Abstract
Deficiency in 25-hydroxyvitamin D (25[OH]D), the main circulating form of vitamin D in blood, could be involved in the pathogenesis of acute coronary syndromes (ACS). To date, however, the possible prognostic relevance of 25 (OH)D deficiency in ACS patients remains poorly defined. The purpose of this prospective study was to assess the association between 25 (OH)D levels, at hospital admission, with in-hospital and 1-year morbidity and mortality in an unselected cohort of ACS patients.
We measured 25 (OH)D in 814 ACS patients at hospital presentation. Vitamin D serum levels >30 ng/mL were considered as normal; levels between 29 and 21 ng/mL were classified as insufficiency, and levels < 20 ng/mL as deficiency. In-hospital and 1-year outcomes were evaluated according to 25 (OH)D level quartiles, using the lowest quartile as a reference.
Ninety-three (11%) patients had normal 25 (OH)D levels, whereas 155 (19%) and 566 (70%) had vitamin D insufficiency and deficiency, respectively. The median 25 (OH)D level was similar in ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI) patients (14.1 [IQR 9.0–21.9] ng/mL and 14.05 [IQR 9.1–22.05] ng/mL, respectively; P = .88). The lowest quartile of 25 (OH)D was associated with a higher risk for several in-hospital complications, including mortality. At a median follow-up of 366 (IQR 364–379) days, the lowest quartile of 25 (OH)D, after adjustment for the main confounding factors, remained significantly associated to 1-year mortality (P < .01). Similar results were obtained when STEMI and NSTEMI patients were considered separately.
In ACS patients, severe vitamin D deficiency is independently associated with poor in-hospital and 1-year outcomes. Whether low vitamin D levels represent a risk marker or a risk factor in ACS remains to be elucidated.
INTRODUCTION
Beyond its fundamental role in bone metabolism and calcium homeostasis, vitamin D may influence several other medical conditions, including cardiovascular disease. Indeed, vitamin D receptors have been found in the myocardium as well as in vascular cells, and hypovitaminosis D, a common finding in many industrialized countries, has been independently associated with increased risk of developing acute myocardial infarction and heart failure.1,2 Moreover, vitamin D deficiency has been linked to conditions, such as hypertension, diabetes mellitus, metabolic syndrome, cardiac hypertrophy, and chronic kidney disease, that predispose to cardiovascular disease.3–7 More importantly, in heart failure patients, vitamin D supplementation has been shown to be associated with improved survival.8 Several studies have also demonstrated a survival benefit in end-stage renal disease patients treated with vitamin D, primarily related to a reduction in cardiovascular death.9 Thus, vitamin D seems to play an important role in cardiac function and in the development and progression of coronary artery disease.
Deficiency of vitamin D, or of 25-hydroxyvitamin D (25[OH]D), its main circulating form in the blood, has been recently reported to be common in patients with acute coronary syndromes (ACS),10 and preliminary studies indicate a possible association with prognosis. Very few studies, however, have investigated the association between vitamin D levels and clinical outcomes in ACS patients thus far; moreover, they were either underpowered to evaluate in-hospital outcomes, or mainly focused on long-term results.11–14 Therefore, convincing data demonstrating the possible impact of vitamin D insufficiency, or deficiency, on morbidity and mortality of ACS patients are still lacking. Notably, vitamin D has been demonstrated to suppress the renin-angiotensin system and to affect endothelial function, inflammatory processes, platelet function, insulin resistance, and blood pressure.3,15–18 All these effects are relevant during ACS, and related to patients’ clinical course. Moreover, low levels of vitamin D have been associated with ventricular dysfunction and cardiac remodeling after ACS, and with heart failure mortality and sudden cardiac death.11,19,20 Thus, both the short- and long-term outcomes of ACS patients could be significantly affected by vitamin D status.
The purpose of this prospective study was to determine the clinical implications of 25 (OH)D levels in an unselected cohort of ACS patients at hospital admission, and their possible association with in-hospital and 1-year morbidity and mortality.
MATERIAL AND METHODS
Study Population
This was a prospective, observational study. All consecutive ACS patients, including both ST-elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI) patients, admitted to the Intensive Cardiac Care Unit of Centro Cardiologico Monzino, between June 1st, 2010 and October 31st, 2012 were recruited. Patients on chronic peritoneal or hemodialysis treatment were excluded. Patients experiencing acute myocardial infarction after elective percutaneous coronary intervention (PCI), those with known malignancy and those with short life expectancy, were also excluded. The study was approved by the Institutional Review Board of our centre (Centro Cardiologico Monzino, Milan, Italy), and written informed consent was obtained from all participants. No extramural funding was used to support this work.
Study Protocol
In all patients a venous blood sample (3.5 mL) was drawn at hospital admission and biological measurement of 25 (OH)D was available for all enrolled patients. Architect 25-OH vitamin D assay (Abbott Diagnostics, Wiesbaden, Germany), with a limit of detection of 7 ng/mL, was used for serum 25 (OH)D measurement; values below this limit were considered 6.9 ng/mL. According to published data, and to the US Endocrine Society guideline recommendations,21 we used the following cut-off values for classifying vitamin D status: >30 ng/mL were considered normal vitamin D levels; between 29 and 21 ng/mL were classified as vitamin D insufficiency, and < 20 ng/mL as vitamin D deficiency. As most patients were expected to present low vitamin D levels,10 the sample was also stratified into 25 (OH)D quartiles, in order to maximize statistical power.
