Supplemental Digital Content is available in the text
Abstract
Previous studies have revealed antibody to hepatitis B core antigen (anti-HBc) levels as a predictor of treatment response in hepatitis B early antigen (HBeAg)-positive chronic hepatitis B (CHB) patients in both interferon and nucleos(t)ide analog therapy cohorts. However, there is no information about anti-HBc levels in the natural history of CHB.
This study aimed to define anti-HBc levels of different phases in the natural history of CHB.
Two hundred eleven treatment-naive CHB patients were included in the study. They were classified into 4 phases: immune tolerance (IT) phase (n = 39), immune clearance (IC) phase (n = 48), low or no-replicative (LR) phase (n = 55), and HBeAg-negative hepatitis (ENH, n = 69). Fifty patients who were HBsAg negative and anti-HBc positive were also recruited as past HBV infection (PBI) control group. Anti-HBc levels were measured by a newly developed double-sandwich immunoassay. Correlation of anti-HBc levels with alanine aminotransferase (ALT) and other HBV-related markers within each phase was performed.
Serum anti-HBc levels were statistically significant between patients in different phases of CHB (P < 0.001). The median anti-HBc levels were: IT (3.17 log10 IU/mL), IC (4.39 log10 IU/mL), LR (3.29 log10 IU/mL), ENH (4.12 log10 IU/mL), and PBI (0.61 log10 IU/mL). There existed a strong correlation in IC (r = 0.489, P < 0.001), a poor correlation in ENH (r = 0.275, P = 0.042), and no correlation in patients with ALT reached 5 times upper limit of normal (r = 0.120, P = 0.616).
Anti-HBc levels show significant differences during the natural course of CHB. These results may provide some potentially useful insights into hepatitis B pathogenesis and immune activation against hepatitis B virus.
INTRODUCTION
Approximately 2 billion people in the world have been infected with hepatitis B virus (HBV) and 350 to 400 million of them are chronic HBV carriers. The spectrum of this disease and natural history of chronic HBV infection are diverse and variable, ranging from an inactive carrier state to progressive chronic hepatitis B (CHB), which may evolve to cirrhosis and hepatocellular carcinoma (HCC).1,2 Chronic HBV infection is a dynamic process. The natural history of CHB can be schematically divided into 4 phases, which are not necessarily sequential. They are immune tolerance (IT) phase, immune clearance (IC) phase, low or no-replicative (LR) phase, and hepatitis B early antigen (HBeAg) (−) hepatitis (ENH).2 These phases have been classified by specific biochemical, HBV-related serological and virological characteristics, including serum alanine aminotransferase (ALT) and spartate aminotransferase (AST) levels, hepatitis B surface antigen (HBsAg) levels, HBeAg serostatus, and HBV–DNA titer.3,4 Till now, treatment for CHB mainly focuses on patients of immune activation against HBV, including IC and ENH phases, while IT and LR phases need long time of follow-up.5 The 4 phases show dynamic changes of immunological status of CHB,6 but specific immunological biomarkers to reflect immune activation against HBV in CHB patients are still unknown.
Antibody to hepatitis B core antigen (anti-HBc) is one of the most classical serological markers in HBV infection. Unlike antibody to HBsAg, it is not a protective marker that appears by itself and cannot be used to differentiate acute infections from chronic ones.7 Only anti-HBc positive can be used to assess the risk of HBV reactivation in patients undergoing immunosuppression or patients who are human immunodeficiency virus (HIV)8 posi tive or hepatitis C virus (HCV)9 positive. Kobyashi et al10 diluted acute hepatitis B (AHB) patients’ serum sample 200 times and found that progressive and sufficient decrease of anti-HBc could predict the disappearance of HBV–DNA with HBsAg clearance in AHB patients, which revealed a possible role of anti-HBc titer in reflecting the status of the disease. But quantitative assays measuring anti-HBc levels is difficult to achieve because of the lack of appropriate standardization. A novel diagnostic immunoassay testing procedure for anti-HBc using homogeneous purified full-length hepatitis B core antigen (HBcAg) capsids obtained from Escherichia coli could quantify the levels of anti-HBc in serum.11 According to the new method, Yuan et al12 found that among CHB patients, the anti-HBc levels in those who had elevated ALT levels were significantly higher than patients with normal levels of ALT. However, further researches proved that baseline of anti-HBc was an independent biomarker for predicting HBeAg seroconversion in CHB patients with therapy of interferon or nucleos(t)ide analogs, regardless of ALT levels. Our pilot study also found that levels of anti-HBc and ALT showed a linear correlation, and baseline levels of anti-HBc could predicate the response to interferon therapy (unpublished data). All results revealed that anti-HBc might be a surrogate parameter in indicating an immune activation against HBV.
