Abstract
The present study evaluated the association of carbon monoxide intoxication (COI) with Parkinson disease (PD).
A total of 9012 adults newly diagnosed with COI were enrolled in this study as the COI cohort. The control (non-COI) cohort, comprising 36,048 participants, was matched for each COI patient according to age, sex, and the year of hospitalization. We calculated the hazard ratios (HR) and 95% confidence intervals by using a Cox proportional hazards regression model.
The overall incidence of PD (per 10,000 person-year) in the COI and non-COI cohorts was 27.4 and 2.53, respectively. After adjustment for age, sex, and comorbidities, the COI patients exhibited a 9.08-fold increased risk for PD. The COI patients without comorbidity exhibited a significantly higher risk of PD (adjusted HR = 15.8) than did the COI patients without comorbidity (adjusted HR = 4.15). Patients with COI and receiving hyperbaric oxygen therapy exhibited a 14.3-fold increased risk of PD; the adjusted HR of patients who did not receive hyperbaric oxygen treatment was increased 7.97-fold.
The risk of PD increased in the COI patients and the significance increased in young people. COI is a crucial factor leading to PD.
INTRODUCTION
Parkinson disease (PD) is a progressive neurodegenerative disorder of the central nervous system. PD affects between 100 and 200 per 100,000 people over 40 years, and over 1 million people in North America alone.1 Initially, patients with PD exhibit tremor, bradykinesia, and rigidity.2–5 Subsequently, approximately 25% of patients become severely disabled or die within 5 years of PD onset.6 The condition of most patients shifts from impairment to disability within 3 to 7 years of PD onset.7 Approximately 77% of patients exhibit a poor outcome 10 years after diagnosis.8,9 The pathogenesis of PD is unknown. Oxidative stress is only one of the proposed pathogenic mechanisms. The cause of PD is unknown; however, the underlying mechanisms of PD have been determined.10 The oxidative stress hypothesis postulates that inappropriate production of reactive oxygen species leads to neurodegeneration.11,12
Carbon monoxide intoxication (COI) occurs after inhalation of excessive amounts of carbon monoxide (CO), a toxic gas. However, because CO is colorless, odorless, tasteless, and initially nonirritating, detecting CO is difficult for humans. COI leads to approximately 40,000 emergency department consultations, and between 5000 and 6000 deaths per year in the United States.13–15 Approximately 40% of patients with considerable COI develop delayed neurologic sequelae (DNS).16–19 These include variable degrees of cognitive deficits, personality changes, movement disorders, and focal neurologic deficits. The mechanism of DNS has not been entirely determined; however, it probably involves lipid peroxidation through oxidative stress and damaged endothelial cells.20–23 Ischemia-reperfusion injury and exposure to high oxygen may exacerbate the initial oxidative damage in those who recover from COI.24,25
Both diseases related to oxidative stress have been rarely discussed together. Therefore, this study evaluated the association of COI with PD.
METHODS
Data Source
The study was based on data from the National Health Insurance Research Database (NHIRD) in Taiwan. The National Health Research Institute (NHRI), which maintains and updates the NHIRD, provided the medical claims data and approved this study. We used scrambled patient identification numbers to link files, including inpatient care claims and the registry for beneficiaries. This study was approved by the Institutional Review Board of China Medical University, Central Taiwan (CMU-REC-101–012). According to the National Health Insurance (NHI) annual statistics report, the coverage of the NHI in 2007 was nearly 99% of the entire population of Taiwan; in total, more than 25 million people were enrolled in this program (http://www.nhi.gov.tw/english/index.aspx). The diagnosis codes in this study were derived from the International Classification of Diseases, Ninth Revision (ICD-9). The NHIRD covers a highly representative sample of Taiwan's general population, because the reimbursement policy is universal and operated by a single buyer, the government in Taiwan. All insurance claims should be scrutinized by medical reimbursement specialists and peer review. COI and PD were accurately diagnosed and coded (ICD-9 codes) by the specialists according to the standard diagnosed criteria including typical symptoms/signs, laboratory data, and imaging findings. In addition, if the doctors or hospitals make the wrong codes or diagnoses will be punished by the National Health Insurance Administration with a lot of penalty. Therefore, the diagnoses of COI and PD in this study were highly reliable. In addition, we have published related studies according to the same diagnoses and ICD-9 codes.26–29
Sampled Participants
We conducted a retrospective cohort study of patients who were newly hospitalized for COI (ICD-9 code 986) between January 1, 2000 and December 31, 2011. The date of the first hospitalization for COI was identified as the index date. We excluded patients who had exhibited PD (ICD-9 code 332) before the index date and those with incomplete age or sex information. For each identified COI patient, four comparison person was randomly identified frequency-matched with age (at 5-years interval), sex,, year of index date and using the same exclusion criteria for the non-COI cohort.