All patients received standard medical treatment and coronary revascularization at the discretion of the attending physician, on the basis of the current standards of care recommended by published guidelines.
Demographical, clinical, biochemical, echocardiographic, and angiographic data were obtained. The estimated glomerular filtration rate was calculated according to the Modification of Diet in Renal Disease (MDRD) formula.22 The left ventricular ejection fraction (LVEF; echocardiogram) was measured in all patients within 24 hours from hospital admission.
In all patients undergoing coronary angiography, the extent of angiographic coronary artery disease was quantified by the SYNTAX score.23 A team of 2 interventional cardiologists, blinded to the patients’ clinical characteristics and vitamin D result, calculated the SYNTAX score.
After hospital discharge, all patients were followed up for at least 1 year. Patient follow-up was mainly obtained through regularly scheduled outpatient visits or, in a minority of cases, by telephone contacts performed by dedicated medical personnel.
Study End Points
The primary end point of the study was 1-year mortality. In-hospital mortality and in-hospital major adverse clinical events (MACEs) (death, major bleeding [requiring blood transfusion], acute pulmonary edema [with or without the need for mechanical ventilation], cardiogenic shock, clinically significant tachyarrhythmias [ventricular fibrillation, sustained ventricular tachycardia, and atrial fibrillation] and bradyarrhythmias requiring pacemaker implantation, and acute kidney injury [defined according to the Acute Kidney Injury Network classification]24) were evaluated as secondary end points. At 1-year follow-up, the following events were also considered: 1) re-hospitalization for ACS; 2) re-hospitalization for acute decompensated heart failure (ADHF); 3) other relevant clinical events [stroke, major bleeding, and life-threatening tachyarrhythmias]; 4) and the composite of death, re-hospitalization for ACS and ADHF, and other relevant clinical events (combined end point).
Statistical Analysis
A sample size of 800 patients was calculated under the following assumptions: 6% overall 1-year mortality, with an expected 8.5% and 2% mortality in patients with the lowest and the highest 25 (OH)D plasma level quartile, respectively. This sample size allowed >80% statistical power in assessing significantly different (α error of 0.05) 1-year mortality between the 2 groups.
Continuous variables are presented as mean±SD. Variables with a skewed distribution are presented as median and interquartile ranges (IQR), and were transformed in logarithm before analysis. Categorical data are presented as n (%). Trends across 25 (OH)D quartiles were assessed by ANCOVA for continuous variables and by Mantel-Haenszel chi-square. The association between 25 (OH)D and in-hospital and 1-year clinical outcomes was assessed by logistic regression and by Cox regression analysis, respectively. Analyses were adjusted for potential confounders selected among the most important recognized clinical predictors of mortality (ie, age, LVEF, diabetes mellitus, and baseline serum creatinine concentration [sCr]), and among correlates of vitamin D levels observed in our cohort (body mass index [BMI], high-sensitivity-C-reactive protein, total cholesterol, and triglycerides); results are presented as odds ratios or hazard ratio with 95% confidence intervals. Patients were grouped into quartiles according to 25 (OH)D levels, using the lowest quartile as a reference.
Kaplan-Meier analysis was employed to generate time-to-event curves for the 1-year end points (mortality and combined end point), stratified according to 25 (OH)D < 9 ng/mL. Log rank test was used to compare strata.
All tests were 2-tailed, and a P value of less than .05 was required for statistical significance. All calculations were computed with the aid of the SAS software package (Version 9.2 SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
A total of 814 consecutive ACS patients (mean age 67 ± 12 years, 584 men; 341 STEMI and 473 NSTEMI patients) were included in this study. Out of them, 93 (11%) patients had normal 25 (OH)D levels at hospital admission, whereas 155 (19%) and 566 (70%) had vitamin D insufficiency and deficiency, respectively. No difference in this proportion was observed between STEMI and NSTEMI patients (Figure 1). The median 25 (OH)D level in the whole population was 14.05 (IQR 9.0–22.0) ng/mL; it was similar in STEMI and NSTEMI patients (14.1 [IQR 9.0–21.9] ng/mL and 14.05 [IQR 9.1–22.05] ng/mL, respectively; P = .88).
FIGURE 1.
