Abstract
Our previous studies have shown that secreted extracellular matrix-associated protein Cysteine rich angiogenic inducer 61 (Cyr61), a novel proinflammatory factor, is involved in the pathogenesis of rheumatoid arthritis (RA). However, whether Cyr61 has any effect in systemic lupus erythematosus (SLE) remains unknown. This study aims to assess the level of serum Cyr61 and to investigate the association of serum Cyr61 and clinical disease activity in SLE. We found the level of serum Cyr61 in patients with SLE was significantly higher than healthy controls (P < 0.001), and Cyr61 was high expressed in renal tubule of lupus nephritis compared to control. The sensitivity of Cyr61 in diagnosis of SLE was 47.3%. In receiver operating characteristic (ROC) curve analysis, the area under the curve (AUC) was 0.830, with a 95% confidence interval (CI) from 0.776 to 0.885. Cyr61 was present in 60.0%, 54.5%, and 41.5% of anti-double stranded DNA (dsDNA), anti-antinuclear antibodies (ANA), and anti-Sm negative SLE patients, respectively. Serum Cyr61 levels were significantly higher in high systemic lupus erythematosus disease activity index (SLEDAI) group than that in low SLEDAI group (P = 0.003). Correlation analyzes showed a significant negative correlation between serum Cyr61 and complements (C3) (P = 0.015), C4 (P = 0.04). Moreover, increased Cyr61 level in SLE was associated with serum level of TNF-α, interleukin 6 (IL-6), and IL-17. In conclusion, serum Cyr61 was increased in patients with SLE which was associated with clinical disease activity and inflammation in SLE, suggesting Cyr61 may be a novel potential auxiliary marker for the diagnosis of SLE.
This work was supported by grants from the “National Natural Science Foundation of China" (81401340) and “Youth Foundation of Fujian Provincial Health and Family Planning Commission" (2014-1-52).
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease associated with an array of autoantibodies, complements (C3), and cytokines.1 Although the etiology and pathogenesis of SLE are still unclear, the chronic inflammatory tissue damage is mediated at least in part by immune complexes, autoantibodies, and autoreactive lymphocytes.1 It is well known that auto-antibodies against nuclear components such as double stranded DNA (dsDNA) or RNA-binding protein Smith (Sm), Ro (SS-A), and La (SS-B) antigens, and so on, are significantly higher in SLE patients.2 Moreover, as a heterogeneous autoimmune disease with a strong genetic component modified by environmental exposures, complement system has been linked to its pathogenesis because low serum complement levels are demonstrated in patients with active disease.3,4 It is found that the deposition of circulating immune complexes (ICs) formed by these autoantibodies induces the damage of endothelia cells of SLE patients.1 Tissue damage is mediated by deposition of pathogenic autoantibodies and ICs in affected organs and the deposition of ICs and infiltration of neutrophils and activation of lymphocytes are central to organ inflammation and dysfunction in SLE.3–5 The formation of ICs is one of the principal ways of activating the classical pathway of the complement system that also has an important role in clearing immune complexes from the circulation.6,7 In SLE, the role of complement is complex because it may both prevent and exacerbate the disease.5 Moreover, complement can affect the activation of both B and T cells, leading to the production of autoantibodies and cytokines.8 Nevertheless, it is getting to know that cytokines such as interleukin 6 (IL-6), IL-17, and tumor necrosis factor α (TNF-α), are intimately involved in the pathogenesis of SLE, which influence uncontrolled lymphocyte activation, increased antigen presenting cell maturation and then defective immune regulation.9,10 However, the diagnosis of SLE remains challenging partly because of the heterogeneity of the disease, its evolutive nature and also because of the limitations with presently available diagnostic tests. To explore novel preinflammatory factors may be helpful to serve as valuable diagnostic tools in combining with the autoantibodies and provide potential insight regarding the pathogenesis of SLE.2,10
Cysteine rich angiogenic inducer 61 (Cyr61,CCN1), is a secreted, cysteine-rich, heparin binding extracellular matrix-associated protein which is known to function in mediating cell adhesion and inducing cell migration.11,12 Recently, Cyr61 is found to act as a new proinflammatory factor and involved in the pathogenesis of rheumatoid arthritis (RA).13,14 In RA, Cyr61 increases fibroblast-like synoviocyte (FLS) proliferation and results in hyperplasia;13 Cyr61 mediates inflammation and tissue damage by promoting IL-6 and IL-8 production by FLS;14,15 somatic p53 mutations in RA synovial tissue are functionally linked to the inflammation process through a mechanism involving miR-22 and Cyr61.16 Moreover, Cyr61 is found to promote type I macrophage and Th17 differentiation,14,17 enhance T cells output from thymus,18 suggesting that Cyr61 plays an important role in mediating the pathogenesis of RA.13,14,16 Nevertheless, whether Cyr61 plays any role in the inflammation processes of SLE has not yet been explored, and if so, whether Cyr61 is associated with clinical disease activity in SLE, evenly, whether it can serve as a potential inflammatory marker for the diagnosis of SLE.
In this study, we aim to explore the level of serum Cyr61 in SLE patients to analyze the association of Cyr61 with clinical disease activity and inflammatory cytokines, also, to evaluate the potential diagnostic and pathogenesis value of Cyr61 in SLE.
