Abstract
A positive association between hypertension or high-normal blood pressure (BP) and risk of nonalcoholic fatty liver disease (NAFLD) is well-known; however, no data have been generated exploring the risk of NAFLD within the normal range of BP. We aimed to assess the association between normal systolic blood pressure (SBP) and risk of NAFLD.
A total of 27,769 subjects from 2 separate medical centers were included. Subjects were divided into 4 groups (G1 to G4) by SBP levels: G1: 90–99 mmHg, G2: 100–109 mmHg, G3: 110–119 mmHg, and G4: 120–129 mmHg. The prevalence, hazard ratios (HRs) and 95% confidence intervals (CIs) for NAFLD were calculated across each group, using the G1 as reference.
Higher SBP was observed in subjects with NAFLD than those without NAFLD. The prevalence of NAFLD in a cross-sectional population from G1 to G4 was 6.1%, 13.6%, 19.6%, and 25.8%, respectively. The HRs for NAFLD in the longitudinal population were 2.17 (95% CI 1.60–2.93), 3.87 (95% CI 2.89–5.16), 5.81 (95% CI 4.32–7.81) for G2, G3, and G4, respectively. After adjusting for known confounding variables, HRs of G2 to G4 were 1.44 (95% CI 1.06–1.96), 1.94 (95% CI 1.44–2.61), 2.38 (95% CI 1.75–3.23), respectively.
This is the first study to demonstrate that increased levels of SBP within the normal range are associated with significantly elevated risks of NAFLD, independent of other confounding factors.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is recognized as the hepatic manifestation of metabolic syndrome (MS) and results from hepatic fat accumulation in patients without excessive alcohol intake or other causes of liver disease.1–3 It is the most common form of chronic liver disease and affects 5% to 42% of the general population in Asian countries.4,5 Recently, increasing attention has be paid to the clinical association between NAFLD and cardiovascular disease (CVD) and evidence from cross-sectional and prospective studies has suggested that NAFLD is independently associated with an increased prevalence and incidence of CVD in patients with metabolic disturbances, even when adjusted for traditional CVD risk factors or MS components.6–9 Insulin resistance (IR) has been suggested as a primary factor in the accumulation of hepatocellular fat, and may play a crucial role in patients at risk of developing CVD.10 Identifying potential risk factors or IR-related risk factors for NAFLD is may become valuable when considering the treatment regime for patients with this condition.
Referred to as an IR state, hypertension has strong associations with the occurrence of all CVDs across a wide age range.11–13 There is increasing evidence that high blood pressure (BP) is not merely associated with cardiometabolic risk factors, but may be an independent causal factor for NAFLD and several studies have shown that NAFLD occurs more frequently in hypertensive patients.14,15 Latea et al16 showed that the altered BP status of hypertension associated both a higher IR and a higher prevalence of NAFLD, suggesting altered hypertension status could be a marker of NAFLD. Further studies have indicated that even high-normal BP may also be a risk for NAFLD. An observational study which included 454 participants, demonstrated that compared with normotensive participants with an systolic blood pressure (SBP) <130 mmHg, NAFLD was independently associated with high-normal SBP (130–139 mmHg), with an adjusted odds ratio of 2.13 (95% CI, 1.08–4.20).17
Though there is strong evidence that hypertension or high-normal BP has a positive association with the prevalence of NAFLD, there is no data examining the association between BP within the normal range and NAFLD. Therefore, in this study, we aimed to determine the strength of the association between normal SBP and NAFLD risk from the large general cross-sectional population. A further validation of our findings was performed in an exterior prospective longitudinal population.
MATERIALS AND METHODS
Study population
We included subjects from 2 separate medical centers with the same medical documentation. The cross-sectional population was consisted of 24,900 individuals who underwent a health examination in the First Affiliated Hospital of Wenzhou Medical University, from January 2009 to December 2009. The longitudinal population was based on a prospective study and conducted from 14,734 initially FLD-free individuals who underwent an annual health screening in the Xinyu People's Hospital of Jiangxi Province. The study period was initiated in January 2010 and concluded in June 2013.
The exclusion criteria were as follows: age <18 years or age >65 years; systolic/diastolic blood pressure <90/60 mmHg or ≥130/85 mmHg; alcohol consumption >140 g/wk for men and 70 g/wk for women; those taking antihypertensive, lipid-lowering agents for medication, or with a history of CVD (including self-reported or diagnosed history of myocardial infarction, stroke, arterial revascularization, heart failure, arrhythmia); any other known potential causes of chronic liver disease, such as viral or autoimmune hepatitis or those using hepatotoxic medications; and subjects who were lost to follow-up.