Outcome and Comorbidities
All subjects were followed for the period beginning at the index date until the end of 2011, loss to follow-up, the date of withdraw from the NHI, or until the occurrence of PD. We used inpatient diagnosis files to ascertain the existence of comorbidities, including diabetes (ICD-9 code 250), hypertension (ICD-9 codes 401–405), head injury (ICD-9 codes 310.2, 800, 801, 803, 804, 850, 851, 853, 854), depression (ICD-9 codes 296.2, 296.3, 296.82, 300.4, 311), stroke (ICD-9 codes 430–438), dementia (ICD-9 codes 290, 294.1, 331.0), and chronic kidney disease (ICD-9 code 585). Acute respiratory failure (ICD-9 code 518.81) was also considered and identified according to diagnoses in the hospitalization records within 3 days of the patient's index date. We also evaluated the risks of PD for the COI patients who received hyperbaric oxygen (HBO) therapy (Procedure Code 93.95).
Statistical Analysis
The differences in demographic variables between COI and non-COI cohorts were analyzed using a chi-square test for categorical variables and a t test for continuous variables. We assessed the cumulative incidence of PD by using the Kaplan–Meier method between the COI cohort and the non-COI cohort and estimated their differences using a log-rank test. The incidence for PD was calculated in both cohorts. Univariate and multivariate Cox proportional hazards regression was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for PD. The multivariate model was simultaneously adjusted for sex, age, and the comorbidities of diabetes, hypertension, head injury, depression, stroke, dementia, and chronic kidney disease. A 2-tailed P value < 0.05 was considered statistically significant. All analyses were performed using SAS statistical software (Version 9.2 for Windows; SAS Institute, Inc., Cary, NC).
RESULTS
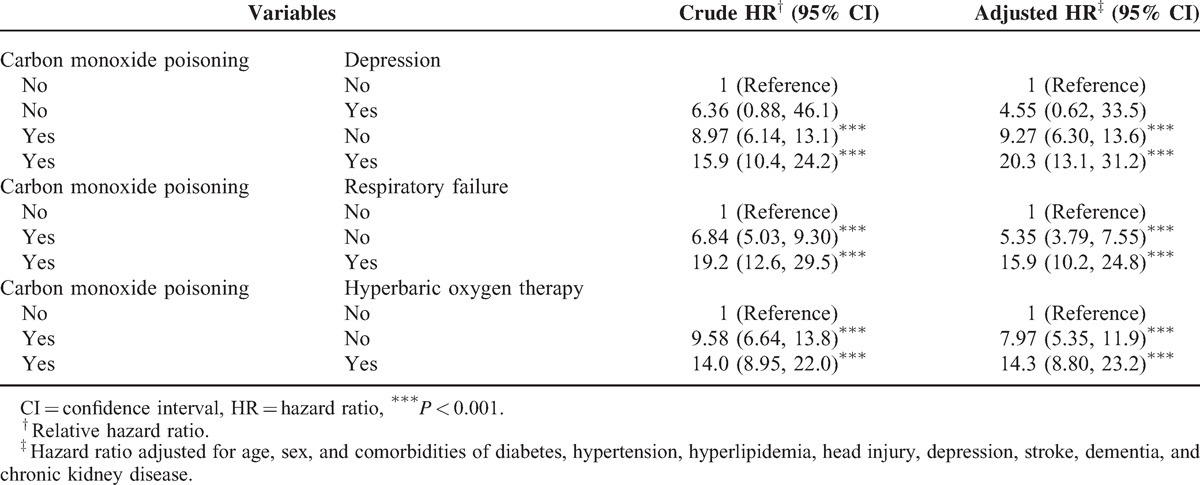