Despite the quantitative monitoring of anti-HBc has already been used for predicating therapeutic response in HBeAg (+) hepatitis patients, there are no studies investigating the clinical significance of anti-HBc in treatment-naive patients. We hypothesize that anti-HBc levels mirror the immune-activation status of chronic HBV infection. Hence, the aim of this study is to evaluate anti-HBc levels in different phases of natural history with CHB who never received antiviral therapy; and the association between anti-HBc and ALT, AST, HBV–DNA, and other biochemical and virological markers in patients with chronic HBV infection.
METHODS
Patient Population
Two hundred eleven patients who were persistent of HBV infection and never received antiviral therapy were included in the study. Patients were recruited from the Sixth Hospital of Shenyang, Liaoning, China. Eighty-seven patients were HBeAg positive and others were HBeAg negative. There were 122 males and 89 females with a median age of 38 years (minimum 18, maximum 65 years). Fifty patients who were HBsAg negative and anti-HBc positive were also recruited as past HBV infection (PBI) control group. Patients combined with other viral hepatitis, autoimmune or metabolic liver disease, hepatic dysfunction, or malignancies were excluded as well as ones who received immunosuppressive therapy. Children or adolescents with CHB were also excluded in this study.
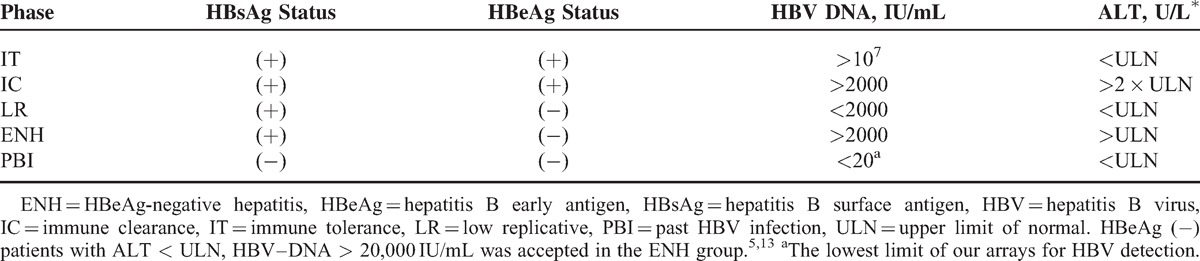
Patients included were classified into different phases of CHB according to Table 1. The criteria were based on the European Association for the Study of the Liver clinical practice guidelines.5 The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the local institutional ethics research committee.
TABLE 1.
Definitions of the Different Phases of Chronic HBV Infection and Past HBV Infection
Serum HBsAg Quantification
Serum HBsAg levels were quantified using the Roche Elecsys HBsAg II assay (Roche Diagnostics, Penzberg, Germany). Quantitative HBsAg levels were tested with a dilution of 1:400 and reported in IU/mL, with a dynamic range of 20 to 52,000 IU/mL. If HBsAg levels >52,000 IU/mL, samples were retested with a stepwise dilution of 1:4000. Samples with HBsAg levels <20 IU/mL were retested without prior dilution.
HBV–DNA Measurement
Serum HBV–DNA was measured according to the manufacturer's instructions (dynamic range 2.0 × 101–1.7 × 108 IU/mL) of COBAS AmpliPrep/COBAS TaqMan, Roche Diagnostics, Basel, Switzerland. Samples with a viral load above the upper limit of the dynamic range were retested at a dilution of 1:1000 to obtain a defined level.
Anti-HBc Measurement
The serum anti-HBc levels were measured by a newly developed double-sandwich immunoassay (Wantai, Beijing, China) that was calibrated using immunoassay.11
Statistical Analysis
Continuous and categorical variables were compared between the groups, using the Mann–Whitney test and Kruskall–Wallis analysis of variance for nonparametric continuous data, and χ2/Fisher exact test for categorical data. Correlation of anti-HBc with ALT, HBsAg, and other parameters was carried out using the method of Pearson and Spearman. The significance level was fixed at 0.05. Statistical analyses were performed by SPSS ver. 16.0 (SPSS, Chicago, IL).