MATERIALS AND METHODS
Study Population
One hundred ten patients with SLE fulfilled at least 4 of the 1997 revised American College of Rheumatology criteria for the diagnosis of SLE were consecutively enrolled in our study.19 Sera from 100 healthy donors (HD) matched for gender and age were recruited from blood donors to serve as controls. Serum samples from 40 patients with RA, 20 patients with Sjogren's syndrome (SS), 20 patients with dermatomyositis (DM), 20 patients with mixed connective tissue disease (MICD), and 20 patients with scleroderma (SSc) were also collected. Patients with RA and SSc were fulfilled the criteria of the American College of Rheumatology.20,21 Patients with SS fulfilled the American–European Group Criteria.22 Patients with MCTD fulfilled the classification criteria described by Alarcon-Segovia.23 Patients with DM fulfilled criteria described by Medsger and Oddis.24 All serum samples were split into aliquots and stored at −80 °C until use. Study protocols were approved by the Institutional Medical Ethics Review Board of The First Affiliated Hospital of Fujian Medical University, Fuzhou, China. Patients provided written or verbal consent before inclusion in the study.
Enzyme-Linked Immunosorbent Assay (ELISA)
Sera Cyr61, IL-6, IL-10, IL-17, and TNF-α were measured by ELISA (R&D System, MN, USA) according to the manufacturer's recommendations. A standard curve was performed for each plate and used to calculate the absolute concentrations of each parameter. Each cytokine analysis was simultaneously performed on all patients and control sera, thereby avoiding a possible defrosting/refreezing bias.
Laboratory Analyses and Assessment of Disease Activity
Laboratory data were obtained from medical records; no analyses were performed on stored samples. Blood samples were originally taken using standard procedures and analyzed in the course of routine treatment. Enzyme-linked immunosorbent assays (EUROIMMUNAG, Lubeck, Germany) were used to quantify serum antinuclear antibodies (ANA) and dsDNA according to the manufacturers’ protocols. Anti-ribonucleoprotein (RNP), -Sm, -SSA, and -SSB antibody levels were measured by line blot techniques (Euroimmun, Lubeck, Germany). Erythrocyte sedimentation rate (ESR) was measured by the Westergren method. Serum Igs (IgG, IgM, and IgA), complements (C3, C4), and C-reactive protein (CRP) were quantified by immunoturbidimetric assay (Dade Behring, Marburg, Germany). The reference intervals for these serologic parameters were: ANA (0–1 s/co), dsDNA (0–100 IU/mL), ESR (0–15 mm/h), C3 (0.90–1.80 g/L), C4 (0.10–0.40 g/L), CRP (0–8 mg/L), IgG (7.0–16.0 g/L), IgA (0.5–4.0 g/L), and IgM (0.4–3.0 g/L).
Lupus activity was classified by systemic lupus erythematosus disease activity index (SLEDAI) scores: no activity (SLEDA = 0), mild activity (SLEDA = 1–5), moderate activity (SLEDA = 6–10), high activity (SLEDAI = 11–19), and very high activity (SLEDAI ≥ 20).25 In this study, we classified disease activity into 2 groups, low (SLEDAI ≤ 5) and high (SLEDAI ≥ 6) groups by SLEDAI-2K.26
Real-Time PCR Analysis
Total RNA was extracted from SLE and HD peripheral blood mononuclear cells (PBMC), and real-time PCR were performed as previously reported.16 The primers used in this study are shown in Supplemental Table 1.
Immunofluorescence Staining
Immunofluorescence staining was performed as previously reported.16 Briefly, frozen renal cryosections from lupus nephritis patients and individuals without renal disease which were used as negative controls were stained for immunological assessment of Cyr61 expression. Rabbit anti-human Cyr61 (Santa Cruz) in conjugation with goat anti-rabbit Alexa Fluor 488 (Invitrogen™), mouse anti-human IgG (Cell Signaling Technology Inc, MA, USA) in conjugation with goat anti-mouse Alexa Fluor 555 (InvitrogenTM, OR, USA) and 4,6-diamidino-2-phenylindole (DAPI) (Fluka, Seelze, Germany) were used. Immunostainings were analyzed with a fluorescence microscope (Zeiss Axioskop 2 plus).
Statistical Analysis
Data were presented as mean ± SD or n (%), differences between groups were analyzed by unpaired Student's t test. Comparisons of categorical variables were conducted using χ2 testing. Correlation analyses were done by Microsoft Excel. To evaluate the diagnostic performance, sensitivity was plotted against (1-specificity) at different cut-off values for Cyr61 levels by receiver operating characteristic (ROC) analysis, which was created by plotting the true positive rate against the false positive rate at various threshold settings (The true-positive rate is also known as sensitivity, the false-positive rate is also known as 1-specificity). For all statistical analyses, 2-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences, version 13.0 (SPSS Inc, Chicago, IL, USA) or GraphPad Prism 4.0 (GraphPad Software, San Diego, CA).