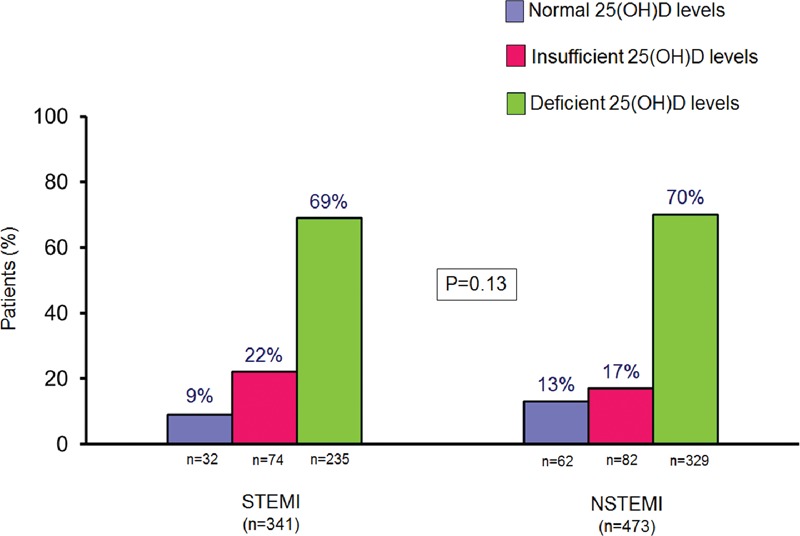
Vitamin D levels in ST-elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI) patients. 25 (OH)D = 25-hydroxyvitamin D. P value was obtained by chi-square test.
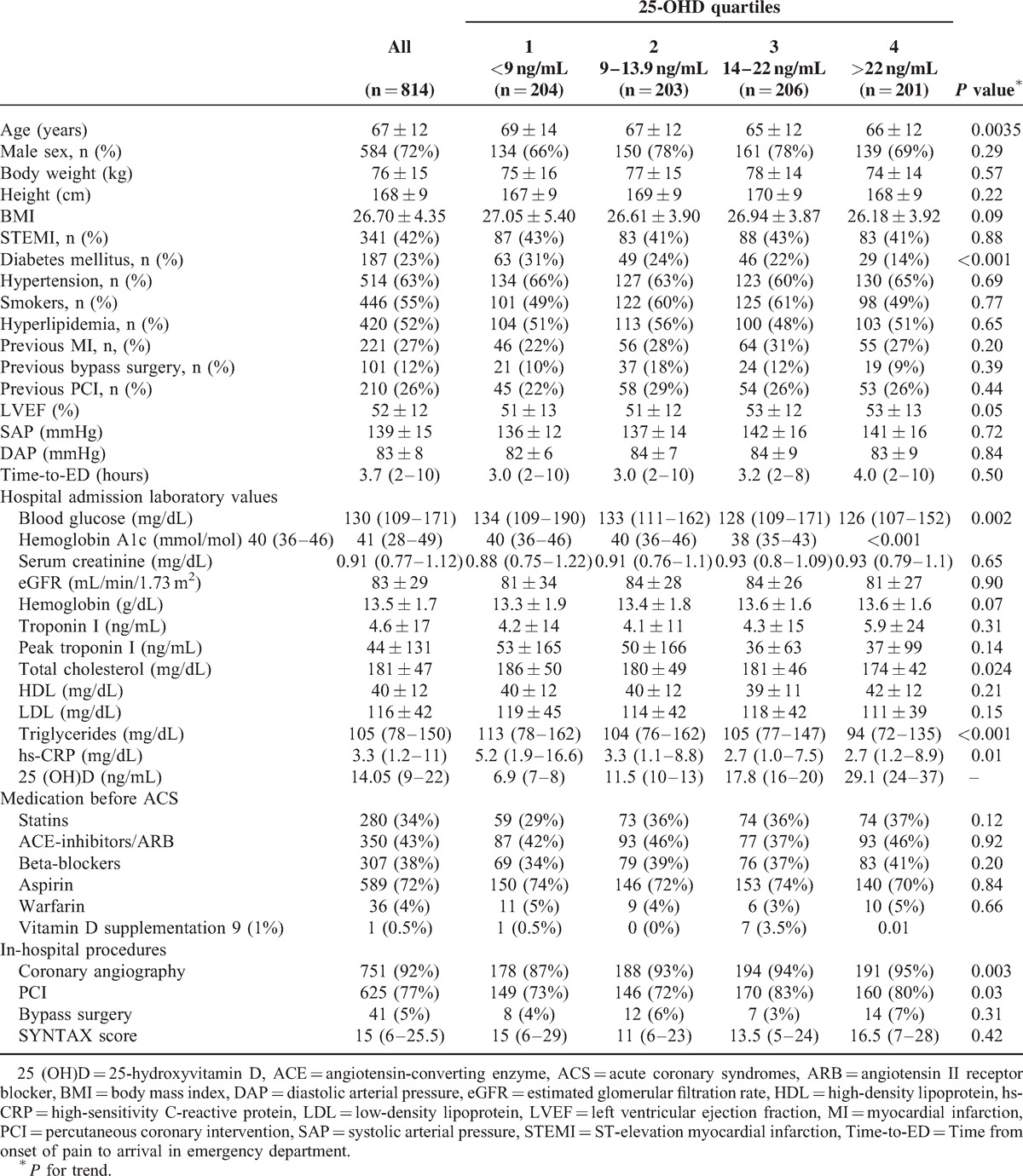
Table 1 reports patient characteristics according to 25 (OH)D quartiles. Patients in the low 25 (OH)D quartiles were older, more frequently diabetics, with a lower LVEF, and with higher high-sensitivity C-reactive protein levels. Notably, the percentage of STEMI patients was similar in the 4 groups, as well as the extent of coronary disease, as evaluated by the SYNTAX score.