RESULTS
Two hundred eleven treatment-naive HBsAg-positive patients and 50 anti-HBc-positive patients as a control group were included in the study. The distributions of patients were: IT (n = 38), IC (n = 49), LR (n = 69), ENH (n = 55), and PBI (n = 50).
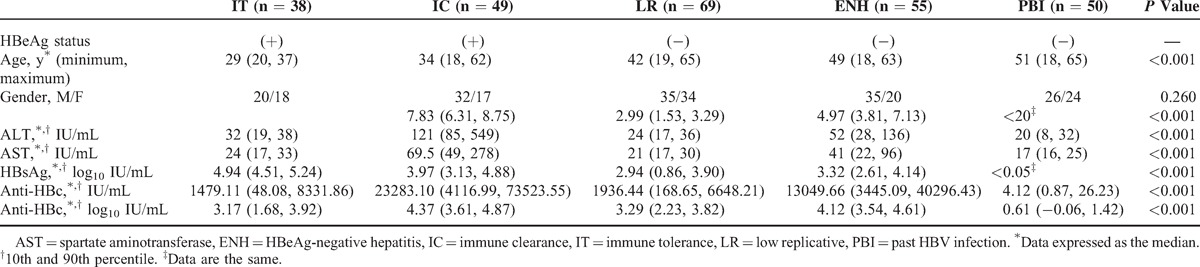
The baseline characteristics are presented in Table 2. In this study, there were more males (57.82%) than females. HBeAg-positive patients were younger than HBeAg-negative patients (P < 0.001). In HBeAg-positive patients, there were no significant differences between IT and IC groups referring to ages, with a similar situation in HBeAg-negative patients.
TABLE 2.
Characteristics of Patients With Chronic HBV Infection (n = 211) and Past HBV Infection (n = 50)
Population Distribution of Anti-HBc Levels
Serum anti-HBc levels were statistically significant between patients in different phases of CHB (P < 0.001). The median anti-HBc levels in each phase of CHB were: IT (3.17 log10 IU/mL), IC (4.37 log10 IU/mL), LR (3.29 log10 IU/mL), ENH (4.12 log10 IU/mL), and PBI (0.61 log10 IU/mL), respectively. The median anti-HBc in IC and ENH were significant higher than IT and LR (P < 0.001) (Table 2 and Figure 1).
FIGURE 1.

Distribution of serum anti-HBc levels in the natural history of CHB. Median values with 95% confidence interval (of median) represented. CHB = chronic hepatitis B, ENH = HBeAg-negative hepatitis, HBc = hepatitis B core antigen, HBeAg = hepatitis B early antigen, IC = immune clearance phase, IT = immune tolerance, LR = low replicative, PBI = past HBV infection.
Correlation Between Serum Anti-HBc and ALT
The correlation between serum anti-HBc and ALT levels is shown in Figure 2 and Table 3. There was a significantly statistical correlation between anti-HBc and serum ALT levels in entire CHB patients (r = 0.568, P < 0.001). The strongest correlation was observed in IC group (r = 0.489, P < 0.001) and a poor correlation in ENH group (r = 0.275, P = 0.042), as well as no correlation in IT (r = −0.031, P = 0.855) and LR group (r = −0.196, P = 0.106). When ALT reached 5 times the upper limit of normal (5 × ULN), there was no correlation in those CHB patients (r = 0.120, P = 0.616).
FIGURE 2.
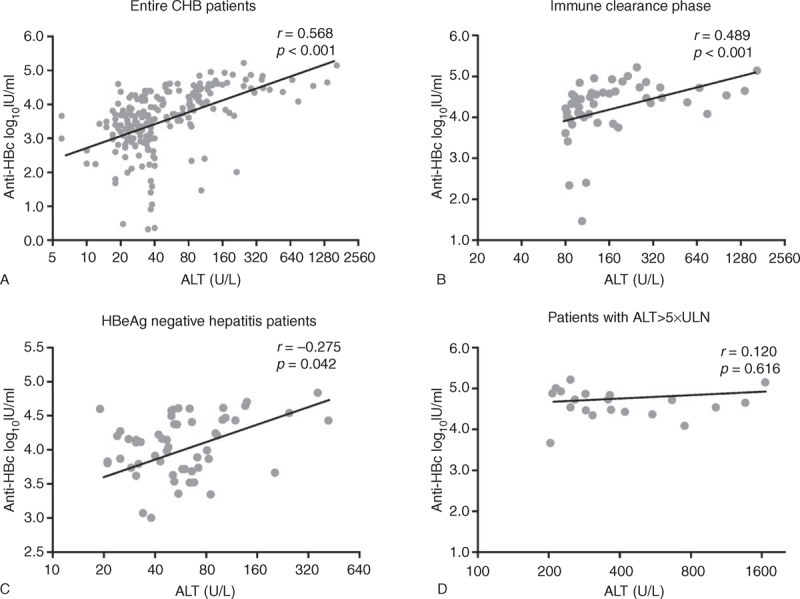
(A–D) Correlation of serum anti-HBc levels and ALT in patients with CHB. CHB = chronic hepatitis B, HBc = hepatitis B core antigen, ULN = upper limit of normal.
