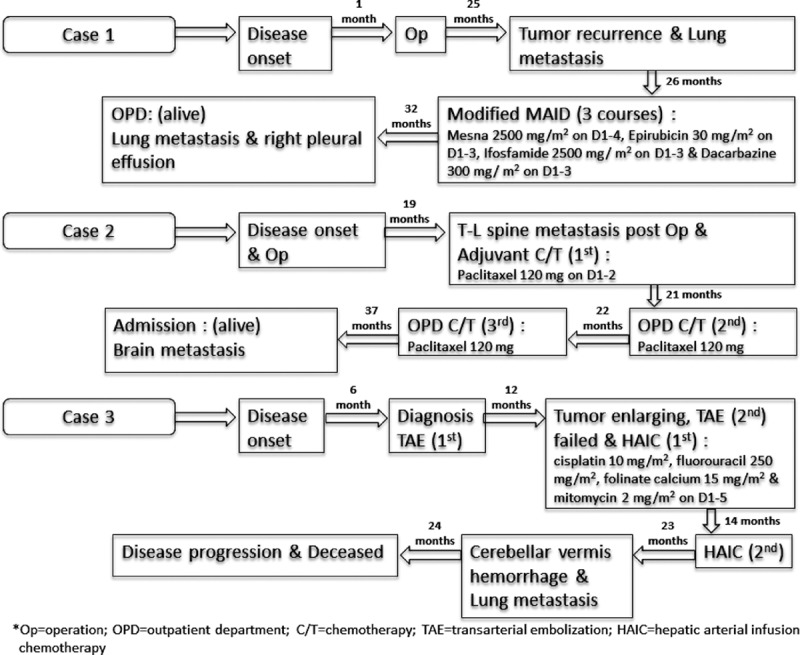
Treatment protocols and outcomes of three individual patients.
Case 1 was a 67-year-old man referred from a local hospital due to the finding of a liver tumor by sonography after having right upper abdominal pain for 1 week and body weight loss of 3 kg in 1 month. Abdominal CT revealed a mass (Figure 2A), and fine needle aspiration led to the diagnosis of sarcoma. Angiography arranged under the suspicion of intracapsular rupture found a hypervascular tumor, and MRI displayed a complex cystic lesion with intracapsular hemorrhage (Figure 2B). He received extended right hepatic lobectomy and cholecystectomy. A surgical specimen of a 16 × 15 × 9.5 cm tumor beneath the liver capsule and a 0.5 cm free margin with intracapsular hemorrhage led to the diagnosis of HAS. After 25 months of regular follow-up, tumor recurrence in the right subphrenic area with peritoneal and lung metastases was found (Figure 2). The second laparotomy failed due to severe adhesions. He received 3 courses of chemotherapy with a modified MAID regimen including mesna 2500 mg/m2 from day 1 to day 4, epirubicin 30 mg/m2 from day 1 to day 3, ifosfamide 2500 mg/m2 from day 1 to 3, and dacarbazine 300 mg/m2 from day 1 to 3 (Figure 1). He tolerated the courses well, although an episode of leukopenia fever with bacteremia occurred after the chemotherapy. The last hospital visit was 32 months after disease onset and revealed progression of lung metastasis with right pleural effusion.
FIGURE 2.
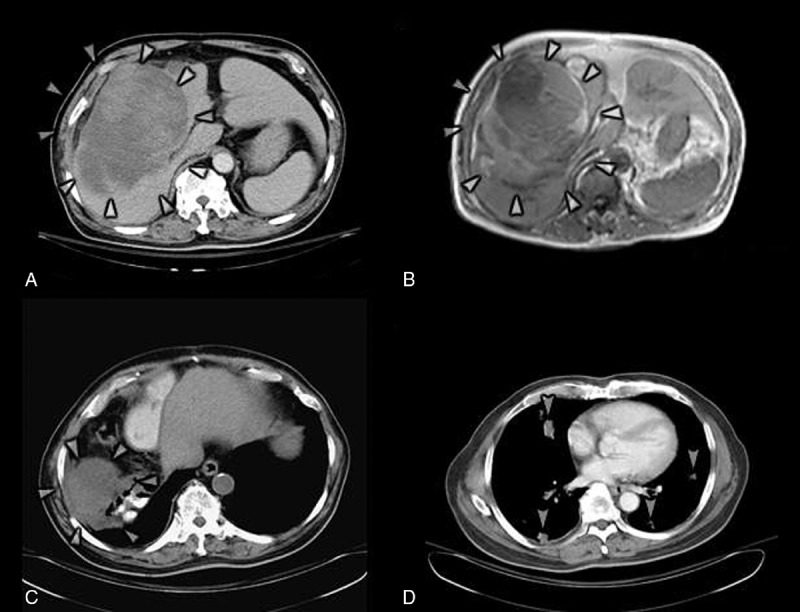
Images of case 1. (A) Abdominal CT scan with contrast shows a 9 × 15 cm mixed high and low-density mass in S5, S7, and S8 (arrowheads). Portal vein and IHDs are compressed. (B) MRI of abdomen (T2WI) displays a 9 × 11 × 15 cm complex cystic lesion in S5 to S8 with right portal vein, right IHD, and hilum compressions (arrowheads). S5–S8 tumor with intracapsular hemorrhage with mass effect over right IHD, and right portal vein. (C) Abdominal CT scan with noncontrast demonstrates a low-density mass lesion approximately 8.4 cm in size in the right subphrenic area which identifies the peritoneal subphrenic tumor recurrence and seeding (arrowheads). (D) Chest CT scan with contrast exhibits numerous nodular lesions within both lungs of up to 2 cm in size, consistent with multiple metastases (arrows). CT = computed tomography, IHD = intrahepatic duct, MRI = magnetic resonance imaging.