Table 1 contains the baseline characteristics of the patients with and without COI. Most patients were aged <34 years (42.9%) and the mean ages of the COI cohort and non-COI cohort were 39.8 (±13.9) and 39.7 (±14.3) years, respectively. Comorbidities at the baseline were more prevalent in the COI cohort than in the non-COI cohort (all P < 0.01). The mean follow-up was 4.50 years for the COI cohort and 4.94 years for the non-COI cohort. After 12 years of follow-up, the cumulative incidence of PD in the COI cohort was approximately 1.47% higher than that in the non-COI cohort (P < 0.001; Figure 1). The overall incidence of PD was 10.6-fold higher in the COI cohort than in the non-COI cohort (27.4 and 2.53 per 10,000 person-year, respectively; Table 2). After adjustments for age, sex, and the comorbidities of diabetes, hypertension, head injury, depression, stroke, dementia, and chronic kidney disease, the COI patients exhibited a 9.08-fold higher risk of developing PD (95% CI = 6.21–13.3) than did the non-COI patients. The sex-specific relative risk of PD in the COI cohort compared with that in the non-COI cohort was significant for both women (adjusted HR = 14.1; 95% CI = 7.20–27.7) and men (adjusted HR = 7.48; 95% CI = 4.66–12.0). The incidence of PD increased with age and in the presence of comorbidities. The age-specific relative risk of PD in the COI patients compared with that in the control patients was higher in all age groups, particularly in patients between 20 and 49 years of age (adjusted HR = 39.4; 95% CI = 16.7–93.0). The risk of PD stratified by comorbidity exhibited a 15.8-fold risk and was observed in patients without comorbidity (95% CI = 9.80–24.8). Table 3 lists the joint effects of COI and depression or respiratory failure on the risk of PD. A higher risk of PD was observed in patients with both COI and depression (adjusted HR = 20.3; 95% CI = 13.1–31.2) or both COI and respiratory failure (adjusted HR = 15.9; 95% CI = 10.2–24.8), compared with patients without COI, depression, or respiratory failure. Patients with COI and receiving HBO therapy were 14.3-fold more likely to develop PD (95% CI = 8.80–23.2) than were patients without COI and not receiving HBO therapy, followed by patients with COI and not receiving HBO (HR = 7.97; 95% CI = 5.35–11.9).
TABLE 1.
Characteristics of Patients With Carbon Monoxide Poisoning and Without Carbon Monoxide Poisoning
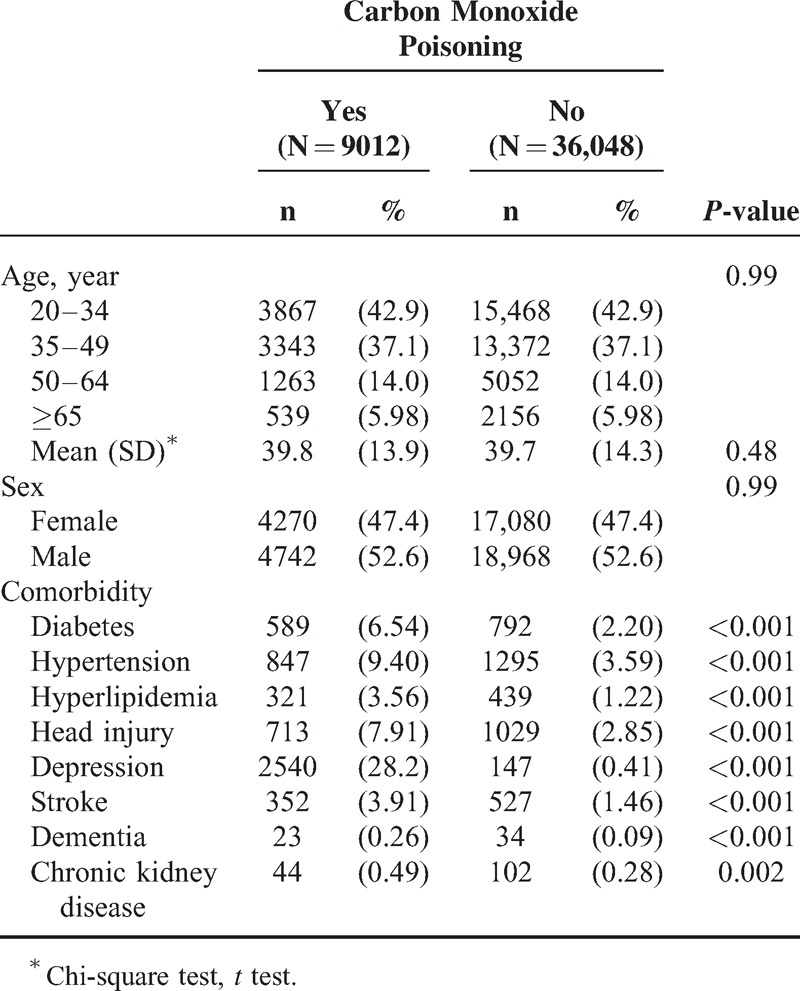
FIGURE 1.
