Abstract
Acute gastric variceal bleeding (GVB) is a catastrophic problem and accounts for one of the major causes of death in cirrhotic patients. Although, N-butyl cyanoacrylate (NBC) has been shown to control bleeding effectively, it still carries up high mortality rate. This study aimed to find the predictors of mortality within 6 weeks after emergent endoscopic treatment with NBC injection.
This retrospective study recruited patients with acute GVB after emergent endoscopic NBC injection between January 2011 and June 2013 in Linkou Medical Center, Chang Gung Memorial Hospital, Linkou, Taiwan. Logistic regression analysis was applied for predictors of mortality within 6 weeks. Statistical significance was set as P < 0.05.
There were 132 patients with acute GVB (83.3% men, median age 51.3 years) with endoscopic NBC injection treatments recruited. Mortality within 6 weeks was noted in 16.7% patients. By multivariate analysis, renal function impairment (odds ratio [OR]: 21.1, 95% confidence interval [CI]: 3.06–146.0, P = 0.002), higher Child–Turcotte–Pugh (CTP) score (OR: 2.49, 95% CI: 1.41–4.38, P = 0.002), higher model for end-stage liver disease (MELD) score (OR: 1.18, 95% CI: 1.03–1.35, P = 0.013), rebleeding within 5 days (OR: 16.4, 95% CI: 3.36–79.7, P = 0.001), and acute on chronic liver failure (ACLF) (OR: 4.67, 95% CI: 1.62–13.33, P = 0.004) were independent predictors of mortality within 6 weeks. A MELD score of ≥18 was associated with Area Under the Receiver Operating Characteristic (AUROC) of 0.79 (P < 0.001, 95% CI: 0.69–0.90) and a CTP score of ≥9 with AUROC of 0.85 (P < 0.001, 95% CI: 0.76–0.94) for determining 6 weeks mortality.
Impaired renal function, deteriorated liver function with CTP score ≥ 9 as well as MELD score ≥18, rebleeding within 5 days, and ACLF are independent predictors of mortality.
INTRODUCTION
Variceal bleeding is a frequently encountered and often catastrophic complication of portal hypertension, accounting for up to 15% to 30% of deaths in patients with cirrhosis.1–3 Successful management of a variceal bleeding relies on early diagnosis and prompt institution of therapy using a combination of aggressive resuscitation, pharmacological treatments to reduce portal pressure, and endoscopic intervention to target the source of bleeding. Although gastric varices (GVs) occur less frequent than esophageal varices (EV), the cumulative risk of GV bleeding is as high as 44% in 5 years.4 It poses a greater mortality rate (30%)5,6 and up to nearly one-third rebleeding rate after spontaneous remission,7,8 which is still far from ideal. The identification of high mortality risk patients may suggest more aggressive intervention such as evaluation of liver transplantation or transjugular intrahepatic portosystemic shunt (TIPS). As for EV bleeding, most consistently reported risk indicators of mortality are Child–Turcotte–Pugh (CTP) score class or its components, model for end-stage liver disease (MELD) score, renal failure, bacterial infection at admission or shortly after, hypovolemic shock, active bleeding at endoscopy, hepatocellular carcinoma (HCC), and hepatic venous pressure gradient (HVPG) above 20 mm Hg.9–14 However, few studies focus on the clinical predictive factors of mortality for acute gastric variceal bleeding (GVB). Therefore, this retrospective study aimed to investigate the possible predictors of early rebleeding within 120 hours, mortality within 6 weeks after emergent Histoacryl treatment, and evaluate clinical outcome of these patients within 6 weeks.