Case 2 was a 70-year-old woman who visited the outpatient clinic due to right upper abdominal pain for 1 week and was found to have a hepatic tumor by CT (Figure 3A). She received cholecystectomy and liver segmentectomy of S5 and S6. A surgical specimen including a well-circumscribed 12.2 × 8.6 × 6.5 cm tumor in the subcapsular area and a 0.7 cm free margin led to the diagnosis of HAS. After regular follow-up for 19 months, she was found to be suffering from progressive lower leg weakness. MRI showed multiple metastases to T-L spine (Figure 3B). She received L4-L5 laminectomy and tumor resection with a pathological proof of angiosracoma. Palliative radiotherapy of T-L spine and postoperative chemotherapy were arranged. She accepted 3 courses of paclitaxel regimen (Figure 1) and suffered from general weakness and urinary incontinence 23 months from disease onset. Urinary tract infection, acute renal failure, and diabetic ketoacidosis were diagnosed. A brain CT showed a bony defect over the left parietal area without metastasis. A follow-up brain CT 1 month later confirmed a recent cerebral infarction over the bilateral basal ganglion, left thalamus, and periventricular area (Figure 3C). With progressive right hemiplegia, she suffered from general weakness, aphasia, and dysphagia 1 week later, and a brain CT demonstrated a metastatic lesion (Figure 3D). She became bedridden and depended on nasogastric tube feeding and Foley catheterization. She accepted hospice care at home and was still alive 37 months after the disease onset when she was discharged.
FIGURE 3.
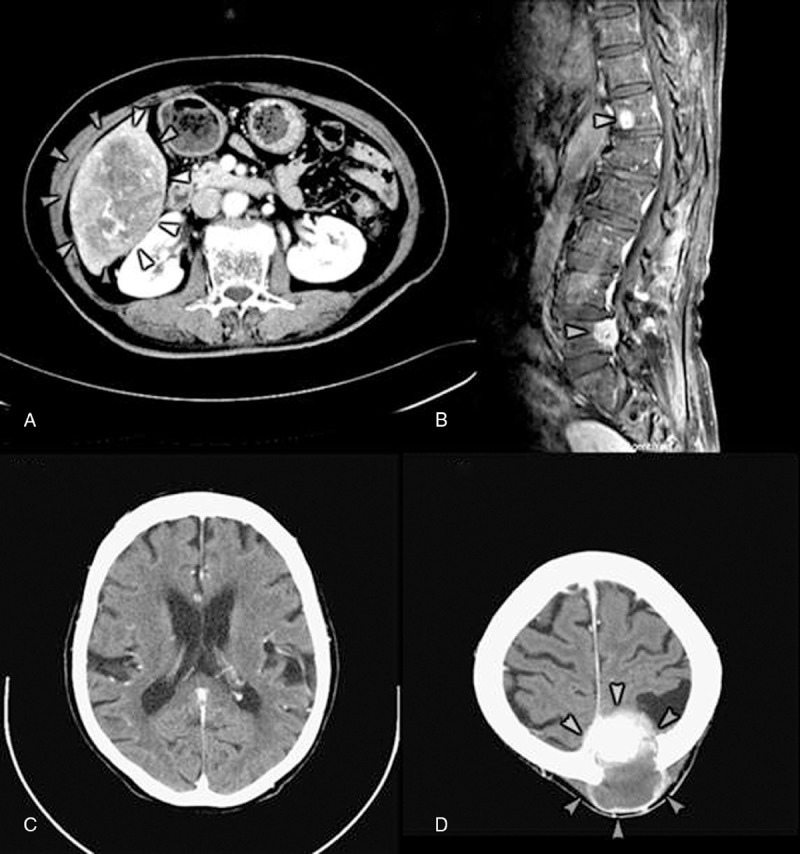
Images of case 2. (A) Abdominal CT with contrast shows a hypervascular tumor sized 11.0 × 7.5 × 9.1 cm over S5 and S6 of liver (arrowheads). (B) MRI of T-L spine (T2WI) shows degenerative spondylolisthesis at L4-L5 and multiple bony metastasis over T-L spine (T4, T6, T12 [arrowhead] and L5 [arrowhead]) with anterior epidural invasion and thecal sac compression at L5. (C) Brain CT with contrast shows small low densities involve bilateral basal ganglion, left thalamus, and left periventricular white matter, in favor of recent ischemic infarction. (D) Brain CT with contrast displays a mass lesion of 5.2 × 5.4 × 5.0 cm, manifesting hypervascularity, partial enhanced solid component and partial cystic change, involving left high parietal bone with skull destruction, epidural extension, and suspicious superior sagittal sinus invasion with compression of adjacent brain parenchyma (arrowheads). Left high parietal bone metastasis with epidural extension is indicated. CT = computed tomography, MRI = magnetic resonance imaging.