TABLE 1.
Baseline characteristics of study patients according to 25(OH)D quartiles
Association Between Vitamin D and in-Hospital Outcomes
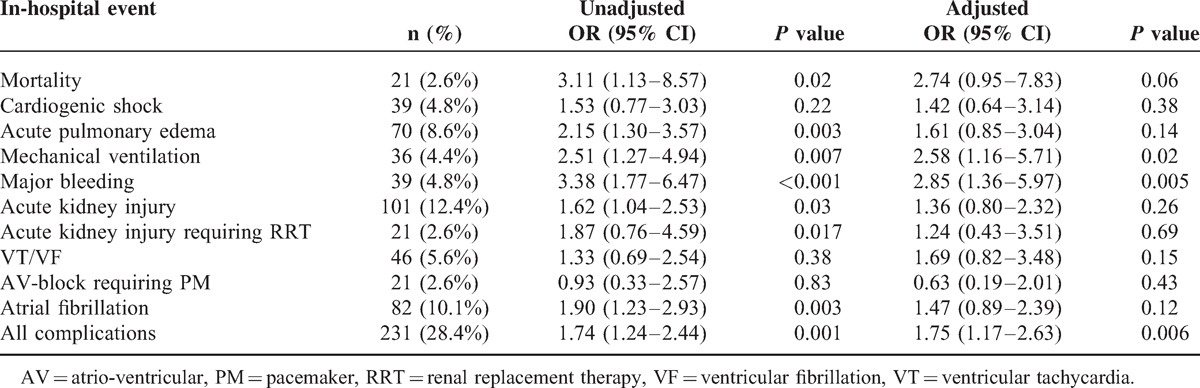
The overall in-hospital mortality in all study patients was 2.6% (n = 21), and it was higher in STEMI than in NSTEMI patients (4.7% vs. 1.1%; P < .01). The lowest quartile of 25 (OH)D was associated with a higher risk for several in-hospital MACEs, including mortality (Table 2). After adjustment for major confounders, vitamin D remained a significant independent predictor of major bleeding and mechanical ventilation only, with a borderline statistical significance with mortality.
TABLE 2.
Association between the lowest 25 (OH)D quartile and in-hospital events. Data are also presented after adjustment for major potential confounding (age, body mass index, diabetes mellitus, left ventricular ejection fraction, serum creatinine, high-sensitivity-C-reactive protein, total cholesterol, and triglycerides)
Association Between Vitamin D and 1-Year Outcomes
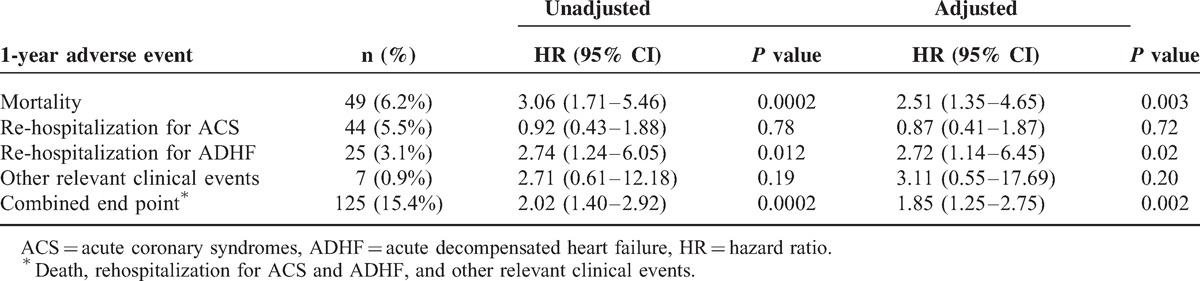
Outcome data at 1-year follow-up were obtained for 796 (98%) patients, with a median follow-up period of 366 (IQR 364–379) days. After hospital discharge, 28 additional patients died, resulting in a cumulative mortality rate of 6% (n = 49) (6.6% in STEMI and 5.8% in NSTEMI patients; P = .63). Forty-four (5.5%) patients were re-admitted for a new ACS, and 25 (3.1%) for ADHF. Finally, there were a total of 7 (0.9%) major adverse events. After adjustment for major confounders, vitamin D remained significantly associated to 1-year mortality and re-hospitalization for ADHF in our population (Table 3).
TABLE 3.
Association between the lowest 25 (OH)D quartile and 1-year adverse events. Data are also presented after adjustment for major potential confounding (age, body mass index, diabetes mellitus, left ventricular ejection fraction, serum creatinine, high-sensitivity-C-reactive protein, total cholesterol, and triglycerides)
The Kaplan–Meier survival curves provided confirmation that the lowest 25 (OH)D quartile was associated with 1-year mortality (Figure 2, Panel A) (P < .01 by log rank test vs. the other 3 quartiles pooled together) and to combined end point of death, re-hospitalizations for ACS and ADHF, and other relevant clinical events (Figure 2, Panel B; P < .01). Similar results were obtained when STEMI and NSTEMI patients were considered separately (Figure 3).
FIGURE 2.