MATERIALS AND METHODS
Patient Selection
This study recruited cirrhotic patients that presented with acute GVB and admitted to our hospitals receiving emergent endoscopic injection of N-butyl-2-cyanoacrylate (NBC) between January 2011 and June 2013 (Figure 1). GVB was diagnosed by clinical signs of hematemesis, coffee ground vomitus, hematochezia, or melena with any of the following criteria15,16: endoscopic signs of active bleeding from the GVs; adherent blood clots, white nipple signs, or GV erosion; and the presence of distinct large GVs with red-color signs (red spots or red wale markings on the varices) and absence of EV and other bleeding sources. The diagnosis of cirrhosis was based on pathology, clinical, and sonographic or computed tomographic findings. Patients were excluded as follows: initial treatment failure (rebleeding within 24 hours), and lost of follow-up within 6 weeks following initial successful hemostasis of GVB. We collected data from all enrolled patients including history, physical examination, and laboratory measurements at arrival to the hospital. This study was carried out with preapproval by the Linkou Chang-Gung Memorial Hospital Institutional Review Board (102–0673B).
FIGURE 1.
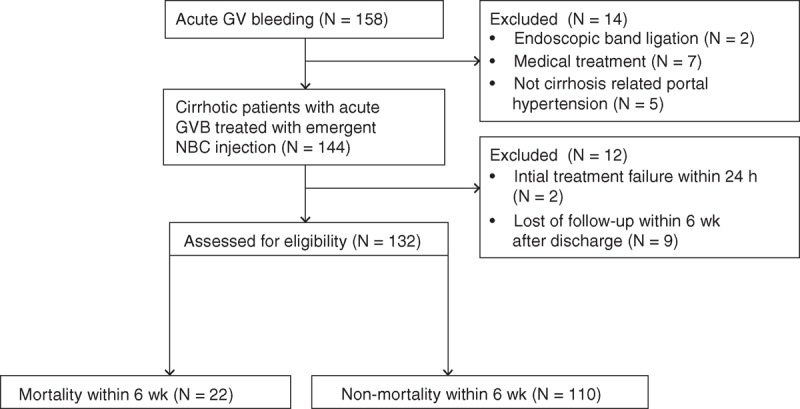
Flowchart of patients admitted to our hospital for GVB and selection of the study. GV = gastric varice, GVB = gastric variceal bleeding, NBC = N-butyl cyanoacrylate.
Definitions
The GVs were classified according to the criteria suggested by Sarin et al7: type 1 (GOV1), varices continuous with EV and extending along the lesser curve for approximately 2 to 5 cm below the gastroesophageal junction; type 2 (GOV2), varices extending from the esophagus below the gastroesophageal junction toward the fundus; and type 3 (IGV, isolated GV), varices located in the fundus or antrum without EV. The size of GV was defined according to the criteria suggested by Hashizume et al17: Form 1 (F1), tortuous winding varices; F2, nodular-shaped varices; and F3, tumorous huge varices. Active bleeding is a state defined endoscopically, when spurting or oozing is seen from the varix.18 According to Asian Pacific Association for the Study of the Liver 2011 guideline,18 protection of airway for esophagogastroduodenoscopy (EGD) examination with endotracheal intubation was suggested in those patients with the following conditions: severe uncontrolled variceal bleeding, hepatic encephalopathy (grades III and IV), aspiration pneumonia, and difficulty in maintaining oxygen saturation above 90%. Achievement of initial hemostasis was defined by stable vital signs and absence of rebleeding within 24 hours. Initial treatment failure was defined as failure to control acute bleeding after 2 attempts with the same endoscopic methods, >1 GV rebleeding episode, and bleeding death or change of modality (ie, if the endoscopist judged that hemorrhage could not be controlled, the modality might be changed to another endoscopic method or operation) within 24 hours. Renal function impairment was defined as a creatinine level >1.5 mg/dL in those without preexisting renal disease and an increase of >50% in those with preexisting renal disease19 rather than universal definition of acute kidney injury (AKI),20 because it was not always available in this tertiary hospital cohort because most of the patients were transferred and lack of previous baseline renal function test. The systemic inflammation response syndrome (SIRS) was defined as fulfilling at least 2 of the following criteria: core temperature >38°C(100.4°F) or <36°C (96.8°F); heart rate ≥90 beats/minute; respiratory rate ≥20 breaths/minute or arterial partial pressure of carbon dioxide <4.3 kPa (32 mm Hg); white blood cell count ≥12 000/mm3 or ≤4000/mm3; or a differential count showing ≥10% immature polymorphonuclear neutrophil cells according to the recommendations of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference.21 Acute on chronic liver failure (ACLF) was based on the presence of acute decompensation (AD; defined by the acute development of one or more major complications of liver disease such as ascites, encephalopathy, or gastrointestinal hemorrhage) and organ failures (including liver, kidney, cerebral, coagulation, circulatory, and respiratory) according to Moreau definition.22
Treatment Methods
In our study, vasoconstrictors such as terlipressin or somatostatin, proton pump inhibitor, and prophylactic antibiotics23,24 were administered as soon as possible after arrival at emergency room. Packed red blood cell (PRBC) was given to maintain hemoglobin of 7 g/dL prior to the EGD treatment.25 Therapeutic endoscopy was performed within 24 hours after bleeding occurred.