Verbal informed consent was obtained from each subject before their participation in the study. The personal information of subjects was erased and replaced by the health examination number. The research protocol of the study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and Xinyu People's Hospital of Jiangxi Province, respectively.
Ultrasonography Test
The diagnosis of NAFLD was performed in reference to Guidelines for the assessment and management of nonalcoholic fatty liver disease in the Asia-Pacific region.18 In general, NAFLD can be diagnosed when imaging tests indicate hepatic steatosis, excluding alcohol abuse and specific diseases that could lead to steatosis. Hepatic steatosis was defined by the presence of at least 2 of 3 abnormal findings on abdominal ultrasonography: diffusely increased echogenicity (“bright”) liver with liver echogenicity greater than kidney or spleen, vascular blurring, or deep attenuation. Abdominal ultrasonography was assessed by 2 experienced imaging specialists who were blinded to the study design during the ultrasonic examination. If the diagnoses made by the 2 specialists were not in agreement or inconclusive, a third specialist was invited.
Data Collection
Clinical examination and data recording was conducted in the morning after an overnight fast and subjects were instructed to refrain from exercise during the day prior to their examination. Medical history and a health habit inventory were performed by trained medical staff using a standardized procedure.
BP, including SBP and diastolic blood pressure (DBP), was measured using a noninvasive automated sphygmomanometer (OMRON, Japan) with the subjects in a quite environment and in a sitting position. Normal BP was defined as BP < 130/85 mmHg.19 Standing height and body weight were measured without shoes or outer clothing. Body mass index (BMI), used as an index of body fat, was calculated as the ratio of weight (kg) to height (m2).
Fasting blood samples were collected from each subject in an antecubital vein and were used for the analysis of biochemical measurements serum samples without frozen. The experimental procedures were consistent throughout the study period and the laboratories in 2 centers were both certified according to International Organization Standardization. The biochemical measurements included albumin, alanine aminotransferase (ALT), aspartate aminotranferase (AST), fasting plasma glucose (FPG), blood urea nitrogen (BUN), creatinine (Cr), uric acid (UA), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). All values were measured by an automated analyzer (Abbott AxSYM) using standard methods.
MS was defined by the presence of 3 or more of the following risk factors19: central obesity: waist circumference >90 cm for men and >80 cm for women and/or BMI > 25 kg/m2 in both genders; hypertriglyceridemia: triglycerides ≥1.7 mmol/L; low HDL-C: HDL-C < 1.03 mmol/L for men and <1.29 mmol/L for women; elevated BP: BP ≥ 130/85 mmHg or previously diagnosed; and elevated FPG: FPG ≥ 5.6 mmol/L or previously diagnosed type 2 diabetes.
In the longitudinal population, subjects underwent their health examination once a year on average. The clinical, laboratory and radiological tests during the follow-up were the same as they had in the first time.
Statistical analysis
In order to derive a deeper understanding of the relationship between normal range of BP level and the prevalence of NAFLD, all subjects were classified into 4 groups by the SBP level (per 10 mmHg increase), since that elevation of SBP has major prevalence in the population as revealed by epidemiological studies.20 SBP was categorized as follows: G 190–99 mmHg, G2 100–109 mmHg, G3 110–119 mmHg, and G4 120–129 mmHg.
Continuous variables were summarized as mean ± standard deviation (SD), and categorical variables were displayed as counts or percentages. The characteristics of the study population according to SBP groups were compared using a one way analysis of variance (ANOVA) or Kruskal-Wallis test for continuous variables and χ2-test for categorical variables. We obtained a P value for linear trend of prevalence of NAFLD in each group by Pearson chi-square. Cox proportional-hazards models were used to assess the association between SBP and the time to the event of NAFLD in the longitudinal population. Multivariable models included sex, age, BMI, FPG, albumin, ALT, AST, BUN, Cr, SUA, TC, TG, HDL-C and LDL-C. The subgroup analysis was performed and stratified by MS and its components. The proportional-hazards assumption was met for all models. Kaplan-Meier analysis was applied to calculate the cumulative hazard of NAFLD during the follow-up. All P-values are 2-sided and a P value of <0.05 was considered statistically significant. Analyses were performed in SPSS version 20.0 (SPSS, Chicago, IL).