RESULTS
Demographic Characteristics of SLE Patients and Healthy Controls
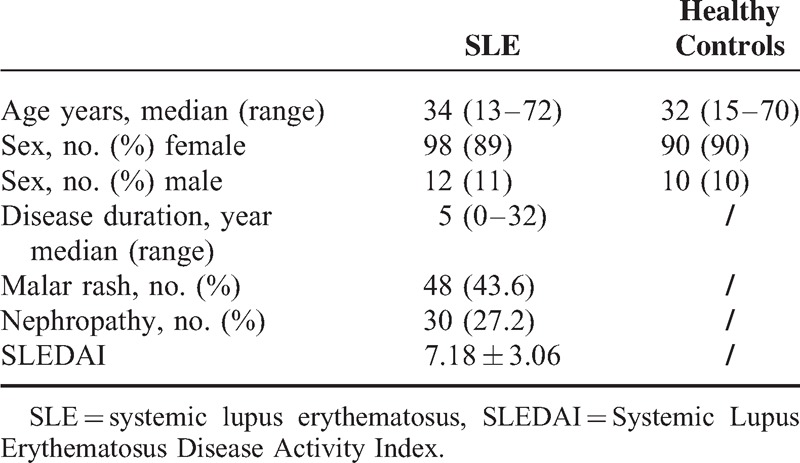
In this cohort of 110 SLE patients, 98 were female (89%) and 12 were male (11%), with ages from 13 to 72 years (median age 34 years). Median disease duration was 6 years (0–32 years). Disease manifestations included malar rash, nephropathy and SLEDAI. Demographic features were shown in Table 1.
TABLE 1.
Demographic Characteristic of SLE Patients and Healthy Controls
Cyr61 is Increased in SLE Patients
Because Cyr61 is normally expressed at a low level in most tissues but becomes highly expressed in tissue damage and repair,27,28 and the pathomechanisms of lupus nephritis often involves renal cell necrosis and proliferation of endothelial and mesangial cells, we hypothesis that Cyr61 may increase in SLE. Indeed, as shown in Figure 1, the positive cut-off value was 134 (represented by the dotted line) which was established by 2 SD above the mean value for healthy controls. The serum level of Cyr61 was higher in SLE patients compared to healthy controls (P < 0.001, Figure 1). In other autoimmune diseases, such as RA, SSc, and DM, serum Cyr61 also increased (Figure 1). Based on the previously defined threshold value of 134, the sensitivity of Cyr61 levels for identification of SLE patients in this cohort were 47.3% (52/110, 52 patients was positive in all 110 SLE patients). Moreover, we investigated Cyr61 expression in frozen renal specimens from lupus nephritis and individuals without renal disease, and found that Cyr61 was high expressed in renal tubule of lupus nephritis compared to control (Figure 2). To further assess the predictive value of Cyr61 in diagnosis of SLE, we performed ROC curve analysis, and the results shown the area under the curve (AUC) was 0.830, with a 95% confidence interval (CI) ranging from 0.776 to 0.885 (Figure 3).
FIGURE 1.
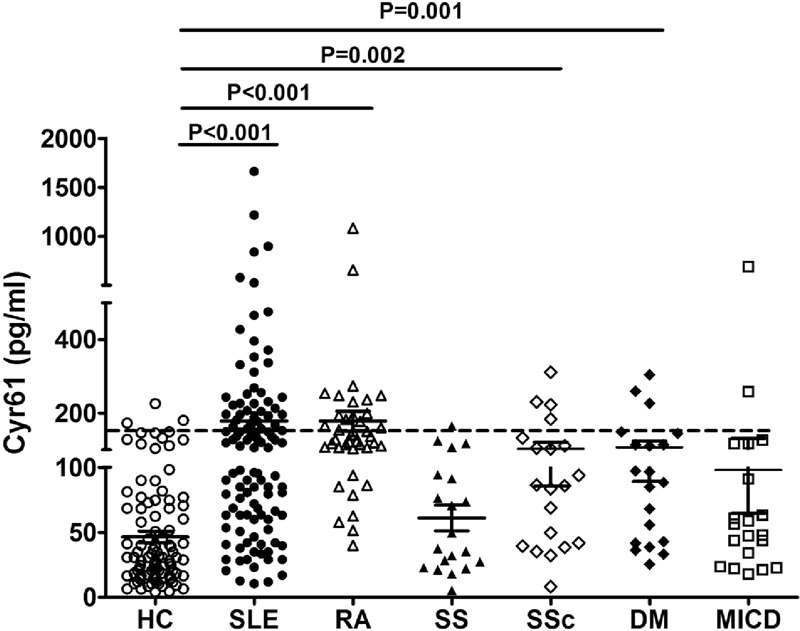
Serum level of Cyr61 in patients with SLE, healthy controls and other autoimmune diseases. The dotted line marks the cut-off value of 134 defined by mean + 2SD from healthy controls. Differences between groups were analyzed by t test. SLE: systemic lupus erythematosus; HC: healthy control; RA: rheumatoid arthritis; SS: Sjogren's syndrome; DM: dermatomyositis; MICD: mixed connective tissue disease; SSc: scleroderma.
FIGURE 2.
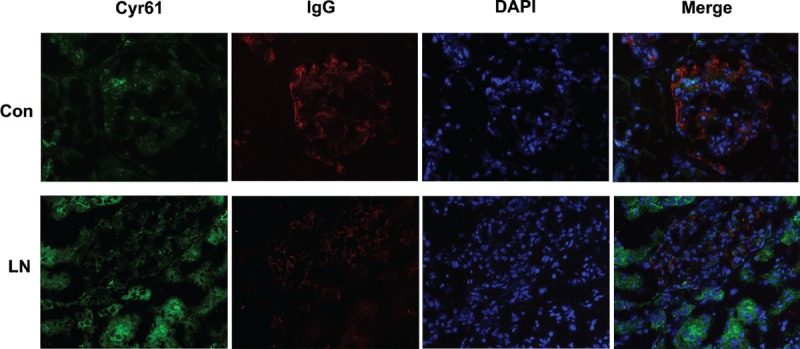
Expression of Cyr61 in the renal specimens of lupus nephritis patients. Frozen renal cryosections from lupus nephritis (LN) patients and individuals without renal disease (Con: control) were stained with Cyr61 (green), IgG (Red), and DAPI (blue). Cyr61 was high expressed in renal tubule of LN.