A standardized Histoacryl injection method was used according to the recommendations of Seewald et al.26 A working solution of Histoacryl was generated by mixing 0.5 mL with 0.8 mL of lipiodol (Guerbert, Roissy, France). The injection catheter (21G or 23G InterjectTM; Boston Scientific, Spencer, IN) was first primed with 0.8 to 1.2 mL of lipiodol to prevent premature solidification of the Histoacryl. Then, the bleeding gastric varix was punctured and the working solution was injected, immediately followed by 1.0 to 2.0 mL of sterile distilled water to ensure delivery of the entire working solution volume into the varix. The needle was retracted and immediately flushed with sterile distilled water to maintain patency. Then, the varix was probed with the injection catheter and if it was found to have remained soft, an additional 1.3 mL injection was initiated to achieve complete obliteration (defined as absolute firmness of the injected varix). All of these procedures were carried out without the aid of fluoroscopic monitoring. All procedures were performed by experienced gastroenterologists or by second-year gastroenterologist trainees under the supervision of an experienced gastroenterologist. Postoperative monitoring included clinical and laboratory examinations to identify development of complications. Most patients (N = 125, 94.7%) keep intravenous bolus of vasopressors (terlipressin) for 5 days following the procedure while 3 patients received intravenous infusion of somatostatin because they could not tolerate side effect of terlipressin. At arrival, 3rd-generation cephalosporin was prescribed for 5 to 7 days in 93 (70.1%) patients.
Statistical Analysis
Statistical analyses were carried out by the SPSS software, version 20.0 (SPSS, Inc, Chicago, IL). Descriptive data with normal distribution are reported as mean ± standard deviation or as percentage otherwise as median [interquartile Range (IQR)]. The Student t test and Mann–Whitney U test were used to assess differences between groups in normal distributed and non-normal distributed groups separately. Chi-square was used for categorical variables between the 2 groups. Stepwise logistic regression analyses, both univariate and multivariate, were used to determine the correlation of the predictive factors to clinical outcomes. A 2-tailed P value <0.05 was considered as statistically significant.