RESULTS
Subject Characteristics
A total of 39,634 subjects were initially enrolled into the study, of which 27,769 subjects remained (Figure 1). In the cross-sectional population, 16,854 eligible subjects were enrolled, including 2537 subjects who were diagnosed with NAFLD. As expected, the SBP was significantly higher in subjects with NAFLD than those without NAFLD (115.2 ± 9.0 vs. 109.8 ± 10.1 mmHg, P < 0.001). BMI, DBP, FPG, ALT, AST, BUN, Cr, TC, TG, LDL-C were significantly higher, while HDL-C was lower, among subjects with NAFLD. A total of 10,915 initially NAFLD-free subjects were included in the longitudinal population. The median follow-up time was 23.7 months. Of the 10,915 eligible subjects, 967 (8.8%) subjects developed into NAFLD, with higher SBP than that in normal subjects (116.6 ± 7.9 vs. 112.8 ± 9.2 mmHg, P < 0.001). A similar change in the measured clinical characteristics was observed with the cross-sectional population (Table 1).
FIGURE 1.
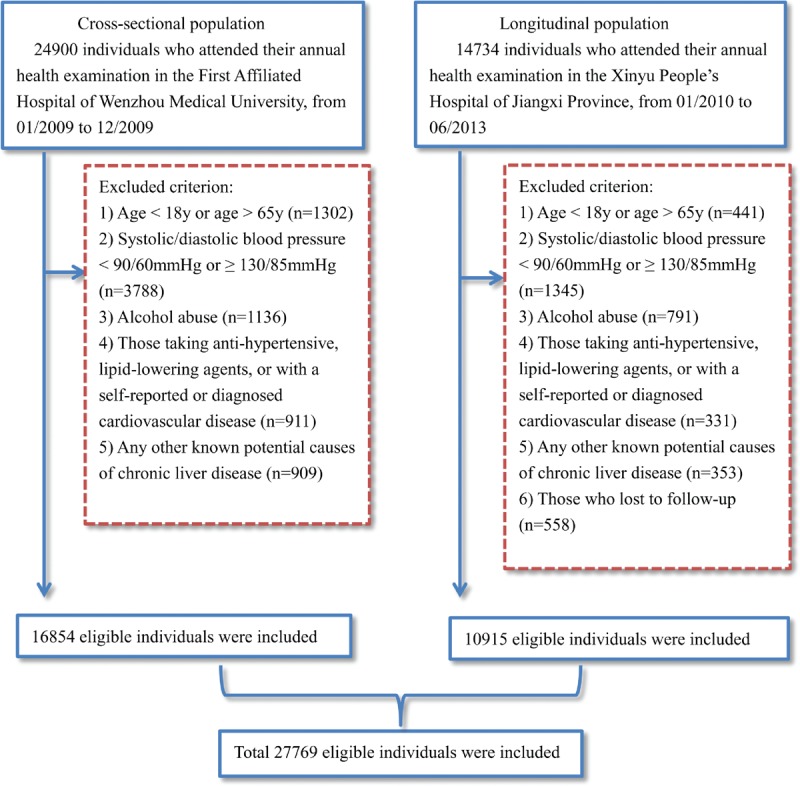
Study flow diagram. A total of 39,634 participants were enrolled initially, while 11,863 participants who did not meet the inclusive criteria were excluded. Finally, 27,769 individuals (16,854 in the cross-sectional population and 10,915 in the longitudinal population) were included.
TABLE 1.
Baseline Characteristics of Cross-Sectional Population and Longitudinal Population
Association of normal SBP with prevalence rate of NAFLD
As shown in Table 1, subjects with NAFLD have a higher BP than those without NAFLD. The prevalence of NAFLD was significantly higher in the subjects with higher SBP than in those with lower SBP. In general, the prevalence of NAFLD in the cross-sectional population from G1 to G4 was 6.1%, 13.6%, 19.6%, and 25.8%, respectively. MS is a well-established risk factor for NAFLD and a subgroup analysis stratified by MS and its components is shown in Figure 2. A higher prevalence of NAFLD was observed in subjects with MS or its abnormal components, than those with normal conditions. Prevalence in most subgroups presented a linear trend increasing from G1 to G4, however numerical but not statistically significant effects were observed in subjects with elevated hypertriglyceride and MS.
FIGURE 2.