FIGURE 3.
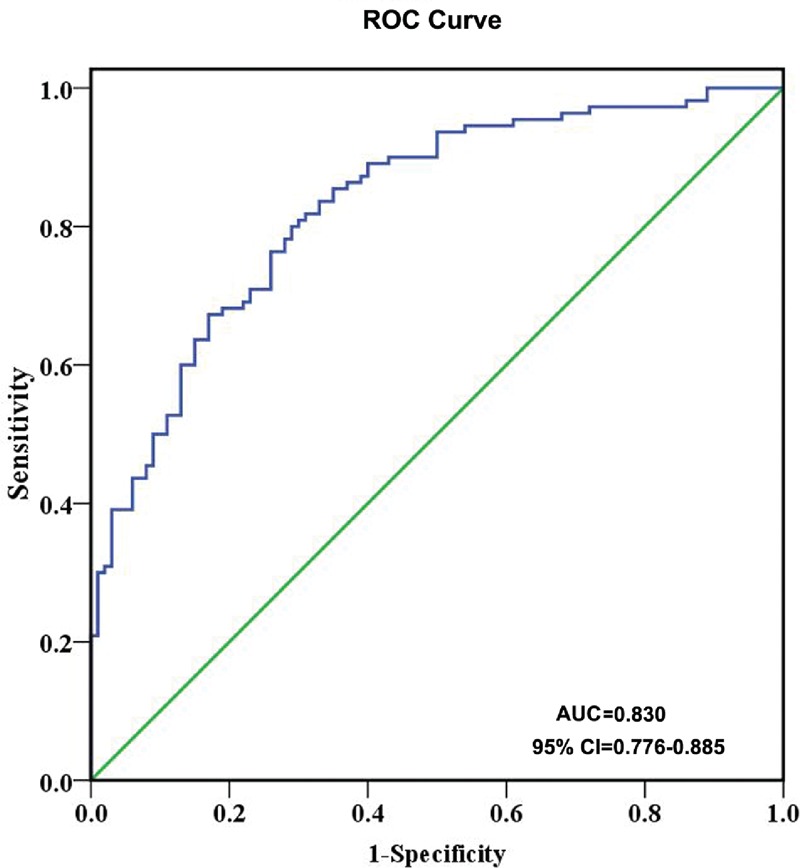
Receiver-operating characteristic (ROC) curves of Cyr61 for the identification of SLE. AUC: area under the curve; CI: confidence interval.
Serum Cyr61 is Associated with Clinical Disease Activity in Patients with SLE
To observe whether the clinical features have relations with the level of Cyr61, we divided the patients into Cyr61 positive and negative subgroup. The results showed in the 110 SLE patients, there were significant differences between Cyr61 negative and Cyr61 positive subgroups with respect to C3, C4, Sm (+), RNP (+), and SLEDAI (P < 0.05, Table 2). Furthermore, correlation analyses showed a significant negative correlation between the serum level of Cyr61 and that of C3, C4 (Figure 4). Moreover, the difference in serum Cyr61 was significant between the low SLEDAI (149.6 ± 136.1) and high SLEDAI (206.7 ± 296.0) groups (P = 0.0132, Figure 5). Other parameters were statistically similar between the 2 groups (P > 0.05 for each parameter, Table 2). Thus, we concluded that serum Cyr61 may be correlated to clinical disease activity of SLE.
TABLE 2.
Comparison of Clinical and Laboratory Parameter of SLE Patients between Cyr61 Positive and Those without Cyr61 Groups
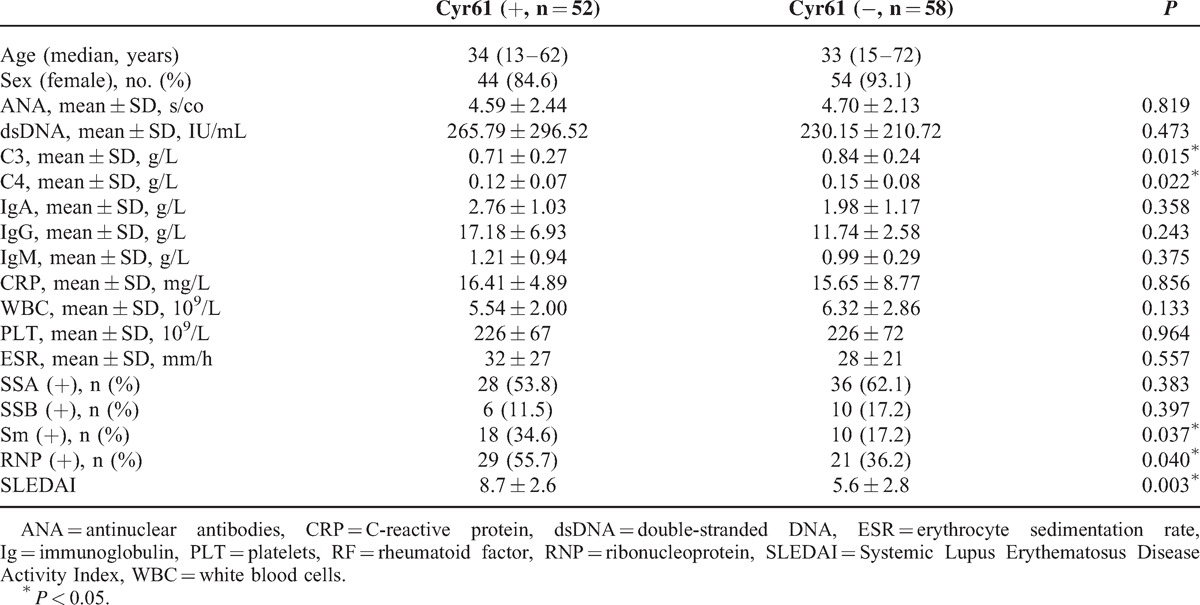
FIGURE 4.