Case 3 was a 56-year-old man admitted through an outpatient clinic for biopsy of two enlarging liver nodules (3.85 × 2.44 cm in S2 and 3.54 × 2.86 cm in S5) found during regular follow-up for liver cirrhosis related to alcoholism and HCV. He had refused the biopsy when the nodules were first observed 6 months previously with an initial impression of hepatocellular carcinoma (HCC). An abdominal CT later showed a pedunculated tumor over S5 (Figure 4). However, an MRI following the CT displayed 3 nodules in the left lobe and another 3 in the right lobe approximately 0.98–2.9 cm with centripetal contrast filling in the dynamic study. Abdominal CTs 4 and 5 months later displayed a low-density mass in the left lobe and several lesions over S5 that were not well demonstrated in the venous phase (Figure 4). A fine needle aspiration led to the diagnosis of HAS. He accepted transarterial embolization (TAE) after refusing an operation or systemic chemotherapy. Abdominal sonography showed a 3.16 × 2.54 cm enlarging mass in the right lobe 3 months later. A second course of TAE failed due to obvious arterioportal shuntings. Hepatic arterial infusion chemotherapy (HAIC) was arranged instead. He accepted 2 courses of HAIC which included cisplatin, fluorouracil, folinate calcium, and mitomycin for 5 days in each course (Figure 1). The abdominal CT after the first course revealed suspected multiple residual or recurrent tumors (Figure 4). An abdominal CT 3 months after the second course found several ill-defined enhancing lesions in both lobes, indicating residual of HAS in stable disease or minimal progressive (Figure 4). A follow-up abdominal CT 21 months after the disease onset found tumor regression (Figure 4). Another episode of acute cerebellar vermis hemorrhage with bilateral lung metastasis occurred 23 months after disease onset, and he died of disease progression 24 months after disease onset.
FIGURE 4.
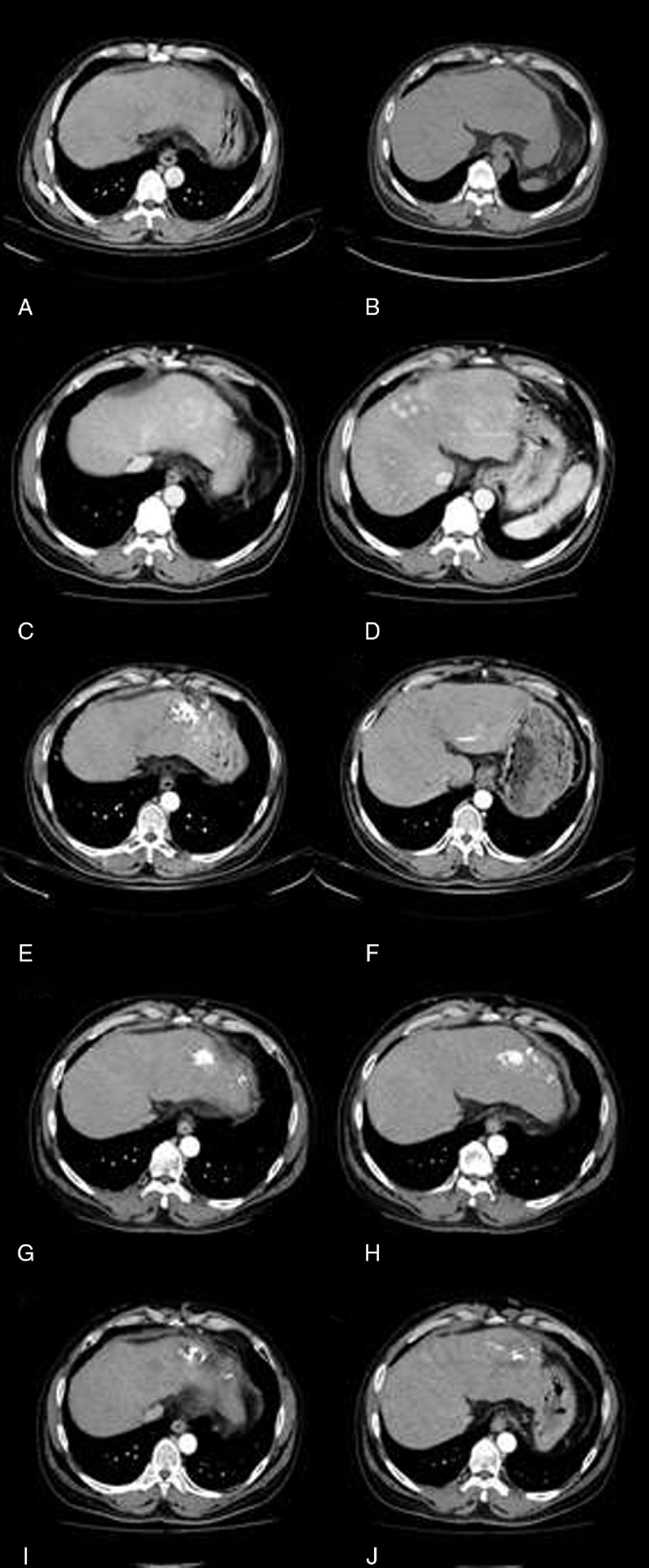
Three-phase abdominal CT scan of case 3. (A, B) Images of initial finding (after follow-up for 1 month) show a pedunculated tumor, approximately 3.5 cm in size, at S5 of liver, with arterial enhancement and equivocal washout in venous phase, suggestive of HCC. (C, D) Images after follow-up for 5 months display a low-density mass of 3.5 cm with persistent contrast enhancement (29/54/68 