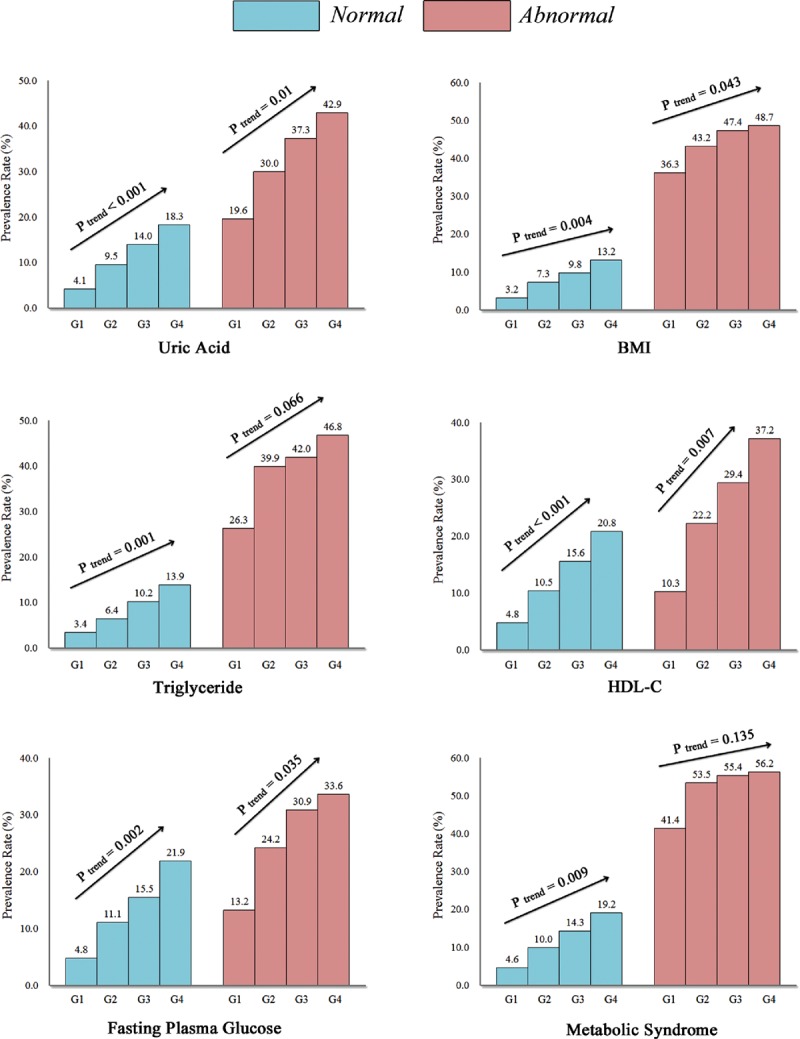
Prevalence rate of nonalcoholic fatty liver disease (NAFLD) in the cross-sectional population with different groups of normal systolic blood pressure. The prevalence rate of NAFLD in the subgroups analysis of serum uric acid, metabolic syndrome and its components including central obesity (BMI ≥ 25 kg/m2), hypertriglyceridemia (TG ≥ 1.7 mmol/L), low high-density lipoprotein cholesterol (HDL-C < 1.03/1.3 mmol/L), and elevated fasting plasma glucose (FPG ≥ 5.6 mmol/L) all showed increasing trends with the increases in normal systolic blood pressure levels. G1: 90–99 mmHg, G2: 100–109 mmHg, G3: 110–119 mmHg, and G4: 120–129 mmHg.
Higher Normal SBP Increases the Incidence Risk of NAFLD
To verify whether an increased level of SBP within the normal range may play a causal role in the development of NAFLD, a longitudinal population was included. Among the 10,915 subjects with complete follow-up data, 967 had developed into NAFLD. There was a positive association of normal SBP level with NAFLD in unadjusted model. Compared with subjects in the G1, those subjects in the G4 had a hazard Ratio (HR) of 5.81 (95% CI 4.32–7.81). It was diminished after adjustment for sex, age, BMI (HR 2.51, 95% CI 1.86–3.40), and was further attenuated with adjustment for the other confounding variables (HR 2.38, 95% CI 1.75–3.23) (Table 2). A stratified analysis for risk factors of MS showed a successive increase in HRs from G1 to G4, as shown in Figure 3. The strongest link between increasing levels of SBP and the incidence of NAFLD was observed in subjects with hypertriglyceride (HRG4 vs. G1 was 3.60, 95% CI 1.99–6.53). The weakest link was presented in subjects with BMI ≥ 25 kg/m2 (HRG4 vs. G1 was 1.44, 95% CI 0.80–2.59). Figure 4 shows the cumulative HR of NAFLD in groups of SBP. Eliminating all the exclusion criteria and including all the subjects in the longitudinal population, with unadjustment or adjustment, also did not alter the findings (unadjusted HRG4 vs. G1 6.03, 95% CI 4.51–8.06; adjusted HR G4 vs. G1 2.45, 95% CI 1.81–3.3).
TABLE 2.