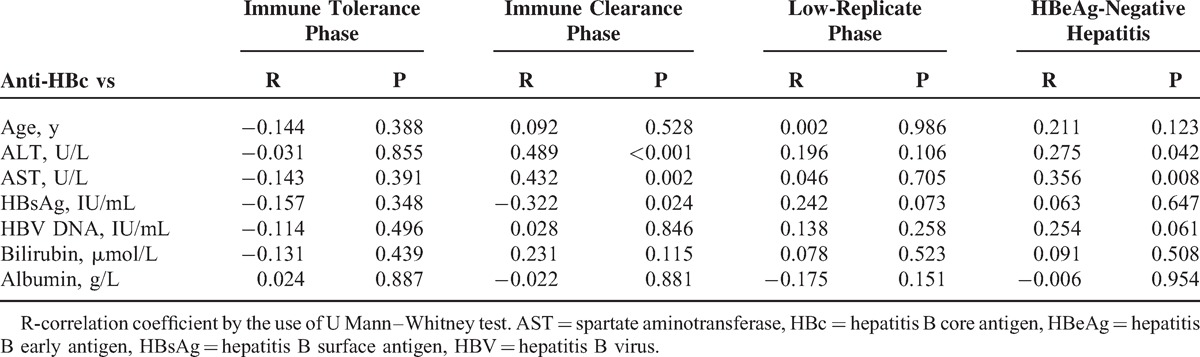
TABLE 3.
Correlation Between Anti-HBc Levels and Clinical as Well as Laboratory Parameters in Different Phases of Chronic HBV Infection
Correlation of Serum Anti-HBc With HBsAg and Other Clinical Parameter
There was no significant statistical correlation of anti-HBc with HBV–DNA or with age, gender, serum bilirubin, and albumin levels in each phase of CHB (P > 0.05, respectively). There was a poor correlation observed in IC (r = −0.322, P = 0.024) between serum anti-HBc and HBsAg. Interestingly, anti-HBc levels were positively associated with spartate aminotransferase in the IC (r = 0.432, P = 0.002) and ENH (r = 0.356, P = 0.008), only in HBV phases with active inflammatory activity (Table 3).
Anti-HBc Levels to Distinguish Different Phases of CHB Patients
In HBeAg-positive patients, anti-HBc had 0.883 area under receiver-operating characteristics (AUROC) curve for IC. The cutoff value of 3.81 log10 IU/mL for IC had a sensitivity of 86.5%, specificity of 87.5%, positive predictive values (PPVs) of 90%, and negative predictive values (NPVs) of 83.3%. In HBeAg-negative patients, anti-HBc had 0.889 AUROC for ENH. The cutoff value of 3.68 log10 IU/mL for ENH had a sensitivity of 81.8%, specificity of 84.1%, PPV of 80.4%, and NPV of 85.3% (Figure 3; Supplement Table 1, http://links.lww.com/MD/A118).
FIGURE 3.