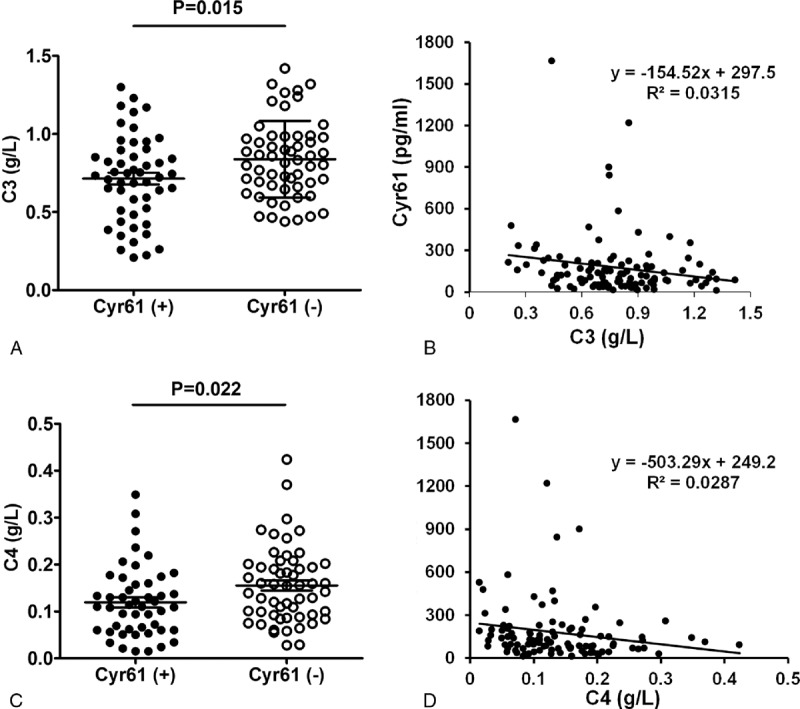
Associations between Cyr61 and C3, C4 of SLE patients. (A) Levels of C3 in SLE patients with Cyr61 positive or negative. (B) Associations between serum Cyr61 and C3 in SLE patients. (C) Levels of C4 in Cyr61 positive or negative subgroup. (D) Associations between serum Cyr61 and C4 in SLE patients. C3: complement 3; C4: complement 4.
FIGURE 5.
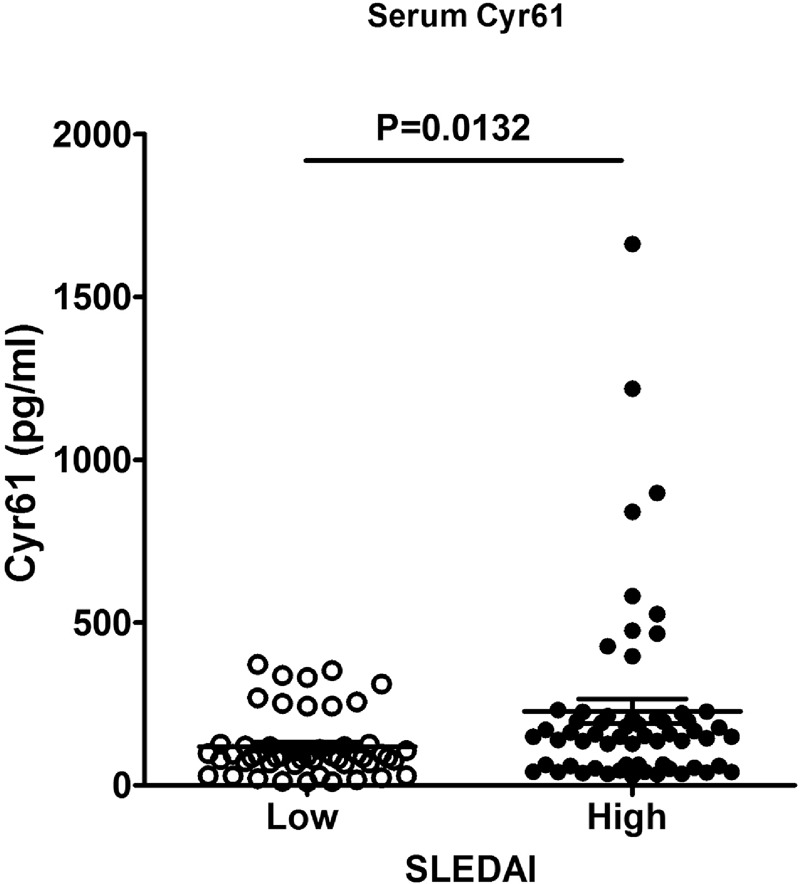
Comparison of serum Cyr61 between low and high SLEDAI groups. Patients with high disease activity showed higher serum Cyr61 levels than those with low SLEDAI (P = 0.0132). SLEDAI: systemic lupus erythematosus disease activity index.