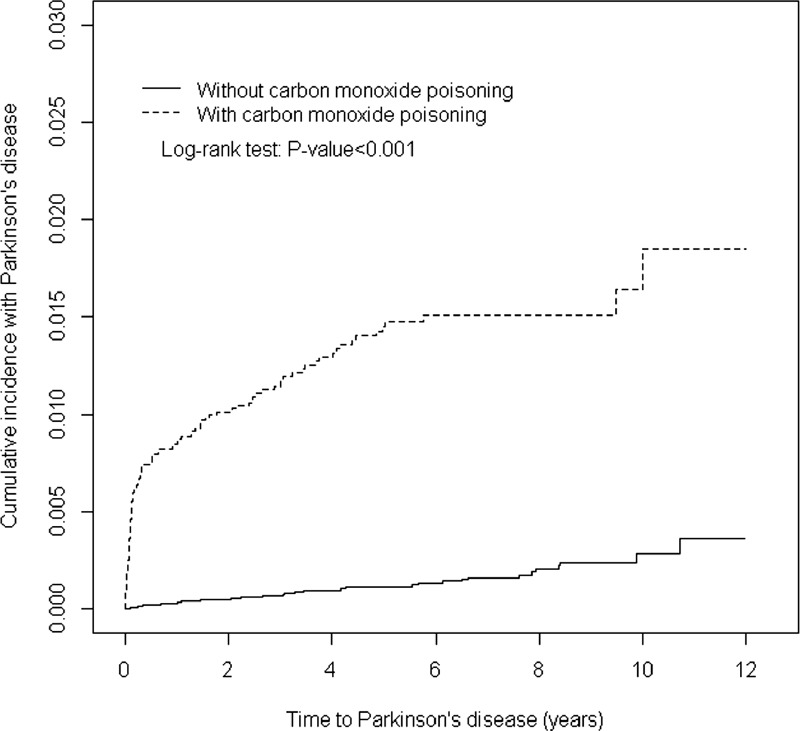
Cumulative incidence of Parkinson disease compared between with and without carbon monoxide poisoning.
TABLE 2.