Hazard Ratio (95% Confidence Interval) for Nonalcoholic Fatty Liver Disease in Longitudinal Population
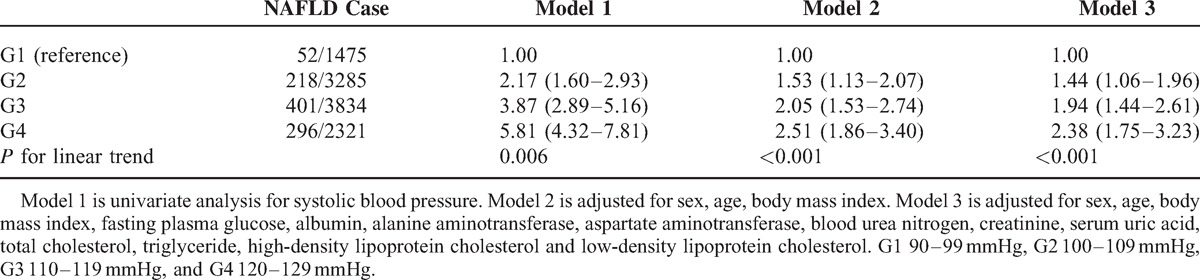
FIGURE 3.
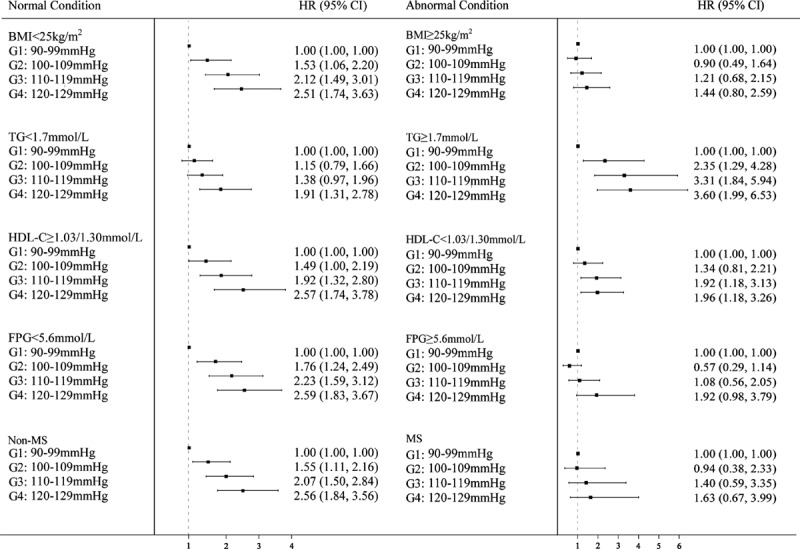
Forest plots of hazard rates (95% confidence interval) for different groups of normal systolic blood pressure in the longitudinal population. The HRs in the subgroups analysis were adjusted for sex, age, body mass index, fasting plasma glucose, albumin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine, serum uric acid, total cholesterol, triglyceride, high-density lipoprotein cholesterol and low-density lipoprotein cholesterol. It showed increasing trends of HRs for NAFLD with the increases in normal systolic blood pressure levels.
FIGURE 4.
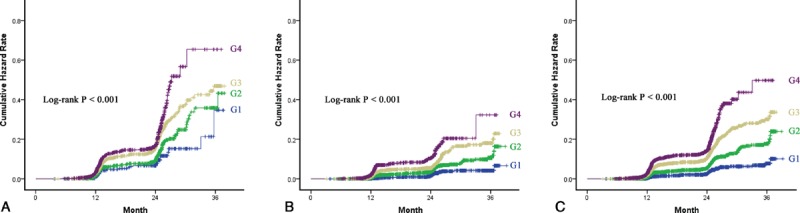
Incidence of nonalcoholic fatty liver disease (NAFLD) in the longitudinal population. (A) Incidence of NAFLD in 4626 men stratified by different groups of SBP. (B) Incidence of NAFLD of 6289 women stratified by different groups of SBP. (C) Incidence of NAFLD of total 10,915 participators stratified by different groups of SBP. G1: 90–99 mmHg, G2: 100–109 mmHg, G3: 110–119 mmHg and G4: 120–129 mmHg.