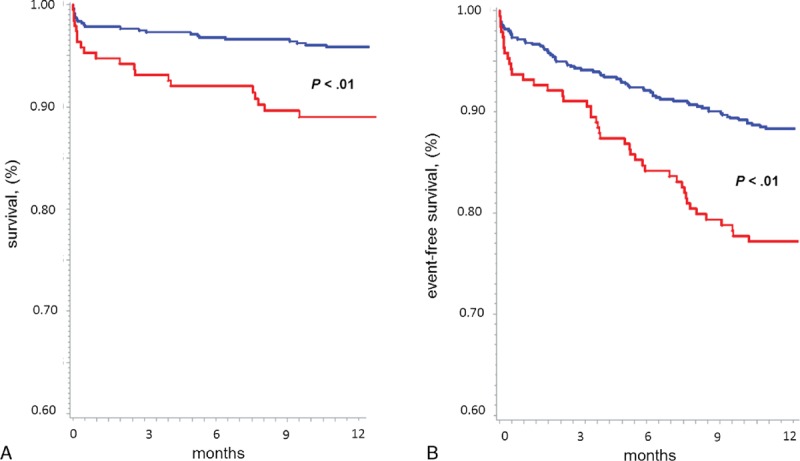
Kaplan-Meier curve analysis stratified according to 25 (OH)D levels (the lowest quartile [red line] vs. the other 3 quartiles pooled together [blue line]) for 1-year mortality (Panel A), and for the combined end point (Panel B), in the whole study population. P value by Log rank test.
FIGURE 3.
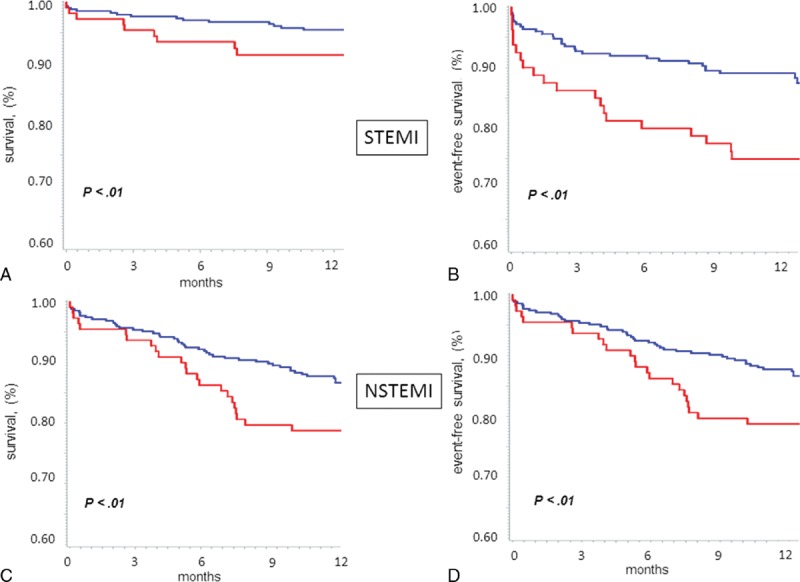
Kaplan-Meier curve analysis stratified according to 25 (OH)D levels (the lowest quartile [red line] vs. the other 3 quartiles pooled together [blue line]) for 1-year mortality (Panels A and C), and for the combined end point (Panels B and D), in patients with ST-elevation myocardial infarction (STEMI) (upper panels) and non-ST elevation myocardial infarction (NSTEMI) (lower panels). P value by Log rank test.
DISCUSSION
The present prospective study, aimed at investigating the role of vitamin D in a consecutive, nonselected cohort of ACS patients, demonstrated an independent association between its severe deficiency (the lowest quartile) and in-hospital and 1-year clinical outcomes.
Multiple lines of evidence suggest a link between vitamin D and cardiovascular disease. Epidemiological studies reported that the rates of coronary artery disease, diabetes, hypertension, as well as of vitamin D deficiency, increase in proportion to increasing distance from the equator.25 Cardiac death has also been reported to be at its highest during periods of decreased sunlight exposure (ie, winter months).26 Moreover, observational studies, small clinical trials, and meta-analyses indicate that vitamin D therapy may reduce cardiovascular events and mortality.8,27,28 Although these data from apparent healthy subjects support a role of vitamin D deficiency as a new potential cardiovascular risk factor, there is still paucity of information regarding the implications of vitamin D deficiency in ACS and its possible association, or causal relationship, with morbidity and mortality. Clinical interest derives from the fact that vitamin D deficiency can be readily determined by blood testing and treated by supplementation. In particular, a single oral ultra-high dose of vitamin D has been shown to restore normal 25 (OH)D levels within 2 days in critically ill patients, without causing adverse effects, thus providing the basis of an easy-to-administer dosing regimen for prospective intervention trials in acute cardiovascular settings.29
In the last few years, growing interest has emerged on the possible implications of low vitamin D levels in ACS. Lee et al10 assessed 25 (OH)D levels in 239 patients enrolled in a multicenter prospective registry, and found a high (96%) prevalence of vitamin D deficiency in patients with acute myocardial infarction. After this first report, further studies have confirmed this observation and investigated its possible clinical and prognostic relevance. In the Khalili et al11 study, a significant inverse relationship between serum levels of matrix metalloproteinase-9 (an early marker of cardiac remodeling), evaluated 72 hours after hospital admission, and vitamin D was found in 139 STEMI patients, suggesting a possible role of vitamin D in influencing cardiac remodeling. The authors also reported a possible correlation between low levels of vitamin D and increased in-hospital mortality. Given the small sample size of their population, however, the study was significantly underpowered to detect any difference in mortality between STEMI patients with normal and low vitamin D levels.12 In another study, Correia et al13 reported a possible independent association between vitamin D deficiency and in-hospital cardiovascular mortality. Again, in this study the sample size was relatively small (n = 206), STEMI patients were underrepresented (7% of all patients), and deaths for complications occurring after bypass surgery were also considered. Therefore, no definite conclusion can be drawn at this time on in-hospital clinical relevance of vitamin D deficiency. Thus far, the largest study evaluating vitamin D and prognosis in ACS patients was that by Ng et al14 They found an association between the lowest vitamin D quartile (<7.3 ng/mL) and long-term major adverse cardiovascular outcomes in 1259 patients. Notably, the association was predominantly with nonfatal adverse outcomes, such as re-hospitalization for ADHF or for another ACS, rather than mortality.