Hu) in left hepatic lobe (C), and several enhancing lesions of 1 to 3 cm in right lobe of liver S5 are noted. These lesions cannot be well demonstrated on venous phase (D). Liver hemangiomas are suspected. (E, F) Images after the first course of TAE and 1 course of HAIC (after follow-up for 12 months) exhibit several incomplete Lipiodol retentions within both lobes of liver, up to 6 × 5 cm in size at S3 (E). Multiple residual or recurrent tumors are noted (F). Progression of viable tumors is indicated. (G, H) Images after the second course of HAIC (after follow-up for 17 months) demonstrate several ill-defined enhancing lesions of 1 to 2 cm in both lobes of liver, compatible with residual angiosarcoma. Post-TAE changes with residual Lipiodol in the liver are noted. Stable disease or minimal progressive changes in comparison with CT before treatment are found. (I, J) Images after two course of HAIC (after follow-up for 21 months) show several incomplete Lipiodol retentions within both lobes of liver, up to 5 cm at S3. Multiple residual or recurrent tumors are indicated. Tumor regressive changes are noted in comparison with the previous study before treatment. CT = computed tomography, HAIC = hepatic arterial infusion chemotherapy, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, TAE = transarterial embolization.
None of the 3 patients had exposure to specific carcinogens such as arsenic, vinyl chloride monomer, Throast, or radium.
Cases Identified Through Literature Review
Through the review of literature on HAS in Taiwan, we found 9 more patients (5 men, 4 women) of HAS whose individual data were available.4,16–23 Eight of them had information on survival. Of those who had survival data, 3 had long-term survival, yielding a 2-year survival rate of at least 33.3% (Table 2). Two patients had received surgery and adjuvant chemotherapy. One died 40 months after disease onset, and the other survived 24 months after disease onset. The third patient received conservative treatment only and survived for 42 months. Through the review of the literature, we also identified 8 cases outside Taiwan with long-term survival, including 1 who had received chemotherapy without surgery and survived for 53 months (Table 2). Among the remaining 7 cases who had received surgery, 4 did not receive postoperative chemotherapy or radiotherapy.