DISCUSSION
High BP was the leading risk factor for the overall global burden of disease.21 Prospective observational studies also have demonstrated that subjects have a greater incidence of CVD with an increase in BP <140/90 mmHg, even with normal BP.11,22 The Framingham Heart Study found that, among 6859 participants with initially free of CVDs, high-normal BP (<139/90 mmHg) conferred a 1.6- to 2.5-fold risk of CVDs event, compared with optimal BP (<120/80 mmHg).13 Furthermore, compared with optimal BP, a prospective cohort analysis including 8960 middle-aged adults in the Atherosclerosis Risk in Communities (ARIC) study demonstrated that the relative risk (RR) of CVD for normal BP (<130/85 mmHg) was significantly higher, with an RR of 1.81 (95% CI 1.47–2.22).23 Likewise, higher BP even within normal range was verified to be associated with a higher risk of other metabolic disorders, which include diabetes, osteoporosis and hyperuricemia.24–27 These studies may suggest that the “normal BP” may be not the real normal as we have previously considered, especially when targeted to special diseases, such as NAFLD.22,23
A number of studies have demonstrated that subjects with high-normal BP or hypertension have an increased risk of NAFLD compared with subjects with optimal BP.14–17 No data is available concerning the association between normal BP and NAFLD. One population-based study showed that early stages of prehypertension, defined as SBP 120–129 mmHg or DBP 80–84 mmHg, was independently associated with an elevated risk of NAFLD, with an adjusted OR of 1.3 (95% CI 1.1–1.6).28 However, this study focused solely on patients with BP ≥ 120/80 mmHg and was based on a cross-sectional population and the interrelation between BP < 120/80 mmHg and risk of NAFLD remained unknown. To our knowledge, this is the first and largest study specifically aimed at evaluating the association between normal BP level (<130/85 mmHg) and NAFLD risk in a nationally representative sample. Subjects were classified into 4 groups by their SBP level. We observed a significant association between SBP level and prevalence of NAFLD in the cross-sectional population, with an increasing prevalence rate from the G1 to G4. Furthermore, a prospective longitudinal population was performed to verify that the elevation of SBP level within the normal range appears to make a significant contribution to an increased risk of developing NAFLD, with an adjusted HRG4 vs. G1 of 2.38 (95% CI 1.75–3.23). When SBP was analyzed as a continuous variable, the positive association with NAFLD also persisted.
In the subgroup analysis, when subjects were divided into 2 groups of BMI <25 and BMI ≥ 25 kg/m2, we could found further interesting results. Subjects with BMI < 25 kg/m2 showed a statistically significant HR for the incidence risk of NAFLD, while subjects with BMI ≥ 25 kg/m2 did not have a significant HR. This finding may indicate that an increase level in normal SBP may not bring a significant risk of NAFLD in subjects with overweight, consistent with previous data.29 Actually, BMI was strongly related with BP. A cohort of more than 700,000 subjects showed a positive near-linear association between BMI and BP with no threshold effect.30 As well, the prevalence and incidence of NAFLD has proved increasing worldwide as the growing epidemic of obesity across the globe.31 Thus, it may be a difficult task to determine the relationship between clinically irrelevant BP and risk of NAFLD, for overweight or other confounding factors could play a vital role in NAFLD and BP. We applied multiple regression models to determine the association between SBP and the incidence of NAFLD, adjusting for BMI and other confounding variables. Result in this study showed that elevated risks of NAFLD brought by the increased levels of normal SBP was not statistically significant in the overweight (BMI ≥ 25 kg/m2). Further studies about overweight, normal range BP, risk of NAFLD should be needed.
Increased BP and NAFLD share some common pathophysiologic mechanisms such as sympathetic nervous system activity, IR, vascular and adipose tissue inflammation, which can produce vascular endothelial dysfunction.30 IR is the most common abnormality linked to the pathogenesis of both NAFLD and hypertension.32 IR can enhance salt absorption, induce lipid peroxidation, and activate the secretion of proinflammatory cytokine such as tumor necrosis factor-α and interleukin-6.30,33,34 These conditions can decrease the vascular elasticity and luminal width to increase BP and cause liver endothelial dysfunction to promote the progression of innocent steatosis to nonalcoholic steatohepatitis and liver fibrosis. Adiponectin has proved to be the only hormone, which is positively associated with insulin sensitization, glucose use, and cardiovascular protection.35 The association between adiponectin and increased BP is also evident in studies by showing that overexpression of adiponectin can decrease the SBP and hypoadiponectinemia is a risk factor for hypertension independent of IR and diabetes.36,37 Also, there is evidence that adiponectin decreases hepatic and systematic IR, and attenuates liver inflammation and fibrosis.38 Thus, therapeutic strategies focused on the indirect upregulation of adiponectin through the administration of various therapeutic agents and/ or lifestyle modifications will be popular and hopeful, even if detailed molecular and cellular mechanisms remain largely uncharacterized.