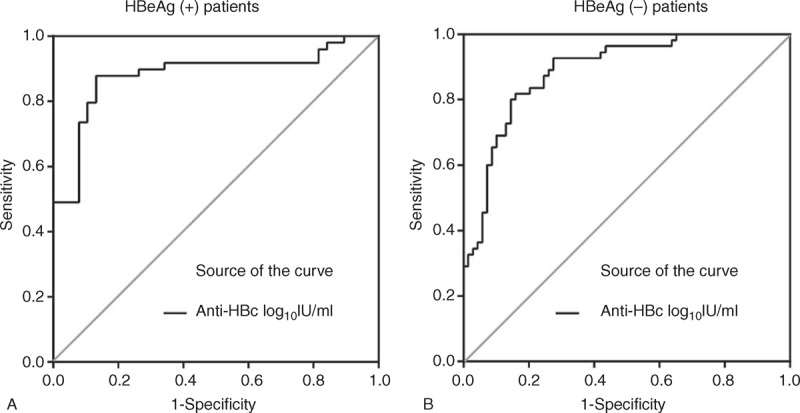
Receiver-operating characteristics curve of anti-HBc levels to distinguish (A) immune clearance from immune-tolerant patients, and (B) HBeAg-negative hepatitis from low-replicate-phase patients. HBc = hepatitis B core antigen, HBeAg = hepatitis B early antigen.
DISCUSSION
The natural history of chronic HBV infection in individuals is complicated. Patients can move from a state of high viral load and mild or no liver necroinflammation to immune-reactive HBeAg-positive phase, followed by inactive disease, and then revert back to active liver disease years later.2,7 The understanding of the pathogenesis and natural history of CHB has been facilitated by the high sensitivity of HBV–DNA viral load assays, and the development of quantitative assays for the detection of HBsAg. HBV–DNA and HBsAg titer has already been used to distinguish different phases of CHB and predicate antiviral treatment response.14–16 Our preliminary study also found that anti-HBc levels at baseline could predicate the antiviral treatment response of interferon therapy in HBeAg-positive CHB patients, and anti-HBc titer showed positive correlation with ALT levels (unpublished data). Hence, an understanding of anti-HBc levels changes throughout chronic HBV infection may provide some potentially useful insights into hepatitis B pathogenesis and immune-activation status. Therefore, it is important to understand the natural history of CHB, which can guide clinicians to decide on the need and optimal timing for initiating antiviral therapy. This is the first article to describe the anti-HBc levels in the natural history of CHB patients, aiming to investigate the baseline of anti-HBc in different phases of CHB. All patients included were treatment naive. The results showed anti-HBc levels were different in the 4 phases. The anti-HBc levels in IC and ENH phases were significantly higher than those in the IT and LR phases, but no significant differences were found between IC and ENH or IT and LR.
In IT phase, HBeAg-positive patients are with no or minor hepatitis activity as well as normal serum ALT concentrations, and this phase can last for a few years to >30 years. During this phase, there is either no or minimal liver disease progression and no immune activation against HBV.2,17 Our study also showed low levels of anti-HBc in IT phase, which may suggest a possible role of low levels of anti-HBc in distinguishing a relative true IT phase. The transition from IT to IC phase characterize a strong cellular immune response, intermittent acute ALT increase, moderate or severe liver necroinflammation, and more rapid progression of fibrosis compared to the previous phase.7 In IC phase, the immune system of the host recognizes HBV as being foreign and results in immune activation of immune system of the host against HBV.18 Our study also showed that anti-HBc levels in IC phase were much higher than those in IT phase, which might reveal an immune-activation status of HBV-infected host. Prior studies have showed that ALT levels could be a pretreatment predictor of anti-HBe seroconversion for interferon and nucleos(t)ide-based treatment in HBeAg-positive CHB patients. Patients with high levels of ALT are usually prone to anti-HBe seroconversion.19,20 Our results also showed that anti-HBc titer were strong positive correlated with ALT levels in IC phase. But when ALT > 5 × ULN, no more possibility of anti-HBe seroconversion was achieved,19,20 while anti-HBc titer showed no more correlation with ALT levels when ALT > 5 × ULN, and it suggested that anti-HBc titer might be related with ongoing immune response of the host against HBV. Pilot studies of small samples revealed that anti-HBc of baseline as a therapy predicting factor was superior to ALT, HBV–DNA and HBsAg.12 Our further study of 119 HBeAg-positive CHB patients showed that high levels of anti-HBc at baseline alone could predicate the anti-HBe seroconversion for interferon treatment (unpublished data). The above results perhaps revealed the possible role of anti-HBc titer in reflecting the immune activation of the host against HBV.7 In the LR phase, infected patients are characterized by very low or undetectable serum HBV–DNA levels and normal serum aminotransferases. The control of HBV replication in the liver does not cause hepatic necroinflammation and immune activation.7 The inactive HBV carrier state confers a favorable long-term outcome with a very low risk of cirrhosis or HCC in the majority of patients.21 The relative low levels of anti-HBc in our study also showed no immune activation in LR phase, which may be a useful supplement to facilitate the acknowledgment of the immune control phase. The HBeAg-negative hepatitis phase represents a later immune-reactive phase in the natural history of chronic HBV infection, which also has immune activation of immune system of the host against HBV. It is characterized by periodic reactivation with a pattern of fluctuating levels of HBV–DNA and aminotransferases and active hepatitis. Our study also showed high levels of anti-HBc in serum of ENH patients, which implied us that high levels of anti-HBc might remind the immune-activation status of host's immune system against HBV. IT and LR are not immune-activation phases with no or mild liver necroinflammation and slow disease progression, while IC and ENH are both immune-activation phases of CHB, characteristic in moderate or severe liver necroinflammation and rapid progression of fibrosis.2,22 The dynamic changes of anti-HBc levels in different phases are in accordance with the immune-activation status of chronic HBV infection. Further analysis using receiver-operating characteristics curve showed that anti-HBc levels with HBeAg status were helpful to differentiate each phase of CHB. Results above revealed a potential role of anti-HBc levels in reflecting different immune-activation status of chronic HBV infection.