Comparison of Taiwanese Patients With and Without Long-Term Survival
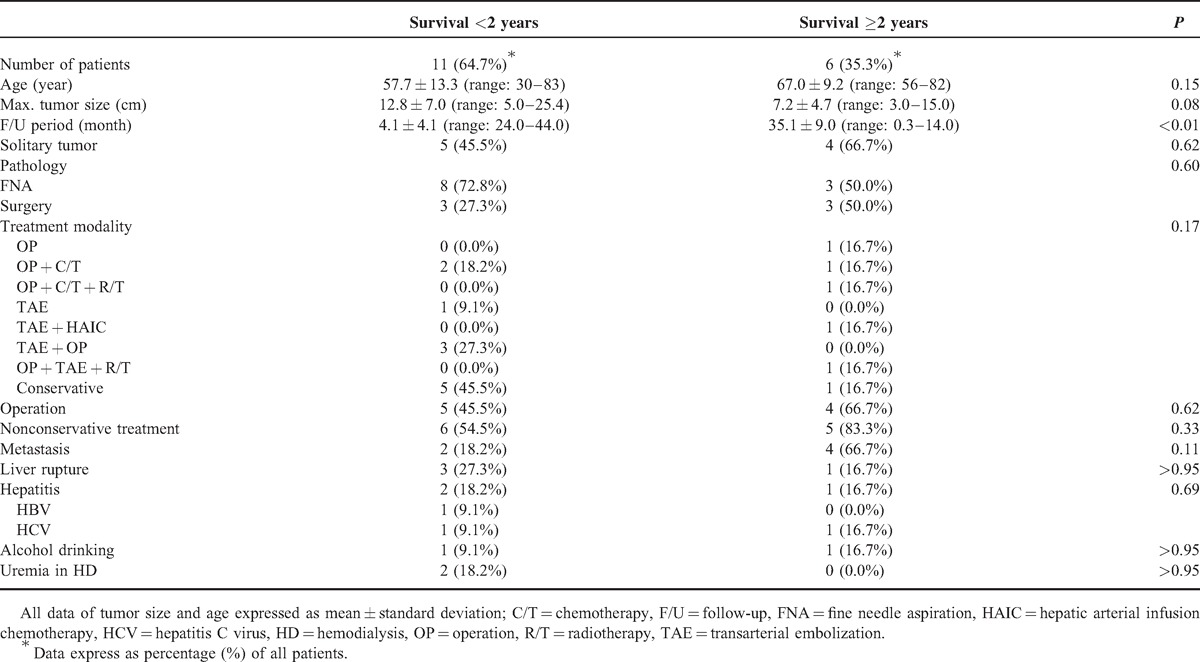
The 9 patients (6 men, 3 women) identified in the current study and 8 (4 men, 4 women) identified in the review of the literature in Taiwan had an average duration of follow-up for 15.1 ± 16.4 months (Table 3). The average age at diagnosis was 61.2 ± 11.2 years, and patients with long-term survival were more than 9 years older (67.0 ± 9.2 vs. 57.7 ± 13.3), although the difference did not reach statistical significance (p = 0.15). Except for survival, none of the differences reached statistical significance, but patients with long-term survival tended to have a smaller maximum tumor size (7.2 ± 4.7 vs. 12.8 ± 7.0, p = 0.08), metastasis (66.7% vs. 18.2%, p = 0.11), and nonconservative treatment (83.3% vs. 54.5%, p = 0.33) (Table 3). None of the differences in the laboratory tests between patients with and without long-term survival reached statistical significances.
TABLE 3.
Demographic Data on 17 Patients of Hepatic Angiosarcoma in Taiwan, Comparisons Between Survival for ≥2 Years or Not
DISCUSSION
Our study showed that the prognosis of HAS patients has improved nowadays with 33.3% having long-term survival, which is compatible with the finding from the review of cases reported in Taiwan that at least 3 of the 9 (also 33.3%) cases (all reported after 1985) had long-term survival (Table 2). In addition to the advancement in medical technology, the fact that Taiwan has a comprehensive and affordable national health insurance may contribute to the improvement.
The treatment of HAS has not been standardized.24 Complete surgical resection of the tumor in a solitary nodule or limited resectable lobes was recognized as the only effective therapy,5,7–11,24–26 but many patients could not meet the criteria due to delayed diagnosis.2,5 Of the 6 Taiwanese patients with long-term survival identified in our study and literature review, 4 had had solitary tumors. From the literature, we identified 8 patients with long-term survival outside Taiwan,7,9,10,27,28 and 7 had descriptions of the number of tumors—all but 1 had solitary tumors (Table 2). Altogether, of the 10 long-term-survival patients with data, 8 with solitary tumors had received surgery and survived for an average of more than (some of the patients were still alive when they were reported, and the durations of survival in some cases were counted starting from the operation instead of disease onset) 61.5 months since disease onset, while the other 2 with multiple tumors had not received surgery and survived for an average of 41.0 months (p = 0.71). When the 3 cases identified by the current study were added, the 10 cases with solitary tumors survived for an average of more than 56.1 months, while the other 3 with multiple tumors survived for an average of only 35.3 months (p = 0.57). The differences did not reach statistical significance, most likely because patient numbers were small.