Subjects were grouped according to SBP levels in this study. It is intrinsically more difficult to normalize patients with elevated SBP than DBP,20 possibly because of the difficulty of reversing pathophysiological abnormalities responsible for elevation of SBP. Thus, elevated SBP has major prevalence and may play a greater impact on BP-related diseases in the general population.20,39 A prior study has demonstrated that among nonhypertensive subjects, high-normal SBP but not high-normal DBP was independently associated with NAFLD.17 As well, no significant risk for NAFLD was also observed with increases in normal DBP (per 5 mmHg increase) after adjusting of confounding factors in this study (data not shown).
Our study may have some limitations and merit comment. The main limitation is the lack of anthropometric parameters about central obesity (ie, waist circumference or/and hip circumference), lifestyle and dietary factors, which may be helpful to better understand the relationship between NAFLD and increased BP. Further studies including more complete personal information were needed. Secondly, due to the observational nature of this study, it is less evident to definite the relationship between normal range BP and the risks of NAFLD, when compared with intervention ones. Thus, studies with intervention should be needed in the further. Finally, a dynamic and continuous detection of BP levels in different stages of NAFLD should be considered, since it is more important and meaningful than a single-point measurement.
In summary, we conclude that increased levels of SBP within the normal range are independently associated with an elevated risk of NAFLD. We propose that the term “normal BP” may be not the real normal for patients with potential risk of NAFLD, thus BP evaluation and control should be an integral component of clinical management of the general population, even in subjects with nonhypertension.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BP = blood pressure, BMI = body mass index, BUN = blood urea nitrogen, CIs = confidence intervals, Cr = creatinine, DBP = diastolic blood pressure, FPG = fasting plasma glucose, HDL-C = high-density lipoprotein cholesterol, HR = hazard ratio, IR = insulin resistance, LDL-C = low-density lipoprotein cholesterol, NAFLD = nonalcoholic fatty liver disease, MS = metabolic syndrome, UA = uric acid, SBP = systolic blood pressure, TC = total cholesterol, TG = triglyceride.
S-JW and HZ are co-first author of the article.
Authors’ contributions: S-JW, data collection and analysis, drafted the manuscript; HZ, data collection and drafted the manuscript; G-QZ, data collection; L-RW, study design and analysis; QZ, data collection; K-QS, participated in its design and helped to draft the manuscript; J-BH, helped to draft the manuscript; W-JH, data analysis and helped to draft the manuscript; MB, helped to draft the manuscript; Y-PC, conceived the study, data collection; M-HZ, conceived the study, participated in its design, study supervision, obtained funding and helped to draft the manuscript. All authors approved the final version of the article.
Funds: This work was supported by grants from the Scientific Research Foundation of Wenzhou, Zhejiang Province, China (H20090014, Y20090269), Health Bureau of Zhejiang Province (2010KYB070), Research Foundation of Education Bureau of Zhejiang Province (Y201009942), Fresh Talent Program for Science and Technology Wenzhou Medical University (wyx201401011, wyx201401087, wyx201401009 and wyx201401085), Research Funds for Tian Qing Liver Diseases (TQGB20120057) and Project of New Century 551 Talent Nurturing in Wenzhou.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Day CP, Saksena S. Non-alcoholic steatohepatitis: definitions and pathogenesis. J Gastroenterol Hepatol 2002; 17 (Suppl 3):S377–384. [DOI] [PubMed] [Google Scholar]
- 2.Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010; 18 163–166). J Dig Dis 2011; 12:38–44. [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005; 143:722–728. [DOI] [PubMed] [Google Scholar]
- 4.Amarapurkar DN, Hashimoto E, Lesmana LA, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol 2007; 22:788–793. [DOI] [PubMed] [Google Scholar]
- 5.Fung J, Lee CK, Chan M, et al. High prevalence of non-alcoholic fatty liver disease in the Chinese - results from the Hong Kong liver health census. Liver Int 2015; 35:542–549. [DOI] [PubMed] [Google Scholar]
- 6.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabetic Med 2007; 24:1–6. [DOI] [PubMed] [Google Scholar]
- 7.Scorletti E, Calder PC, Byrne CD. Non-alcoholic fatty liver disease and cardiovascular risk: metabolic aspects and novel treatments. Endocrine 2011; 40:332–343. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care 2007; 30:2119–2121. [DOI] [PubMed] [Google Scholar]
- 9.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008; 49:600–607. [DOI] [PubMed] [Google Scholar]