Our study supports the close association between low vitamin D levels at hospital presentation and worse prognosis in ACS patients. Indeed, patients in the 25 (OH)D lowest quartile had a 2-fold higher mortality risk, even after adjustment for important independent variables associated with mortality in ACS.30–32 Notably, patients with STEMI and NSTEMI had a similar vitamin D status and portrayed a comparable mortality risk. Thus, our results strengthen the evidence of a close association between low vitamin D levels and poor outcome, and they pave the way for studies based on pharmacologic supplementation of vitamin D in selected high-risk ACS patients, namely those with severe vitamin D deficiency, in order to improve their prognosis.
The possible causal relationship underlying the association between vitamin D status and outcomes in ACS remains to be elucidated. Mechanisms by which vitamin D deficiency may confer an increased cardiovascular risk, directly or indirectly leading to hyperparathyroidism, include renin-angiotensin-system activation and disorders of insulin synthesis, secretion, and sensitivity, that influence glycemic control and may favor the onset of diabetes.3,6,15,33 Of note, low blood 25OH-D concentrations have been associated with an increased risk of both macrovascular and microvascular disease events in type 2 diabetes.34 However, in our study, the prognostic implications of vitamin D were confirmed also after adjustment for diabetes.
Other potential consequences of vitamin D deficiency involve exacerbation of atherogenesis, inflammation, acceleration of arterial calcification, cardiac remodeling and systolic dysfunction, higher risk of restenosis following PCI, and influence on lipid levels.9,17–20,35–37 Additionally, vitamin D deficiency has been associated with endothelial dysfunction,38 which is, in turn, linked to an increased risk of cardiovascular events.39 In particular, the endothelium mediates vascular tone control, platelet aggregation, endothelium permeability, and neoangiogenesis40–42; thus, its dysfunction may exacerbate coronary thrombosis and vasoconstriction occurring during ACS. Finally, in our study, patients with lower vitamin D levels had higher high-sensitivity C-reactive protein values, suggesting a possible link between low vitamin D levels and inflammation. However, controversial data exist on this possible relationship, as recently reported by Eren et al.,43 who found no association between calcidiol levels and inflammatory markers in ACS. Overall, all these features, associated with older age, higher cholesterol and triglycerides levels, and less aggressive coronary reperfusion strategy, may contribute to explain the worse outcome of ACS patients with vitamin D deficiency in our study. Whether vitamin D serves as a risk factor or as a risk marker in ACS, however, cannot be deduced from our study, and future investigation is warranted to clarify this issue. Indeed, frail patients with high cardiovascular risk burden, because of their health status may spend more time indoors and have less sun exposure, leading to vitamin D deficiency. Although a clear evidence that patients with cardiovascular disease have lower levels of 25 (OH)D, a similar association exists for a large number of other medical conditions, like cancer, multiple sclerosis, and psychiatric diseases, supporting the concept that vitamin D is simply a general marker of health.44
An important novelty of our study was the link between vitamin D levels and in-hospital outcomes. Only a trend toward a higher in-hospital mortality rate of patients having the lowest 25 (OH)D quartile was observed, possibly due to the relatively low in-hospital mortality rate of our population. An interesting finding was the association between lowest vitamin D levels and some major in-hospital clinical complications. In particular, patients in the lowest 25 (OH)D quartile had a significantly higher risk of bleeding requiring transfusion, despite similar hemoglobin levels at hospital admission. This represents a critical issue in ACS, where potent antithrombotic therapy is the mainstay of treatment, and where major bleeding, as well as the need for transfusion, has a relevant negative impact on prognosis. The reason (s) for this association is unclear. Possible explanations might involve anticoagulant effects by up-regulating thrombomodulin and down-regulating tissue factor, and interaction with platelet function.45–47
Another interesting association found in our study was between the lowest 25 (OH)D quartile and the risk of acute respiratory insufficiency. This finding could be explained by left ventricular dysfunction, muscle weakness and increased infectious risk associated with low vitamin D levels and/or with transfusion-related acute lung injury.6,44,48 The higher incidence of all these life-threatening complications might contribute, at least in part, to the higher in-hospital mortality risk observed in ACS patients with vitamin D deficiency. Obviously, these findings should be confirmed in larger studies, and future investigation is also needed to elucidate whether supplementation of vitamin D, aimed at rapidly normalizing 25 (OH)D levels in ACS patients, may reduce the incidence of these complications and, finally, improve patients’ in-hospital outcome.