Quantitative HBsAg has been used in the management of CHB patients in the past years.14–16 Prior studies have showed that HBsAg level is highest in the IT phase, starts to decline during the IC phase, decreases slowly in HBeAg-negative hepatitis, and lowest in inactive carriers.3,4 Our study showed similar results. The HBsAg levels change dynamically in different phases, which facilitates itself to be used to distinguish CHB patients into different phases.23 The anti-HBc levels also change dynamically in the 4 phases, which is different from HBsAg. It suggested us that anti-HBc could be an effective supplement of HBsAg in further defining different phases of CHB patients.
Anti-HBc is regularly produced by virtually 100% of HBV-infected patients. Although low levels of anti-HBc can be produced in the absence of T helper (Th) cell support, its production is greatly enhanced by HBcAg-specific Th cell activation.2,24 Other studies also revealed that high levels of anti-HBc are related to the high serum IL-2R levels, which is mainly released from sensitization of T lymphocytes.25 All of the above showed the potential correlation of anti-HBc levels with the immune response of the host against HBV. The future work is to investigate correlation of cytokine and T cells with anti-HBc levels.
Our research still has certain limitations that are presented further. First, we did not include occult HBV infection patients. According to some studies, anti-HBc was considered to be relevant with occult HBV infection.26 However, occult HBV infection is rare in the clinical work, and it is difficult to get serum sample of occult HBV infection. So in the study, we did not include these patients. It will be a future work for us. Second, it is a cross-sectional study. As an observation study, a longitudinal study is more powerful. However, such longitudinal follow-up is difficult, because many patients started antiviral treatment later. We did a pilot follow-up study before based on a small sample size. Results showed that the anti-HBc levels were independently associated with the ALT levels, whereas there was no independent association with the HBV–DNA levels or the HBsAg levels (unpublished data). Third, we did not test HBV genotype or HBeAg-suppressing mutants (precore and base core promoter mutation), because a pilot study showed that there was no relationship between anti-HBc levels and genotype, as well as precore and base core promoter mutation (unpublished data). Finally, the definition of different phases of CHB does not include all the situations of CHB mainly because some situations are difficult to confirm which group should be in. In our study, no liver biopsy was done to these patients, so we cannot decide the phase of these patients. In order to make sure the accuracy of included patients to appropriate phase, we excluded these patients.
In conclusion, our study defines that the levels of anti-HBc vary in different phases of treatment-naive CHB patients. There is a close relationship between anti-HBc and ALT, which reveals a possibility to reflect ongoing anti-HBV immune activation. Overall, our pilot study shows that anti-HBc may be an important factor in illustrating the immune-activation status of CHB patients. Anti-HBc is still a novel biomarker and more relevant studies need to be exerted.
Acknowledgments
The authors would like to thank Professor Ni Wei for her help in recruiting patients, and Professor Si-Yan Zhan (Peking University) for her guidance with the statistics.
Footnotes
Abbreviations: AHB = acute hepatitis B, anti-HBc = antibody to hepatitis B core antigen, CHB = chronic hepatitis B, ENH = HBeAg-negative hepatitis, HBeAg = hepatitis B early antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HIV = human immunodeficiency virus, IC = immune clearance, IT = immune tolerance, LR = low or no replicative, PBI = past HBV infection.