Incidence and Hazard Ratio of Parkinson Disease Between Patients With Carbon Monoxide Poisoning and Without Carbon Monoxide Poisoning
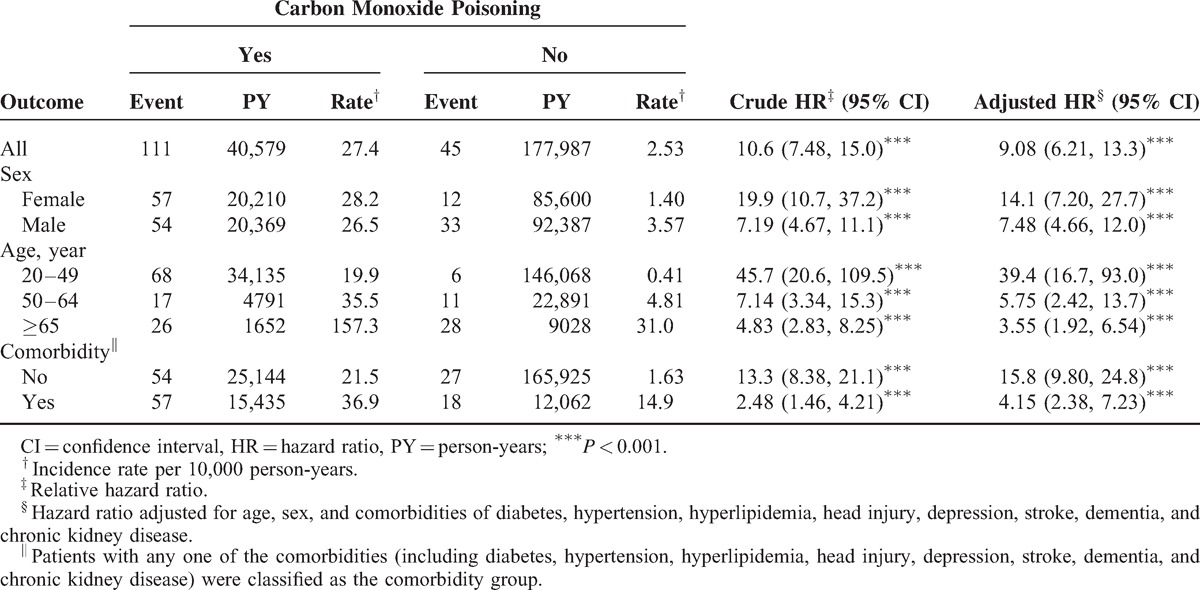
TABLE 3.
Cox Proportional Hazard Regression Analysis for the Risk of Parkinson Disease-Associated Carbon Monoxide Poisoning With Interaction of Depression and Respiratory Failure
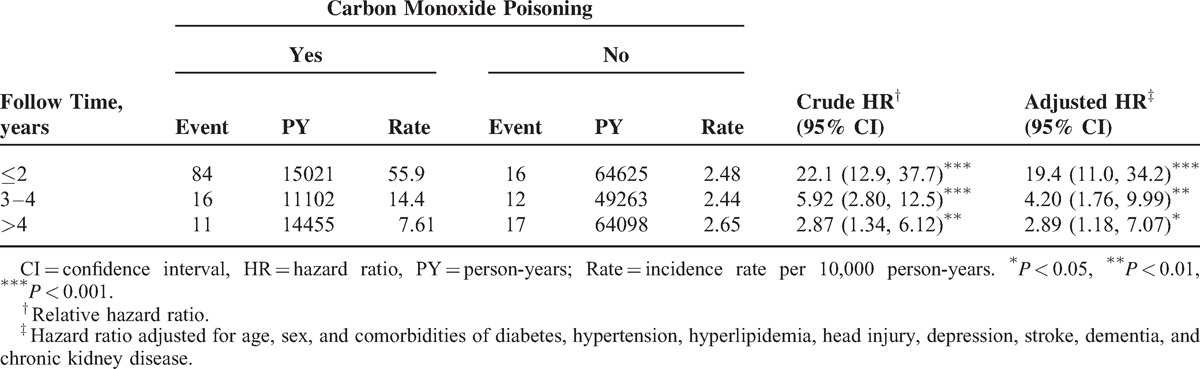
A stratified analysis of the follow-up duration revealed that the adjusted HR of PD decreased with the follow-up length (Table 4). The adjusted HR of PD was significantly higher in the first 2 follow-up years (adjusted HR = 19.4; 95% CI = 11.0–34.2) than after the first 2 years (adjusted HR = 4.20; 95% CI = 1.76–9.99: within 3 to 4 follow-up years; adjusted HR = 2.89; 95% CI = 1.18–7.07: >4 follow-up years).
TABLE 4.
Trends of Parkinson Disease Event Risks by Stratified Follow-Up Years
DISCUSSION
The COI cohort group comprised mostly younger people and people with more comorbidities than those present in the general population (Table 1). Whether young age and comorbidities increased the likelihood of people consciously or accidentally poisoning themselves with CO requires further research.