Some limitations warrant mention. First, we included a population admitted to a single center. Second, the study population was possibly underpowered to detect a significant difference in in-hospital mortality. Third, our data are only hypothesis generating, because they do not provide evidence to support a causal relationship, and they require confirmation in suitably designed clinical trials. Finally, many factors that affect vitamin D status (eg, latitude, season, sunlight exposure, skin color, vitamin D intake, serum albumin, etc.) were not taken into account in our study, and may have influenced, at least in part, our results.
In conclusion, this study demonstrated the presence of an association between vitamin D deficiency and poor in-hospital and 1-year outcomes in patients with ACS. The correction of vitamin D deficiency and maintenance of an optimal status may be a promising approach for acute treatment and secondary prevention of ACS that requires confirmation in interventional trials with vitamin D supplementation.
Acknowledgments
Funding. Funding for this study was provided by the Centro Cardiologico Monzino, I.R.C.C.S., Milan, Italy.
Footnotes
Abbreviations: 25 (OH)D = 25-hydroxyvitamin D, ACS = acute coronary syndromes, ADHF = acute decompensated heart failure, BMI = body mass index, IQR = interquartile ranges, LVEF = left ventricular ejection fraction, MACE = major adverse clinical events, NSTEMI = non-ST-elevation myocardial infarction, PCI = percutaneous coronary intervention, sCr = serum creatinine, STEMI = ST-elevation myocardial infarction.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Lavie CJ, Lee J, Milani R. Vitamin D and cardiovascular disease. Will it live up to its hype? J Am Coll Cardiol 2011; 58:1547–1556. [DOI] [PubMed] [Google Scholar]
- 2.Vanga S, Good M, Howard P, et al. Role of vitamin D in cardiovascular health. Am J Cardiol 2010; 106:798–805. [DOI] [PubMed] [Google Scholar]
- 3.Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 2007; 92:2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nainby-Luxmoore JC, Langford HG, Nelson NC, et al. A case-comparison study of hypertension and hyperparathyroidism. J Clin Endocrinol Metab 1982; 55:303–306. [DOI] [PubMed] [Google Scholar]
- 5.Saleh FN, Schirmer H, Sundsfjord J, et al. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J 2003; 24:2054–2060. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez EA, Sachdeva A, Oliver DA, et al. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 2004; 24:503–510. [DOI] [PubMed] [Google Scholar]
- 8.Gotsman I, Shauer A, Zwas DR, et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 2012; 14:357–366. [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D and vascular calcification. Curr Opin Lipidol 2007; 18:41–46. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Gadi R, Spertus JA, et al. Prevalence of vitamin D deficiency in patients with acute myocardial infarction. Am J Cardiol 2011; 107:1636–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalili H, Talazaz AH, Salarifar M. Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clinical Res Cardiology 2012; 101:321–327. [DOI] [PubMed] [Google Scholar]
- 12.De Metrio M, Milazzo V, Marenzi G. Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin Res Cardiol 2012; 101:771–772. [DOI] [PubMed] [Google Scholar]
- 13.Correia LCL, Sodrè F, Garcia G, et al. Relation of severe deficiency of vitamin D to cardiovascular mortality during acute coronary syndromes. Am J Cardiol 2013; 111:324–327. [DOI] [PubMed] [Google Scholar]
- 14.Ng LL, Sandhu JK, Squire IB, et al. Vitamin D and prognosis in acute myocardial infarction. Int J Cardiol 2013; 168:2341–2346. [DOI] [PubMed] [Google Scholar]
- 15.Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 2003; 88:327–331. [DOI] [PubMed] [Google Scholar]
- 16.Borges AC, Feres T, Vianna LM, et al. Effect of cholecalciferol treatment on the relaxant responses of spontaneously hypertensive rat arteries to acetylcholine. Hypertension 1999; 134:897–901. [DOI] [PubMed] [Google Scholar]
- 17.Schleithoff SS, Zittermann A, Tenderich G, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006; 83:754–759. [DOI] [PubMed] [Google Scholar]
- 18.Ortega A, Perez de Prada MT, González-Armengol JJ, et al. Effect of parathyroid-hormone-related protein on human platelet activation. Clinical Science 2007; 113:319–327. [DOI] [PubMed] [Google Scholar]
- 19.Mancuso P, Rahman A, Hershey SD, et al. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol 2008; 51:559–564. [DOI] [PubMed] [Google Scholar]