Tumor recurrence or metastasis in nonvital organs does not necessarily lead to immediate mortality in HAS patients. Four (28.6%) of the 14 patients with long-term survival (identified in our study and literature) had recurrence or metastasis after follow-up for 26.5 months on average since disease onset and had survived for an average of more than 6.8 months thereafter, with 1 for more than 18 months. Taiwanese patents with long-term survival tended to have a larger proportion of patients with metastasis (66.7% vs. 18.2%, p = 0.11) (Table 3), most likely because the longer survival provided more time for developing metastases. Soft tissue sarcomas are generally recognized as radioresistant,29 and therefore, radiotherapy is not considered as a part of therapeutic modality7 but can be used as a palliative measure.13,30–32 In our study, the palliative radiotherapy of T-L spine for case 2 successfully relieved the symptoms.
TAE is widely used in traumatic laceration of liver33 and bleeding from rupture of hepatic tumors, including HAS.34–37 TAE has been applied to the treatment of HCC.38–40 Transcatheter arterial chemoembolization (TACE) has been adopted as a palliative therapy for unresectable hepatic tumors, especially for HCC or hepatic metastatic tumor,34,38,41–44 but comparisons between TACE and TAE in randomized controlled trials did not yield consistent results.41,45,46 For the treatment of multinodular HCC, in addition to TACE,38,42,43,47 HAIC was an another choice.42,48 In comparison with systemic chemotherapy, HAIC and TACE had regional effects of high hepatic extraction rate by first pass effect and prolonged drug exposure.42,48 Like HCC, HAS is a hypervascular tumor,26,49 and similar modalities had been applied to HAS patients. In our study, case 3 had a response duration of 9 months and a survival of 32 months since disease onset, and case 9 had a survival of at least 64 months with surgery and postoperative TACE.9 Chemotherapy alone, even regional chemotherapy such as HIAC or TACE, may still lead to a favorable survival in cases with solitary tumors without metastasis. The experiences in successful cases in our study and the literature33–35,50 call for further large scale studies to confirm the efficaciousness of systemic chemotherapy and regional HAIC.
In HAS patients, unresectability of tumor, multinodular and diffuse tumor, tumor-infiltrated resection margin, poor-differentiated pattern of tumor, advanced stage, and hemoperitoneum with tumor rupture have been recognized as poor prognostic factors.5–11,24–26,34–37 A good ECOG performance status, effective chemotherapy (systemic chemotherapy and hepatic artery infusion) and a well-differentiated tumor have been proposed as good prognostic factors.6 Of the 10 long-term survival patients, only 3 (cases 1–3) had enough data for calculating ECOG scores and they were all relatively well (0, 1, and 1; Table 1). Among patients in Taiwan (from our study and the literature combined), patients with long-term survival tended to have smaller tumor size (7.2 vs. 12.8 cm, p = 0.08; Table 3). Although tumor size seemed to be a key factor for survival (overall therapeutic effect), our study does not have enough number of cases to categorize them further by tumor size to evaluate its effects on each type of treatment modality. In fact, in our literature review, we did not identify any study that has evaluated the effects of tumor size on a treatment modality. Further studies are needed to identify the factors affecting the survival after different treatment modalities. Tumor staging systems have been widely used to describe severity, but no consensus has been reached on HAS. Further studies should be conducted to evaluate their application to HAS, and the development of a specific staging for HAS might be necessary.
Our study used a retrospective design, which limits the amount of available data. Even with the inclusion of 3 patients reported in Taiwan and 4 outside Taiwan, the total number of patients with long-term survival was not large, which limits further analyses. Nonetheless, we believe our work has provided the most comprehensive individual data on HAS patients who survived for 2 years or more so far.
In conclusion, we can reasonably infer that a long-term survival of HAS patients can be achieved through aggressive treatment, especially complete resection of a resectable solitary tumor or a multinodular but confined tumors. A combination of surgery and adjuvant chemotherapy may be able to achieve long-term survival in some of the patients nowadays, and it is even possible to obtain limited but favorable survival in cases having multinodular tumors with metastasis using chemotherapy alone.
Acknowledgments
The authors thank the support from the National Science Council of Taiwan and Sin-Lau Christian Hospital.
Footnotes
Abbreviations: CT = computed tomography, ECOG = Eastern Cooperative Oncology Group, HAIC = hepatic arterial infusion chemotherapy, HAS = hepatic angiosarcoma, HCC = hepatocellular carcinoma, MRI = magnetic resonance imaging, TACE = transcatheter arterial chemoembolization, TAE = transarterial embolization.
This work was supported by Grant No. NSC100–2314-B-006–061 from the National Science Council of Taiwan and a grant from the Sin-Lau Christian Hospital.