RESULTS
Patient Demographics
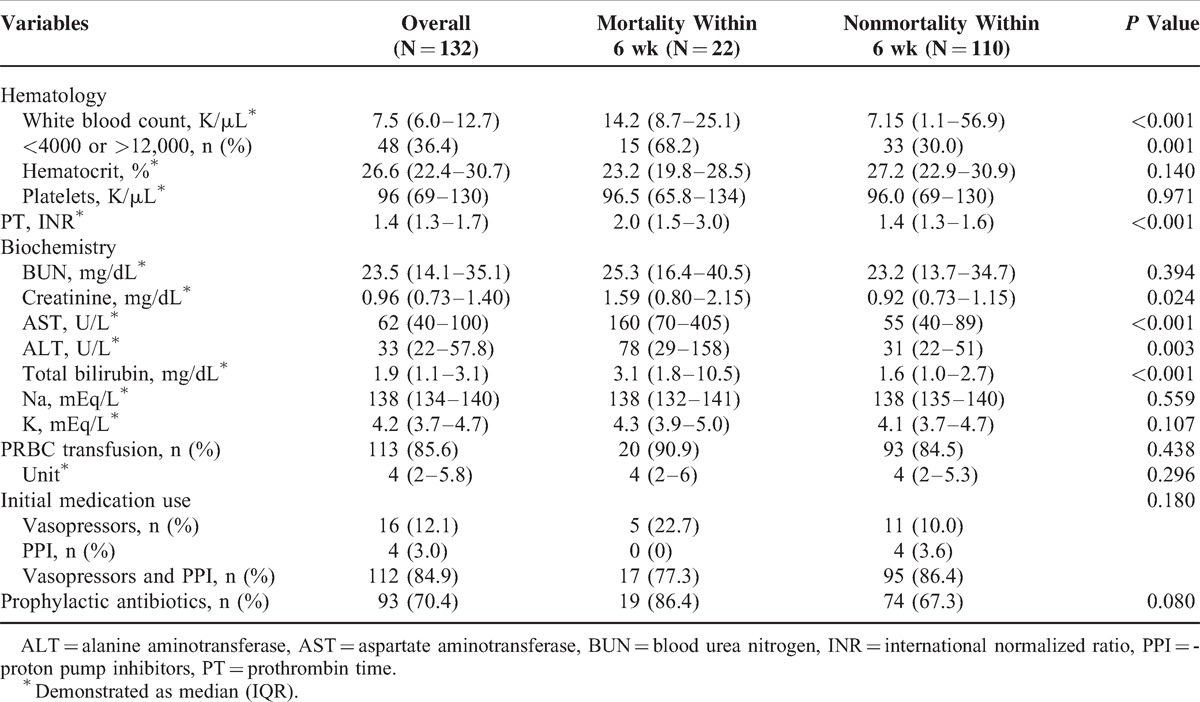
Total 158 patients with acute GVB were screened for eligibility (Figure 1). Among them, 26 patients were excluded because of receiving endoscopic band ligation (N = 2), only medication treatment (N = 7), not cirrhosis-related portal hypertension (N = 5), no achievement of initial hemostasis (N = 3), and loss of follow-up within 6 weeks after discharge (N = 9). Surgical intervention was considered for 3 patients without achievement of initial hemostasis but only 1 received devascularization with splenectomy procedure while the other 2 patients received conservative treatment. However, all the 3 patients eventually died. The remaining 132 cirrhotic patients with active GVB were analyzed. Their demographic characteristics are shown in Tables 1 and 2. The majority was male (n = 110, 83.3%) and median (IQR) age was 51.3 (46.3–64.2) years. The etiologies of liver cirrhosis were alcohol alone in 48 (36.4%) patients, combination of hepatitis B virus (HBV)/ hepatitis C virus (HCV) infection and alcohol in 24 (18.2%) patients, and HBV/HCV infection alone in 60 (45.4%) patients. Thirty-seven (28.0%) patients had previous GVB history treated with NBC injection and 43 (32.6%) patients had previous EVB history treated with endoscopic variceal ligation. The median (IQR) MELD score and CTP score at the time of hospital arrival was 14 (11–20) and 8 (6–10). Ninety-three (70.5%) patients presented with hematemesis or coffee ground vomitus upon arrival. One hundred thirteen (85.6%) patients with GVB received packed red blood cell transfusion prior to the EGD procedure according to their hematocrit level. None of these patients had complication after histoacryl injection such as systemic embolism, mesenteric hematoma, hemoperitoneum, fistula, and pericarditis as reported by previous literature.27–31
TABLE 1.