Frequency of Cyr61 in SLE Patients Lacking Disease-Specific Antibodies
It is well known that some SLE patients were found ANA, anti-dsDNA, or Sm negative, whether Cyr61 can capture some patients with these specific autoantibodies negative? Of note, Cyr61 was present in 54.5% (6/11) of ANA negative SLE patients, 60% (9/15) of anti-dsDNA negative SLE patients, and 41.5% (34/82) of anti-Sm negative SLE patients. Combined with these alternative antibodies, Cyr61 was found positive in an additional 5 SLE patients (Table 3).
TABLE 3.
Frequency of Cyr61 in SLE Patients with the Absence of SLE-Specific Autoantibodies
Serum Cyr61 is Associated with Cytokines IL-10, TNF-α, and IL-17 in SLE Patients
Furthermore, to determine whether the presence of Cyr61 was related to a particular cytokine profile in SLE patients, the mRNA expression of IL-10, TNF-α, IL-17, and IL-6 was evaluated. The results showed that TNF-α, IL-6, and IL-17 expression was increased in SLE patients and correlated with serum Cyr61 (Figure 6). Moreover, the serum level of TNF-α (84.96 ± 54.26 vs 54.97 ± 32.15, P = 0.040), IL-6 (89.62 ± 44.89 vs 63.21 ± 33.30, P = 0.036), and IL-17 (37.99 ± 22.95 vs 23.43 ± 15.83, P = 0.028) was higher in Cyr61 positive group compared to Cyr61 negative group (Table 4). No significant differences between patients and controls or related to Cyr61 were detected regarding IL-10 expression and serum IL-10 (42.69 ± 25.98 vs 46.91 ± 29.37, P = 0.641). Based on these data, we suggested that increased serum Cyr61 may be associated with serum TNF-α, IL-6, and IL-17 level in SLE patients.
FIGURE 6.
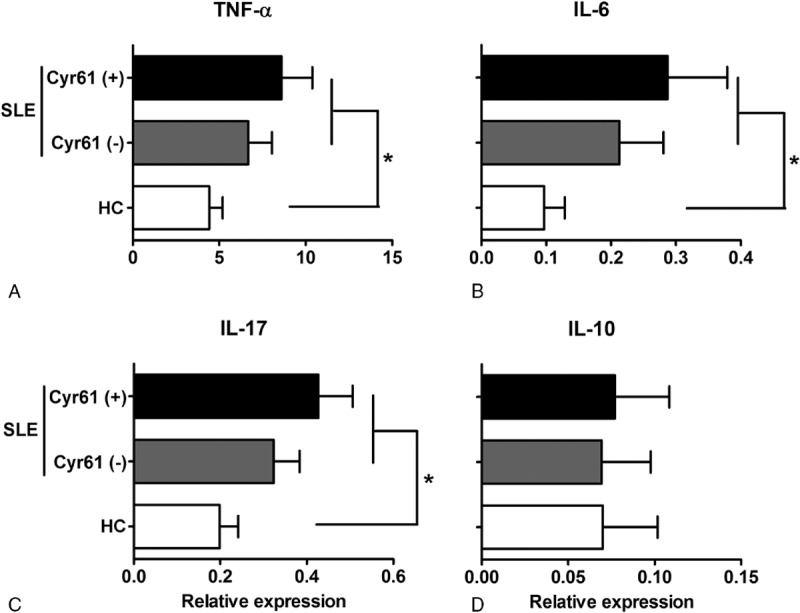
Expression of TNF-α, IL-17, IL-6, and IL-10 in SLE patients and healthy controls. Expression of TNF-α (A), IL-6 (B), and IL-17 (C) was significantly higher in SLE patients, and the presence of Cyr61 was associated with the up-regulated expression of TNF-α, IL-6, and IL-17. IL: interleukin, TNF: tumor necrosis factor; SLE: systemic lupus erythematosus; HC: healthy controls. ∗P < 0.05.
TABLE 4.
Cytokines Level Between Cyr61 Positive and Those without Cyr61 Groups

DISCUSSION
Cyr61 is a multifunctional matricellular protein that plays essential roles in cardiovascular development, wound healing, inflammation, and fibrogenesis.29,30 Aberrant Cyr61 expression is associated with various cancers31–33 and diseases associated with chronic inflammation.34 We recently showed that Cyr61 was overexpressed in RA synovial tissue and that it played a critical role in IL-17-stimulated proliferation of RA FLS.13 We further showed that Cyr61 stimulated IL-6 production by FLS via the αvβ5/Akt/NF-κB signaling pathway, which in turn promoted Th17 cell differentiation.14 Moreover, we have found that Cyr61 can promote IL-8 production by FLS and involved in the inflammation and tissue damage of RA.15 However, whether Cyr61 plays any effect in the pathogenesis of SLE remains unknown.