- 10.Loria P, Lonardo A, Bellentani S, et al. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis 2007; 17:684–698. [DOI] [PubMed] [Google Scholar]
- 11.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014; 383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrannini E, Buzzigoli G, Bonadonna R, et al. Insulin resistance in essential hypertension. N Engl J Med 1987; 317:350–357. [DOI] [PubMed] [Google Scholar]
- 13.Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001; 345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 14.Donati G, Stagni B, Piscaglia F, et al. Increased prevalence of fatty liver in arterial hypertensive patients with normal liver enzymes: role of insulin resistance. Gut 2004; 53:1020–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasunta RL, Kesaniemi YA, Ylitalo AS, et al. High ambulatory blood pressure values associated with non-alcoholic fatty liver in middle-aged adults. J Hypertens 2012; 30:2015–2019. [DOI] [PubMed] [Google Scholar]
- 16.Latea L, Negrea S, Bolboaca S. Primary non-alcoholic fatty liver disease in hypertensive patients. Australas Med J 2013; 6:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Suarez A, Guerrero JM, Elvira-Gonzalez J, et al. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur J Gastroenterol Hepatol 2011; 23:1011–1017. [DOI] [PubMed] [Google Scholar]
- 18.Farrell GC, Chitturi S, Lau GK, et al. Asia-Pacific Working Party on N. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007; 22:775–777. [DOI] [PubMed] [Google Scholar]
- 19.Fan JG, Saibara T, Chitturi S, et al. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol 2007; 22:794–800. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Seravalle G, Grassi G. Systolic blood pressure: an underestimated cardiovascular risk factor. J Hypertens (suppl) 2002; 20:S21–S27. [PubMed] [Google Scholar]
- 21.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panza JA. High-normal blood pressure—more “high” than “normal”. N Engl J Med 2001; 345:1337–1340. [DOI] [PubMed] [Google Scholar]
- 23.Kshirsagar AV, Carpenter M, Bang H, et al. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med 2006; 119:133–141. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Tsumura K, Suematsu C, et al. High normal blood pressure, hypertension, and the risk of type 2 diabetes in Japanese men. The Osaka Health Survey. Diabetes Care 1999; 22:1683–1687. [DOI] [PubMed] [Google Scholar]
- 25.Lee HT, Shin J, Min SY, et al. The relationship between bone mineral density and blood pressure in the Korean elderly population: the Korea National Health and Nutrition Examination Survey. Clin Exp Hypertens 2008–2011; 2014:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Egan BM, Papademetriou V, Wofford M, et al. Metabolic syndrome and insulin resistance in the TROPHY sub-study: contrasting views in patients with high-normal blood pressure. Am J Hypertens 2005; 18:3–12. [DOI] [PubMed] [Google Scholar]
- 27.Leite ML. Uric acid and fibrinogen: age-modulated relationships with blood pressure components. J Hum Hypertens 2011; 25:476–483. [DOI] [PubMed] [Google Scholar]
- 28.Aneni EC, Oni ET, Martin SS, et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J Hypertens 2015; doi:10.1097/HJH.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 29.Ryoo JH, Ham WT, Choi JM, et al. Clinical significance of non-alcoholic fatty liver disease as a risk factor for prehypertension. J Korean Med Sci 2014; 29:973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chorin E, Hassidim A, Hartal M, et al. Trends in adolescents obesity and the association between BMI and blood pressure: a cross-sectional study in 714 922 healthy teenagers. Am J Hypertens 2015; doi:10.1093/ajh/hpv007. [DOI] [PubMed] [Google Scholar]
- 31.Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev 2007; 65:S57–S63. [DOI] [PubMed] [Google Scholar]
- 32.Brookes MJ, Cooper BT. Hypertension and fatty liver: guilty by association? J Hum Hypertens 2007; 21:264–270. [DOI] [PubMed] [Google Scholar]
- 33.Tarantino G, Conca P, Pasanisi F, et al. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol 2009; 21:504–511. [DOI] [PubMed] [Google Scholar]
- 34.Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int 2015; 87:497–499. [DOI] [PubMed] [Google Scholar]
- 35.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension 2008; 51:8–14. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi K, Kihara S, Ouchi N, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 2006; 47:1108–1116. [DOI] [PubMed] [Google Scholar]
- 37.Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension 2004; 43:1318–1323. [DOI] [PubMed] [Google Scholar]
- 38.Finelli C, Tarantino G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J Gastroenterol 2013; 19:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lloyd-Jones DM, Evans JC, Larson MG, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension 2000; 36:594–599. [DOI] [PubMed] [Google Scholar]