In the present study, the COI patients exhibited a 9.08-fold higher risk of PD (95% CI = 6.21–13.3) than did the non-COI patients. This suggests a stronger association between COI and PD than previous subjects with a family history of PD (odds ratio [OR] from 4.45 to 3.25; 95% CI = 2.43–4.35),30 exposure to pesticides (adjusted relative risk = 1.7; 95% CI = 1.2–2.3; P = .002),31–37 brain concussion (OR = 1.57; 95% CI = 1.35–1.83),38 midlife migraine (OR = 3.6; 95% CI = 2.7–4.8),39 exposure to environmental toxins (high manganese release, relative risk = 1.78; 95% CI = 1.54, 2.07; high copper release, relative risk = 1.1; 95% CI = 0.94, 1.31),40,41 milk consumption (2.3-fold excess of PD; 95% CI = 1.3–4.1),42 high dietary intake of iron (increased risk of PD; OR = 1.7; 95% CI = 1.0, 2.7),43 excess body weight,44 higher levels of education,45 and a history of anemia46 (Table 2).
Compared with patients in the non-COI cohort, the incidence of PD was as much as 39.4-fold higher in young COI patients, 5.75-fold higher in the middle-age patients, and 3.55-fold higher in the elderly patients, higher than in those in the same age group (Table 2). PD is uncommon in people younger than 40.47
In addition, we observed that the COI patients with no comorbidity were more likely to develop PD than were the non-COI patients. The incidence of PD was as much as 15.8-fold higher in the patients with no comorbidity, and 4.15-fold higher in COI patients with comorbidities. This suggests that PD is caused mainly by COI itself, not relative to comorbidity factors (Table 2).
Depression is the most common psychiatric symptom of PD.48 A higher risk of PD was observed for patients with both COI and depression (adjusted HR = 20.3; 95% CI = 13.1–31.2; Table 3). COI combined with depression amounted for 28.2%; however, COI without depression leading to PD seemed to be a more prominent cause than depression without COI. Previous studies have reported that COI with respiratory failure was a strong aggravating factor for PD (adjusted HR = 15.9; 95% CI = 10.2–24.8; Table 3). The results of the current study suggest that oxidative stress damage to the brain plays a major role in the development of PD.
HBO treatment has been recommended as a therapy for COI patients.13,14,49,50 Compared with patients without COI who did not receive HBO therapy in our study, patients with COI who received HBO therapy were 14.3-fold more likely to develop PD (95% CI = 8.80–23.2), followed by patients with COI who did not receive HBO therapy (adjusted HR = 7.97; 95% CI = 5.35–11.9; Table 3). HBO treatment did not appear to be an effective preventative measure for PD. In addition, HBO treatment seemed unable to prevent reperfusion injury for neurodegeneration. There may be a severe degree of COI in patients who received HBO treatment, leading to less favorable results. The treatments with hyperbaric oxygen were received by the patients with more severe symptoms of COI. Therefore, we found influence of the severity of COI in the development of PD (Table 3). Certain aspects of COI possibly cause irreversible oxidative damage and neurodegeneration. Moreover, additional randomized controlled trials are required to determine the efficacy and differences between the methods for treating PD with and without COI. A stratified analysis according to follow-up durations revealed that the adjusted HR of PD decreased with the follow-up length (Table 4), and harmful COI effects rapidly developed.
LIMITATIONS
In the present cohort study, the degree of COI could not be determined. The NHI database provides no detailed information regarding the frequency of CO exposure (ie, whether acute or chronic poisoning had occurred) or the level of CO exposure. Therefore, further analysis is required to facilitate a deeper understanding.
The strengths of our study are the population-based design, the generalizability of findings, and the use of population-based data and NHIRD records with a large sample size including study and control cohorts. In addition, the NHIRD comprises a highly representative sample of Taiwan's general population, because the reimbursement policy is universal and operated by a single buyer, the government of Taiwan. All insurance claims are typically scrutinized by medical reimbursement specialists and peer reviewed.
Nevertheless, this study was subject to limitations. First, the NHIRD provides no detailed information on factors such as patient lifestyle, habits, body mass index, physical activity level, socioeconomic status, or family history, all of which are possible confounding factors in this study. Second, the evidence derived from a cohort study is generally of lower methodological quality than that obtained from randomized trials, because a cohort study is subject to numerous biases related to the necessary adjustments for confounding factors. Despite the meticulous design of this study and adequate control of confounding factors, biases could remain because of possibly unmeasured and unknown confounding factors. Third, the registries in the NHI claims are primarily used for administrative billing and are not verified for scientific purposes. Because of the anonymity of the identification numbers, obtaining additional information by directly contacting the patients was not possible. The accuracy of medical coding in the claims data may affect the data validity. However, the data on the diagnoses in the NHIRD are highly reliable. The insurance system has mechanisms to monitor the insurance claims.