In the present study, we first showed clinically significant increase of serum Cyr61 level in SLE. Moreover, we found high expression of Cyr61 in renal tubule of lupus nephritis by immunofluorescence staining of renal specimens. As Cyr61 is highly expressed in tissue damage and repair, and lupus nephritis often involves renal cell necrosis and proliferation of endothelial cells, we supposed the high expression of Cyr61 in renal tubule may contribute to the proliferation of renal tubule epithelial cells of lupus nephritis. The increased expression of Cyr61 by epithelial cells may secreted and released into the blood by renal blood circulation which may result in high serum Cyr61 level in SLE patients. Also, we speculated Cyr61 level may increase in urine from patients of SLE. However, the hypothesis needs to be proved by more experiments. Further analysis showed a strong overall performance characteristics of Cyr61 in the diagnosis of SLE (ROC curve analysis: AUC = 0.830, 95% CI 0.776–0.885), with an optimal sensitivity of 47.3%. Compared to 116 SLE-associated antibodies described in the literature,2 most of which have a sensitivity well below 45%, Cyr61 has good sensitivity. Moreover, when combined with other relatively specific autoantibodies for SLE, such as ANA, anti-Sm, and anti-dsDNA, Cyr61 can capture additional patients testing negative for these autoantibodies, suggesting the complementary potential of Cyr61 assessment for the diagnosis of SLE.
To further assess whether increased serum Cyr61 in SLE was associated with clinical disease activity, the patients were divided into Cyr61-positive and Cyr61-negative subgroup based on the cut-off value. Although there were no significant correlations with parameters such as age, ANA, IgA, IgM, IgG, CRP, ESR, WBC, or PLT, Cyr61 was negative correlated with C3 and C4. It is known that complement is often strongly activated in patients with SLE. Patients with active SLE often have hypocomplementemia. Low total complement hemolytic activity and decreased C3 and C4 levels have been found in SLE patients which were associated with disease activity.3,5,35 In this study, we found C3 and C4 levels were lower in Cyr61-positive group compared to that of Cyr61-negative group, and the Cyr61 level was negative associated with C3 and C4. We also analyzed the association of serum Cyr61 with SLEDAI which was an index reflecting the disease activity of SLE. The results showed the serum Cyr61 was higher in high SLEDAI group. Combined with these founding, we thus suggested that high Cyr61 level in SLE may correlate with heightened disease activity in SLE patients.
The pathogenesis of SLE is complex. Cytokines play key roles in the regulation of systemic inflammation and local tissue damage. Some studies have shown that serum TNFα, IL-6, and IL-17 levels are elevated in SLE patients and correlate with disease activity.10,36–38 So, we further investigated the association of serum Cyr61 with the inflammatory factors. The results showed that TNFα, IL-6, and IL-17 expression was increased in PMBC of SLE compared to controls. Moreover, mRNA expression and serum level of these cytokines was higher in Cyr61 positive subgroup than in Cyr61 negative subgroup. These findings indicated that serum Cyr61 was associated with the inflammation in patients with SLE. However, whether the Cyr61 could induce cytokines production as that in RA14 remained unknown. We know Cyr61 could elicit diverse functions by binding to different types of integrin molecules such as αvβ3, αvβ5, αIIbβ3, and α6β1 on different cells.39,40 In human, we have identified that ανβ5 is Cyr61 receptor on RA FLS.13,14 We thus hypothesized that Cyr61 may be associated with inflammation through induced production of cytokines by binding to the integrin receptors. In the present study, we found that with increased Cyr61 expression, integrin α6, and β1 expression on PBMC derived from SLE patients increased (data was not shown), suggesting that elevated serum level of Cyr61 may induce the expression of TNFα, IL-6, or IL-17 in PBMC of SLE patients by binding to the receptor α6β1. However, the evidence was poor, more data was needed to prove ours hypothesis.
As our results were from a single-center study with a relatively small sample size, it may bias the association between Cyr61 and SLE. However, we presented initial evidence that Cyr61 was increased in serum of SLE patients which was associated with clinical disease activity and inflammation in SLE patients. Cyr61 appear to be a potential useful adjunct to the existing SLE associated autoantibodies. In addition, serum Cyr61 was also found increased in RA, SSc, and DM in this study; however, whether increased Cyr61 was associated with clinical disease activity of these autoimmune diseases need to be studied.
In summary, our results showed serum Cyr61 was elevated in SLE patients with relative high sensitivity. The increased Cyr61 level in SLE was associated with the clinical disease activity and inflammatory factors. Given the critical role of disease activity and inflammation in diagnosis of SLE, we suggest that Cyr61 may be a novel potential auxiliary marker for the diagnosis of SLE.