Baseline Clinical Characteristics of Patients With Acute Gastric Variceal Bleeding
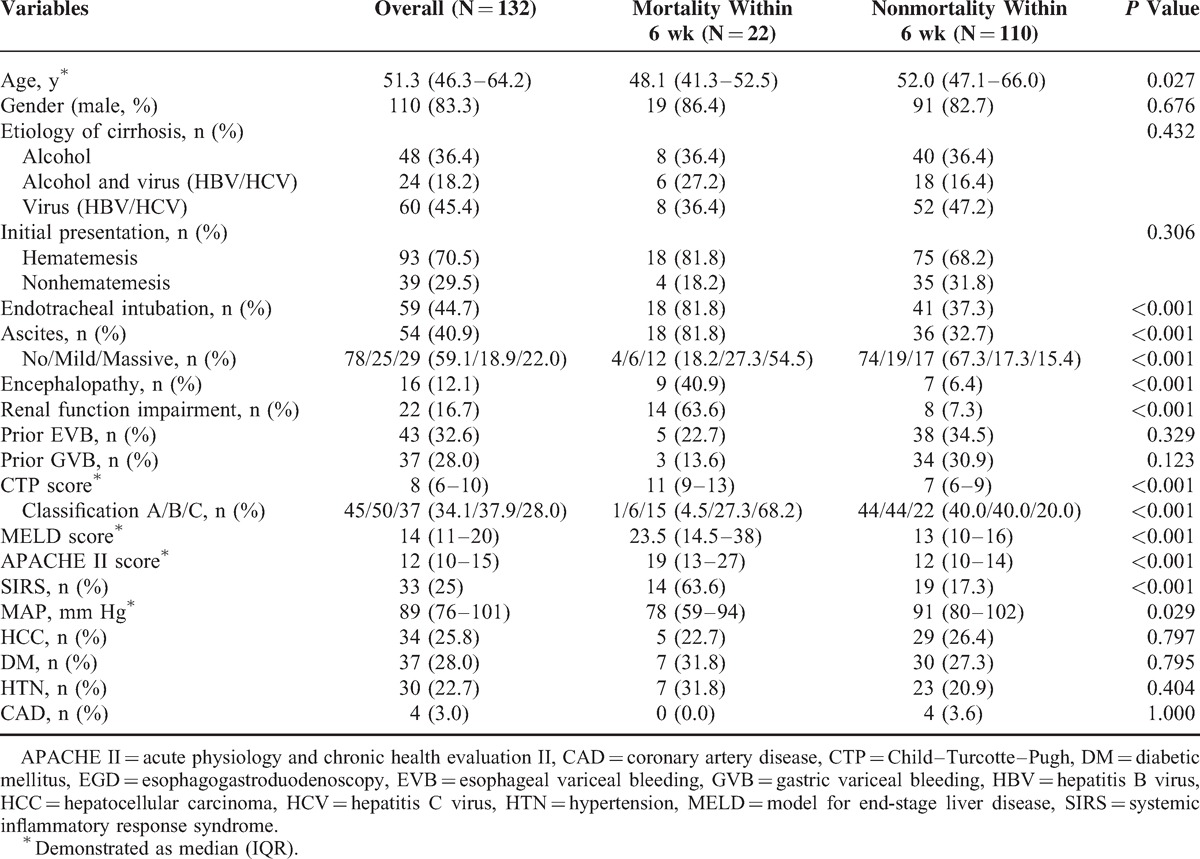
TABLE 2.