- 20.Pilz S, Marz W, Wellnitz B, et al. Association of Vitamin D deficiency with heart failure and sudden cardiac death in a large cross sectional study of patient referred for coronary angiography. J Clin Endocrinol Metab 2008; 93:3927–3935. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 23.Palmerini T, Genereux P, Caixeta A, et al. Prognostic value of the SYNTAX score in patient with acute coronary syndromes undergoing percutaneos coronary intervention. J Am Coll Cardiol 2011; 57:2389–2397. [DOI] [PubMed] [Google Scholar]
- 24.Levin A, Warnock DG, Mehta RL, et al. Improving outcomes from acute kidney injury: report of an initiative. Am J Kidney Dis 2007; 50:1–4. [DOI] [PubMed] [Google Scholar]
- 25.Fabsitz R, Feinleib M. Geographic patterns in county mortality rates from cardiovascular diseases. Am J Epidemiol 1980; 111:315–328. [DOI] [PubMed] [Google Scholar]
- 26.Zipes DP. Warning: the short days of winter may be hazardous to your health. Circulation 1999; 100:1590–1592. [DOI] [PubMed] [Google Scholar]
- 27.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis: a historical cohort study. J Am Soc Nephrol 2005; 16:1115–1125. [DOI] [PubMed] [Google Scholar]
- 28.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1730–1737. [DOI] [PubMed] [Google Scholar]
- 29.Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care 2011; 15:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005; 45:1397–1405. [DOI] [PubMed] [Google Scholar]
- 31.Marenzi G, Moltrasio M, Assanelli E, et al. Impact of cardiac and renal dysfunction on in hospital morbidity and mortality of patients with acute myocardial infarction undergoing primary angioplasty. Am Heart J 2007; 153:755–762. [DOI] [PubMed] [Google Scholar]
- 32.Saltzman AJ, Stone GW, Claessen BE, et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. The HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. J Am Coll Cardiol Intv 2011; 4:1011–1019. [DOI] [PubMed] [Google Scholar]
- 33.Pittas AG, Chung M, Trikalinos T, et al. Systemic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 2010; 152:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrmann M, Sullivan DR, Veillard AS, et al. FIELD Study Investigators. Serum 25-hydroxyvitamin d: a predictor of macrovascular and microvascular complications in patients with type 2 diabetes. Diabetes Care 2015; 38:521–528. [DOI] [PubMed] [Google Scholar]
- 35.Monraats PS, Fang Y, Pons D, et al. Vitamin D receptor: a new risk marker for clinical restenosis after percutaneous coronary intervention. Expert Opin Ther Targets 2010; 14:243–251. [DOI] [PubMed] [Google Scholar]
- 36.Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Prog Lipid Res 2011; 50:303–312. [DOI] [PubMed] [Google Scholar]
- 37.Wu L, Liu L. Systematic Review and Meta-Analysis Evaluating the Impact of Vitamin D on the Risk of Heart Failure: New Evidence from Population-based Studies. JCvD 2014; 2:159–173. [Google Scholar]
- 38.Stach K, Kalsch AI, Nguyen XD, et al. 1alpha,25-dihydroxyvitamin D3 attenuates platelet activation and the expression of VCAM-1 and MT1-MMP in human endothelial cells. Cardiology 2011; 118:107–115. [DOI] [PubMed] [Google Scholar]
- 39.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001; 104:191–196. [DOI] [PubMed] [Google Scholar]
- 40.Kinlay S, Ganz P. Relation between endothelial dysfunction and the acute coronary syndrome: implications for therapy. Am J Cardiol 2000; 86:10J–13J.discussion 13J–14J. [DOI] [PubMed] [Google Scholar]
- 41.Santulli G, Campanile A, Spinelli L, et al. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol 2011; 107:1125–1130. [DOI] [PubMed] [Google Scholar]
- 42.Iaccarino G, Ciccarelli M, Sorriento D, et al. AKT participates in endothelial dysfunction in hypertension. Circulation 2004; 109:2587–2593. [DOI] [PubMed] [Google Scholar]
- 43.Eren E, Ellidag HY, Yılmaz A, et al. No association between vitamin D levels and inflammation markers in patients with acute coronary syndrome. Adv Med Sci 2015; 60:89–93. [DOI] [PubMed] [Google Scholar]
- 44.Rosen CJ. Vitamin D insufficiency. N Engl J Med 2011; 364:248–254. [DOI] [PubMed] [Google Scholar]
- 45.Ohsawa M, Koyama T, Yamamoto K, et al. 1alpha,25-Dihydroxyvitamin D (3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation 2000; 102:2867–2872. [DOI] [PubMed] [Google Scholar]
- 46.Benigni A, Livio M, Dodesini P, et al. Inhibition of human platelet aggregation by parathyroid hormone: is cyclic AMP implicated? Am J Nephrol 1985; 5:243–247. [DOI] [PubMed] [Google Scholar]
- 47.WuWong JR, Nakane M, Chen Y, et al. Different Effects of Calcidiol and Calcitriol on Regulating Vitamine D Receptor Target Gene Expression in Human Vascular Smooth Muscle Cells. JCvD 2013; 1:15–20. [Google Scholar]
- 48.Jaworski K, Maslanka K, Kosior DA. Transfusion-related acute lung injury: A dangerous and underdiagnosed noncardiogenic pulmonary edema. Cardiol J 2013; 20:337–344. [DOI] [PubMed] [Google Scholar]