Footnotes
Abbreviations: ANA = antinuclear antibodies, C3 = complements, Cyr61 = cysteine rich angiogenic inducer 61, DM = dermatomyositis, dsDNA = double stranded DNA, IL = interleukin, MICD = mixed connective tissue disease, RA = rheumatoid arthritis, ROC = receiver operating characteristic, SLE = systemic lupus erythematosus, SLEDAI = systemic lupus erythematosus disease activity index, SS = Sjogren's syndrome, SSc = scleroderma.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med 2008; 358:929–939. [DOI] [PubMed] [Google Scholar]
- 2.Sherer Y, Gorstein A, Fritzler MJ, et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 2004; 34:501–537. [DOI] [PubMed] [Google Scholar]
- 3.Barilla-Labarca ML, Toder K, Furie R. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol 2013; 148:313–321. [DOI] [PubMed] [Google Scholar]
- 4.Leffler J, Bengtsson AA, Blom AM. The complement system in systemic lupus erythematosus: an update. Ann Rheum Dis 2014; 73:1601–1606. [DOI] [PubMed] [Google Scholar]
- 5.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun 2010; 34:J276–286. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey PW, Allison ME, Akkaraju S, et al. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science 1996; 271:348–350. [DOI] [PubMed] [Google Scholar]
- 7.Davies KA, Schifferli JA, Walport MJ. Complement deficiency and immune complex disease. Springer Semin Immunopathol 1994; 15:397–416. [DOI] [PubMed] [Google Scholar]
- 8.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol 2000; 18:393–422. [DOI] [PubMed] [Google Scholar]
- 9.Apostolidis SA, Lieberman LA, Kis-Toth K, et al. The dysregulation of cytokine networks in systemic lupus erythematosus. J Interferon Cytokine Res: Off J Int Soc Interferon Cytokine Res 2011; 31:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res Ther 2011; 13:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure–function relationships. Trends Biochem Sci 2008; 33:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kireeva ML, Mo FE, Yang GP, et al. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol 1996; 16:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Wu J, Cao Q, et al. A critical role of Cyr61 in interleukin-17-dependent proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Rheum 2009; 60:3602–3612. [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Zhou Z, Huo R, et al. Cyr61 induces IL-6 production by fibroblast-like synoviocytes promoting Th17 differentiation in rheumatoid arthritis. J Immunol 2012; 188:5776–5784. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Xiao L, Huo R, et al. Cyr61 is involved in neutrophil infiltration in joints by inducing IL-8 production by fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res Ther 2013; 15:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Huo R, Xiao L, et al. A novel p53/microRNA-22/Cyr61 axis in synovial cells regulates inflammation in rheumatoid arthritis. Arthritis Rheumatol 2014; 66:49–59. [DOI] [PubMed] [Google Scholar]
- 17.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol 2010; 184:3223–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emre Y, Irla M, Dunand-Sauthier I, et al. Thymic epithelial cell expansion through matricellular protein CYR61 boosts progenitor homing and T-cell output. Nat Commun 2013; 4:2842. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 20.Johnson SR. New ACR EULAR Guidelines for Systemic Sclerosis Classification. Curr Rheumatol Rep 2015; 17:506. [DOI] [PubMed] [Google Scholar]
- 21.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 2002; 61:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alarcon-Segovia D, Cardiel MH. Comparison between 3 diagnostic criteria for mixed connective tissue disease. Study of 593 patients. J Rheumatol 1989; 16:328–334. [PubMed] [Google Scholar]
- 24.Medsger TA, Jr, Oddis CV. Classification and diagnostic criteria for polymyositis and dermatomyositis. J Rheumatol 1995; 22:581–585. [PubMed] [Google Scholar]
- 25.Cook RJ, Gladman DD, Pericak D, et al. Prediction of short term mortality in systemic lupus erythematosus with time dependent measures of disease activity. J Rheumatol 2000; 27:1892–1895. [PubMed] [Google Scholar]
- 26.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29:288–291. [PubMed] [Google Scholar]
- 27.Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem 2001; 276:47329–47337. [DOI] [PubMed] [Google Scholar]
- 28.Hadjiargyrou M, Ahrens W, Rubin CT. Temporal expression of the chondrogenic and angiogenic growth factor CYR61 during fracture repair. J Bone Miner Res: Off J Am Soc Bone Miner Res 2000; 15:1014–1023. [DOI] [PubMed] [Google Scholar]
- 29.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 2010; 12:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev 2010; 29:295–307. [DOI] [PubMed] [Google Scholar]
- 31.Sun ZJ, Wang Y, Cai Z, et al. Involvement of Cyr61 in growth, migration, and metastasis of prostate cancer cells. Br J Cancer 2008; 99:1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gery S, Xie D, Yin D, et al. Ovarian carcinomas: CCN genes are aberrantly expressed and CCN1 promotes proliferation of these cells. Clin Cancer Res: Off J Am Assoc Cancer Res 2005; 11:7243–7254. [DOI] [PubMed] [Google Scholar]
- 33.Lin MT, Chang CC, Chen ST, et al. Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J Biol Chem 2004; 279:24015–24023. [DOI] [PubMed] [Google Scholar]
- 34.Kular L, Pakradouni J, Kitabgi P, et al. The CCN family: a new class of inflammation modulators? Biochimie 2011; 93:377–388. [DOI] [PubMed] [Google Scholar]
- 35.Valentijn RM, van Overhagen H, Hazevoet HM, et al. The value of complement and immune complex determinations in monitoring disease activity in patients with systemic lupus erythematosus. Arthritis Rheum 1985; 28:904–913. [DOI] [PubMed] [Google Scholar]
- 36.Esposito P, Balletta MM, Procino A, et al. Interleukin-6 release from peripheral mononuclear cells is associated to disease activity and treatment response in patients with lupus nephritis. Lupus 2009; 18:1329–1330. [DOI] [PubMed] [Google Scholar]
- 37.Studnicka-Benke A, Steiner G, Petera P, et al. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol 1996; 35:1067–1074. [DOI] [PubMed] [Google Scholar]
- 38.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000; 9:589–593. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Du XY. Functional properties and intracellular signaling of CCN1/Cyr61. J Cell Biochem 2007; 100:1337–1345. [DOI] [PubMed] [Google Scholar]
- 40.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci: CMLS 2011; 68:3149–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]