Footnotes
Abbreviations: CI = confidence interval, COI = carbon monoxide intoxication, HR = hazard ratio, ICD-9 = International Classification of Diseases, Ninth Revision, NHIRD = National Health Insurance Research Database, PD = Parkinson disease.
Conception and design: C-YL, M-CC, C-HK. Administrative support: C-HK. Collection and assembly of data, Data analysis and interpretation, Manuscript writing, and Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212–124–002, Taiwan).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
C-YL and M-CC are contributed equally to this work.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med 1998; 339:1044–1053. [DOI] [PubMed] [Google Scholar]
- 2.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56:33–39. [DOI] [PubMed] [Google Scholar]
- 3.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 1992; 7:2–13. [DOI] [PubMed] [Google Scholar]
- 5.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson's disease. Adv Neurol 1990; 53:245–249. [PubMed] [Google Scholar]
- 6.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967; 17:427–442. [DOI] [PubMed] [Google Scholar]
- 7.Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord 2008; 23:790–796. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 2013; 84:1258–1264. [DOI] [PubMed] [Google Scholar]
- 9.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2014; 29:1615–1622. [DOI] [PubMed] [Google Scholar]
- 10.Jankovic J, Sherer T. The future of research in Parkinson disease. JAMA Neurol 2014; 71:1351–1352. [DOI] [PubMed] [Google Scholar]
- 11.Greenamyre JT, Hastings TG. Biomedicine. Parkinson's – divergent causes, convergent mechanisms. Science 2004; 304:1120–1122. [DOI] [PubMed] [Google Scholar]
- 12.Ahlskog JE. Challenging conventional wisdom: the etiologic role of dopamine oxidative stress in Parkinson's disease. Mov Disord 2005; 20:271–282. [DOI] [PubMed] [Google Scholar]
- 13.Ernst A, Zibrak JD. Carbon monoxide poisoning. N Engl J Med 1998; 339:1603–1608. [DOI] [PubMed] [Google Scholar]
- 14.Weaver LK. Carbon monoxide poisoning. Crit Care Clin 1999; 15:297–317.viii. [DOI] [PubMed] [Google Scholar]
- 15.Tibbles PM, Perrotta PL. Treatment of carbon monoxide poisoning: a critical review of human outcome studies comparing normobaric oxygen with hyperbaric oxygen. Ann Emerg Med 1994; 24:269–276. [DOI] [PubMed] [Google Scholar]
- 16.Thom SR, Taber RL, Mendiguren II, et al. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med 1995; 25:474–480. [DOI] [PubMed] [Google Scholar]
- 17.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983; 40:433–435. [DOI] [PubMed] [Google Scholar]
- 18.Kwon OY, Chung SP, Ha YR, et al. Delayed postanoxic encephalopathy after carbon monoxide poisoning. Emerg Med J 2004; 21:250–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampson NB, Little CE. Hyperbaric treatment of patients with carbon monoxide poisoning in the United States. Undersea Hyperb Med 2005; 32:21–26. [PubMed] [Google Scholar]
- 20.Thom SR, Bhopale VM, Fisher D, et al. Delayed neuropathology after carbon monoxide poisoning is immune-mediated. Proc Natl Acad Sci U S A 2004; 101:13660–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldbaum LR, Ramirez RG, Absalon KB. What is the mechanism of carbon monoxide toxicity? Aviat Space Environ Med 1975; 46:1289–1291. [PubMed] [Google Scholar]
- 22.Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest 1992; 90:1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thom SR, Xu YA, Ischiropoulos H. Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol 1997; 10:1023–1031. [DOI] [PubMed] [Google Scholar]
- 24.Weaver LK. Carbon monoxide poisoning. Crit Care Clin 1999; 15:297–317.viii. [DOI] [PubMed] [Google Scholar]
- 25.Tomaszewski C. Carbon monoxide poisoning. Early awareness and intervention can save lives. Postgrad Med 1999; 105:39–40.43-8, 50. [DOI] [PubMed] [Google Scholar]