H-RG and S-YH contributed equally to this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bardwil JM, Mocega EE, Butler JJ, Russin DJ. Angiosarcomas of the head and neck region. Am J Surg 1968; 116:548–553. [DOI] [PubMed] [Google Scholar]
- 2.Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore) 1979; 58:48–64. [DOI] [PubMed] [Google Scholar]
- 3.Neshiwat LF, Friedland ML, Schorr-Lesnick B, Feldman S, Glucksman WJ, Russo RD., Jr Hepatic angiosarcoma. Am J Med 1992; 93:219–222. [DOI] [PubMed] [Google Scholar]
- 4.Ho S-Y, Tsai C-C, Tsai Y-C, Guo H-R. Hepatic angiosarcoma presenting as hepatic rupture in a patient with long-term ingestion of arsenic. J Formos Med Assoc 2004; 103:374–379. [PubMed] [Google Scholar]
- 5.LaDou J. LaDou J. Liver cancer: Hepatic angiosarcoma. Current Occupational & Environmental Medicine 4th ed.New York: McGraw-Hill; 2007. 251–253. [Google Scholar]
- 6.Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol 2009; 20:780–787. [DOI] [PubMed] [Google Scholar]
- 7.Timaran CH, Grandas OH, Bell JL. Hepatic angiosarcoma: long-term survival after complete surgical removal. Am Surg 2000; 66:1153–1157. [PubMed] [Google Scholar]
- 8.Arima-Iwasa S, Chijiiwa K, Makino I, Tanabe R, Ohuchida J, Kondo K. A case of hepatic angiosarcoma surviving for more than 16 months after hepatic resection. Hepatogastroenterology 2007; 54:533–535. [PubMed] [Google Scholar]
- 9.Ozden I, Bilge O, Erkan M, Cevikbaş U, Acarli K. Five years and 4 months of recurrence-free survival in hepatic angiosarcoma. J Hepatobiliary Pancreat Surg 2003; 10:250–252. [DOI] [PubMed] [Google Scholar]
- 10.Louagie YA, Gianello P, Kestens PJ, Bonbled F, Haot JG. Vinylchloride induced hepatic angiosarcoma. Br J Surg 1984; 71:322–323. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Hao X. Hepatic angiosarcoma: a review of twelve cases. Chin J Clin Oncol 2005; 2:457–461. [Google Scholar]
- 12.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5:649–655. [PubMed] [Google Scholar]
- 13.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg 1992; 163:515–518. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Staging Manual. 7th ed.New York: Springer; 2010. [Google Scholar]
- 16.Weng Y-J, Chao Y, Wang S-S, et al. Hepatic angiosarcoma: report of a case. Gastroenterol J Taiwan 1995; 12:208–215. [Google Scholar]
- 17.Tsai M-H, Chien R-N, Hsieh S-Y, Hung C-F, Chen T-C, Sheen I-S. Primary hepatic angiosarcoma: report of a case involving environmental arsenic exposure. Changgeng Yi Xue Za Zhi 1998; 21:469–474. [PubMed] [Google Scholar]
- 18.Tsai C-C, Hsieh J-F, Han S-J, Mo L-R. Hemoperitoneum secondary to biopsy of the hepatic angiosarcoma. Chin J Radiol 1999; 24:37–40. [Google Scholar]
- 19.Liang W-B, Wang T-E, Lin S-C, et al. Primary angiosarcoma of the liver – a case report. J Intern Med Taiwan 2003; 14:290–294. [Google Scholar]
- 20.Tsai C-C, Cheng K-S, Lai H-C, Yu C-J, Hsu C-H. Ruptured angiosarcoma of the liver treated by emergency TAE – case report. China Med Univ Reposit. 2007. [Google Scholar]
- 21.Hsiao V-C. Hepatic angiosarcoma. Clin Forum Inst Hepato-Gastroenterol 2008; l9:1–3. [Google Scholar]
- 22.Lee F-C, Lee C-L, Hsu W-H, Lou F-J. Angiosarcoma of liver: report of a case with literature review. In: Proceedings of the 67th Annual Meeting of Taiwan Surgical Association; Mar 29–30, 2008, Kaohsiung, Taiwan. [Google Scholar]
- 23.Cheng S-P, Jeng K-S. Hepatic angiosarcoma: report of a case. Formos J Surg 2003; 36:179–183. [Google Scholar]
- 24.Lee SW, Song CY, Gi YH, et al. Hepatic angiosarcoma manifested as recurrent hemoperitoneum. World J Gastroenterol 2008; 14:2935–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weitz J, Klimstra DS, Cymes K, et al. Management of primary liver sarcomas. Cancer 2007; 109:1391–1396. [DOI] [PubMed] [Google Scholar]
- 26.Zhou YM, Li B, Yin ZM, et al. Results of hepatic resection for primary hepatic angiosarcoma in adults. Med Sci Monit 2010; 16:CR61–CR66. [PubMed] [Google Scholar]