Biochemistry Finding and Treatment Strategy Prior to Esophagogastroduodenoscopy
Prognostic Indicators of 6 Weeks Mortality
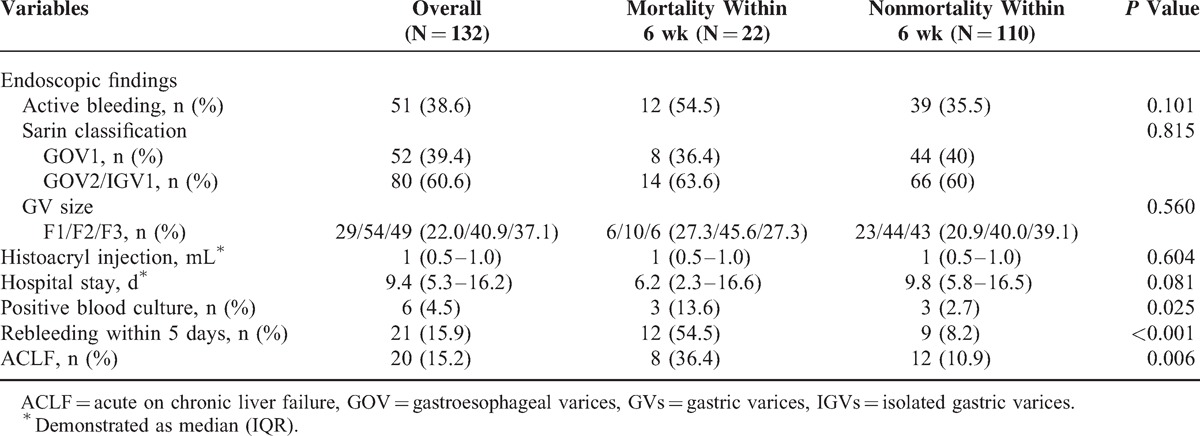
The clinical findings and outcome of these patients are shown in Table 3. The overall mortality rate within 6 weeks was 16.7% (n = 22) after initial successful treatment. As shown in Tables 1 and 2, the significant differences between mortality and nonmortality were younger age (48.1 vs 52.0, P = 0.027), endotracheal intubation (81.8% vs 37.3%, P < 0.001), renal function impairment (63.6% vs 7.3%, P < 0.001), higher CTP score (11 vs 7, P < 0.001), higher MELD score (23.5 vs13, P < 0.001), higher Acute Physiology and Chronic Health Evaluation II (APACHE II) score (19 vs12, P < 0.001), presence of SIRS (63.6% vs 17.3%, P < 0.001), lower mean artery pressure (78 vs 91, P = 0.029), and elevated alanine aminotransferase (ALT) (78 vs 31, P = 0.003). As shown in Table 3, positive blood culture (13.6% vs 2.7%, P = 0.025), early rebleeding within 5 days (54.5% vs 8.2%, P < 0.001), and ACLF (36.4% vs 10.9%, P = 0.006) had significant differences between the 2 groups.
TABLE 3.
Endoscopic Findings and Outcome of Acute Gastric Variceal Bleeding
By multivariate analysis, renal function impairment (odds ratio [OR]: 21.12, 95% confidence interval [CI]: 3.055–146.0, P = 0.002), higher CTP score (OR: 2.488, 95% CI: 1.414–4.378, P = 0.002), higher MELD score (OR: 1.181, 95% CI: 1.033–1.350, P = 0.013), rebleeding within 5 days (OR: 16.37, 95% CI: 3.362–79.66, P = 0.001), and ACLF (OR: 4.67, 95% CI: 1.62–13.33, P = 0.004) were the independent predictive factors for 6 weeks mortality (Table 4). ROC curves were performed to decide the optimal cut-points for 6 weeks mortality. A MELD score of ≥18 was associated with Area Under the Receiver Operating Characteristic (AUROC) of 0.794 (P < 0.001, 95% CI: 0.690–0.897) and a CTP score of ≥9 with AUROC of 0.848 (P < 0.001, 95% CI: 0.755–0.942) for determining 6 weeks mortality (Figure 2). The sensitivity, specificity, positive predictive value, and negative predictive value for predicting of 6 weeks mortality are 68.2% vs 90.9%, 80.9% vs 69.1%, 39.5% vs 37.0%, and 92.6% vs 97.4% by MELD score ≥18 points and CTP score ≥9 points, respectively.
TABLE 4.
Multivariate Analysis of 6 wk Mortality and 5 d Rebleeding
FIGURE 2.