- 26.Lee FY, Chen WK, Lin CL, et al. Carbon monoxide poisoning and subsequent cardiovascular disease risk: a nationwide population-based cohort study. Medicine (Baltimore) 2015; 94:e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung WS, Lin CL, Kao CH. Carbon monoxide poisoning and risk of deep vein thrombosis and pulmonary embolism: a nationwide retrospective cohort study. J Epidemiol Community Health 2015; pii: jech-2014-205047. doi:10.1136/jech-2014-205047. (in press). [DOI] [PubMed] [Google Scholar]
- 28.Wang HC, Lin CC, Lau CI, et al. Angiotensin-converting enzyme inhibitors and bacterial pneumonia in patients with Parkinson disease. Mov Disord 2015; 30:593–596. [DOI] [PubMed] [Google Scholar]
- 29.Huang HC, Tsai CH, Muo CH, et al. Risk of Parkinson's disease following zolpidem use: a retrospective, population-based cohort study. J Clin Psychiatry 2015; 76:e104–e110. [DOI] [PubMed] [Google Scholar]
- 30.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 2012; 72:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ascherio A, Chen H, Weisskopf MG, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol 2006; 60:197–203. [DOI] [PubMed] [Google Scholar]
- 32.Frigerio R, Sanft KR, Grossardt BR, et al. Chemical exposures and Parkinson's disease: a population-based case-control study. Mov Disord 2006; 21:1688–1692. [DOI] [PubMed] [Google Scholar]
- 33.Costello S, Cockburn M, Bronstein J, et al. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 2009; 169:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Firestone JA, Smith-Weller T, Franklin G, et al. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol 2005; 62:91–95. [DOI] [PubMed] [Google Scholar]
- 35.Elbaz A, Clavel J, Rathouz PJ, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol 2009; 66:494–504. [DOI] [PubMed] [Google Scholar]
- 36.Weisskopf MG, Knekt P, O’Reilly EJ, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 2010; 74:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pezzoli G, Cereda E. Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 2013; 80:2035–2041. [DOI] [PubMed] [Google Scholar]
- 38.Jafari S, Etminan M, Aminzadeh F, et al. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013; 28:1222–1229. [DOI] [PubMed] [Google Scholar]
- 39.Scher AI, Ross GW, Sigurdsson S, et al. Midlife migraine and late-life parkinsonism: AGES-Reykjavik study. Neurology 2014; 83:1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willis AW, Evanoff BA, Lian M, et al. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol 2010; 172:1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman SM, Quinlan PJ, Ross GW, et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol 2012; 71:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park M, Ross GW, Petrovitch H, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 2005; 64:1047–1051. [DOI] [PubMed] [Google Scholar]
- 43.Powers KM, Smith-Weller T, Franklin GM, et al. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 2003; 60:1761–1766. [DOI] [PubMed] [Google Scholar]
- 44.Hu G, Jousilahti P, Nissinen A, et al. Body mass index and the risk of Parkinson disease. Neurology 2006; 67:1955–1959. [DOI] [PubMed] [Google Scholar]
- 45.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology 2005; 65:1575–1583. [DOI] [PubMed] [Google Scholar]
- 46.Savica R, Grossardt BR, Carlin JM, et al. Anemia or low hemoglobin levels preceding Parkinson disease: a case-control study. Neurology 2009; 73:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 2003; 157:1015–1022. [DOI] [PubMed] [Google Scholar]
- 48.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson's disease. J Neurol Neurosurg Psychiatry 2006; 77:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao LW, Nañagas KA. Carbon monoxide poisoning. Emerg Med Clin North Am 2004; 22:985–1018. [DOI] [PubMed] [Google Scholar]
- 50.Hampson NB, Dunford RG, Kramer CC, et al. Selection criteria utilized for hyperbaric oxygen treatment of carbon monoxide poisoning. J Emerg Med 1995; 13:227–231. [DOI] [PubMed] [Google Scholar]