- 27.Dannaher CL, Tamburro CH, Yam LT. Chemotherapy of vinyl chloride-associated hepatic angiosarcoma. Cancer 1981; 47:466–469. [DOI] [PubMed] [Google Scholar]
- 28.Duan XF, Li Q. Primary hepatic angiosarcoma: a retrospective analysis of 6 cases. J Dig Dis 2012; 13:381–385. [DOI] [PubMed] [Google Scholar]
- 29.McNeer GP, Cantin J, Chu F, Nickson JJ. Effectiveness of radiation therapy in the management of sarcoma of the soft somatic tissues. Cancer 1968; 22:391–397. [DOI] [PubMed] [Google Scholar]
- 30.Turek-Maischeider M, Kazem I. Palliative irradiation for liver metastases. JAMA 1975; 232:625–628. [PubMed] [Google Scholar]
- 31.Høyer M, Swaminath A, Bydder S, et al. Radiotherapy for liver metastases: a review of evidence. Int J Radiat Oncol Biol Phys 2012; 82:1047–1057. [DOI] [PubMed] [Google Scholar]
- 32.Lock MI, Hoyer M, Bydder SA, et al. An international survey on liver metastases radiotherapy. Acta Oncol 2012; 51:568–574. [DOI] [PubMed] [Google Scholar]
- 33.Rotenberg L, Tubiana JM, Porcel A, Bouras T, Monnier-Cholley L, Arrivé L. Interventional radiology and abdominal emergencies. Ann Radiol (Paris) 1996; 39:89–103. [PubMed] [Google Scholar]
- 34.Park YS, Kim JH, Kim KW, et al. Primary hepatic angiosarcoma: imaging findings and palliative treatment with transcatheter arterial chemoembolization or embolization. Clin Radiol 2009; 64:779–785. [DOI] [PubMed] [Google Scholar]
- 35.Leowardi C, Hormann Y, Hinz U, et al. Ruptured angiosarcoma of the liver treated by emergency catheter-directed embolization. World J Gastroenterol 2006; 12:804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stambo GW, Guiney MJ. Hepatic angiosarcoma presenting as an acute intraabdominal hemorrhage treated with transarterial chemoembolization. Sarcoma 2007; 2007:90169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthaei H, Boelke E, Eisenberger CF, et al. Interdisciplinary treatment of primary hepatic angiosarcoma: emergency tumor embolization followed by elective surgery. Eur J Med Res 2007; 12:591–594. [PubMed] [Google Scholar]
- 38.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208–1236. [DOI] [PubMed] [Google Scholar]
- 39.Chuang VP, Wallace S. Hepatic artery embolization in the treatment of hepatic neoplasms. Radiology 1981; 140:51–58. [DOI] [PubMed] [Google Scholar]
- 40.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998; 27:1578–1583. [DOI] [PubMed] [Google Scholar]
- 41.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002; 35:1164–1171. [DOI] [PubMed] [Google Scholar]
- 42.Dudeck O, Ricke J. Advances in regional chemotherapy of the liver. Expert Opin Drug Deliv 2011; 8:1057–1069. [DOI] [PubMed] [Google Scholar]
- 43.Takayasu K. Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol 2012; 42:247–255. [DOI] [PubMed] [Google Scholar]
- 44.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002; 359:1734–1739. [DOI] [PubMed] [Google Scholar]
- 46.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007; 30:6–25. [DOI] [PubMed] [Google Scholar]
- 47.Makuuchi M, Kokudo N, Arii S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res 2008; 38:37–51. [DOI] [PubMed] [Google Scholar]
- 48.Shao Y-Y, Huang C-C, Liang P-C, Lin Z-Z. Hepatic arterial infusion of chemotherapy for advanced hepatocellular carcinoma. Asia Pac J Clin Oncol 2010; 6:80–88. [DOI] [PubMed] [Google Scholar]
- 49.Kojiro M, Nakashima T, Ito Y, Ikezaki H, Mori T, Kido C. Thorium dioxide-related angiosarcoma of the liver. Pathomorphologic study of 29 autopsy cases. Arch Pathol Lab Med 1985; 109:853–857. [PubMed] [Google Scholar]
- 50.Huang N-C, Wann S-R, Chang H-T, Lin SL, Wang JS, Guo HR. Arsenic, vinyl chloride, viral hepatitis, and hepatic angiosarcoma: a hospital-based study and review of literature in Taiwan. BMC Gastroenterol 2011; 11:142. [DOI] [PMC free article] [PubMed] [Google Scholar]