WJ and L-WS contributed equally to this work. They are the co-first authors.
This study was funded by the key disciplines of Beijing and the key clinical medical disciplines of China.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Fattovich G. Natural historysand prognosis of hepatitis B. Semin Liver Dis 2003; 23:47–58. [DOI] [PubMed] [Google Scholar]
- 2.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology 2009; 49:S45–S55. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen T, Thompson AJ, Bowden S, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol 2010; 52:508–513. [DOI] [PubMed] [Google Scholar]
- 4.Jaroszewicz J, Calle SB, Wursthorn K, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol 2010; 52:514–522. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 6.Ren YY, Liu YZ, Ding YP, et al. Immune characteristics of different immune phases in natural course of chronic HBV infection. Hepatogastroenterology 2013; 60:789–795. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009; 373:582–592. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Rodriguez MT, Sopena B, Crespo M, et al. Clinical significance of “anti-HBc alone” in human immunodeficiency virus-positive patients. World J Gastroenterol 2009; 15:1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wedemeyer H, Cornberg M, Tegtmeyer B, et al. Isolated anti-HBV core phenotype in anti-HCV-positive patients is associated with hepatitis C virus replication. Clin Microbiol Infect 2004; 10:70–72. [DOI] [PubMed] [Google Scholar]
- 10.Kobyashi M, Chayama K, Arase Y, et al. Progressive and sufficient decrease of hepatitis B core antibody can predict the disappearance of hepatitis B virus DNA in Japanese patients with hepatitis B surface antigen clearance. J Gastroenterol 2000; 35:753–757. [DOI] [PubMed] [Google Scholar]
- 11.Li A, Yuan Q, Huang Z, et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol 2010; 17:464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Q, Song LW, Liu CJ, et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut 2013; 62:182–184. [DOI] [PubMed] [Google Scholar]
- 13.Papatheodoridis GV, Manesis EK, Manolakopoulos S, et al. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology 2008; 48:1451–1459. [DOI] [PubMed] [Google Scholar]
- 14.Martinot-Peignoux M, Carvalho-Filho R, Lapalus M, et al. Hepatitis B surface antigen serum level is associated with fibrosis severity in treatment-naive, e antigen-positive patients. J Hepatol 2013; 58:1089–1095. [DOI] [PubMed] [Google Scholar]
- 15.Brunetto MR, Moriconi F, Bonino F, et al. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009; 49:1141–1150. [DOI] [PubMed] [Google Scholar]
- 16.Seto WK, Lam YF, Fung J, et al. Changes of HBsAg and HBV DNA levels in Chinese chronic hepatitis B patients after 5 years of entecavir treatment. J Gastroenterol Hepatol 2014; 29:1028–1034. [DOI] [PubMed] [Google Scholar]
- 17.Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012; 61:i6–i17. [DOI] [PubMed] [Google Scholar]
- 18.Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol 2003; 18:246–252. [DOI] [PubMed] [Google Scholar]
- 19.Buster EH, Hansen BE, Lau GK, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009; 137:2002–2009. [DOI] [PubMed] [Google Scholar]
- 20.Perrillo RP, Lai CL, Liaw YF, et al. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology 2002; 36:186–194. [DOI] [PubMed] [Google Scholar]
- 21.Hsu YS, Chien RN, Yeh CT, et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology 2002; 35:1522–1527. [DOI] [PubMed] [Google Scholar]
- 22.Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2001; 34:617–624. [DOI] [PubMed] [Google Scholar]
- 23.Brunetto MR, Oliveri F, Colombatto P, et al. Hepatitis B surface antigen serum levels help to distinguish active from inactive hepatitis B virus genotype D carriers. Gastroenterology 2010; 139:483–490. [DOI] [PubMed] [Google Scholar]
- 24.Milich DR, McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 1986; 234:1398–1401. [DOI] [PubMed] [Google Scholar]
- 25.Xiao P, Chen QF, Yang YL, et al. Serum soluble interleukin-2 receptor levels in patients with chronic hepatitis B virus infection and its relation with anti-HBc. World J Gastroenterol 2006; 12:482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbani S, Fagnoni F, Missale G, et al. The role of anti-core antibody response in the detection of occult hepatitis B virus infection. Clin Chem Lab Med 2010; 48:23–29. [DOI] [PubMed] [Google Scholar]