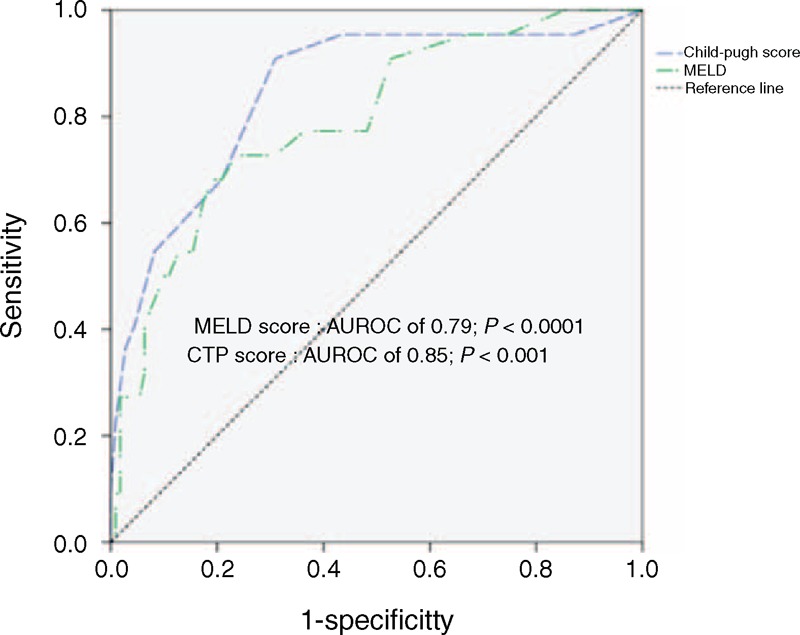
Receiver operating characteristic curve of model for end-stage liver disease (MELD) scores and Child–Turcotte–Pugh (CTP) scores in predicting 6 wk mortality after acute gastric variceal bleeding.
In addition, we also analyzed the predictors of rebleeding within 5 days that we found the presence of SIRS (OR: 9.351, 95% CI: 3.082–28.37, P < 0.001) and active bleeding during EGD (OR: 6.682, 95% CI: 2.068–21.59, P = 0.002) are associated factors of rebleeding within 5 days (Table 4).
DISCUSSION
In this study, several important prognostic factors were associated with the mortality within 6 weeks after initial successful hemostasis. Impaired renal function, deteriorated liver function with CTP score ≥9 as well as MELD score ≥18, rebleeding within 5 days, and ACLF were prone to have higher mortality rate. Besides, SIRS and active bleeding during EGD are associated with rebleeding within 5 days.
Currently, there are few well-established models for accurate prediction of survival in patients with cirrhosis following an episode of GVB compared to EV bleeding. In cirrhotic patients with esophageal variceal hemorrhage, a high MELD score has been demonstrated to be a predictor of short (in-hospital and 30 days) and long-term (1 year and overall) mortality.32,33 In our study, we recruited patients with GV bleeding only. The impacts of liver reserve have significant influence on the survival in patients with GVB. The deterioration of MELD and CTP scores are significantly predictive of mortality in cirrhotic patients with an acute GVB. The MELD and CTP scores are composed by components reflecting severity of the underlying liver disease. The 6-week mortality rate was higher in the MELD score ≥18 points group (68.2% vs 31.8%, P < 0.001) (Figure 3A) and CTP score ≥9 points group (90.9% vs 9.1%, P < 0.001) (Figure 3B). By comparison of AUROC between MELD and CTP scores, the predictive value is similar (P = 0.437). The higher ALT level at the beginning of acute GVB was observed in the mortality group (78 vs 31, P = 0.003), but not significant when using logistic regression analysis (OR: 1.003, 95% CI: 0.996–1.009, P = 0.394) that is compatible with the findings of the EV mortality studies.33,34 The increase of ALT level at the bleeding time may relate to liver hypoperfusion, but not represent the extent of liver injury secondary to bleeding event.
FIGURE 3.
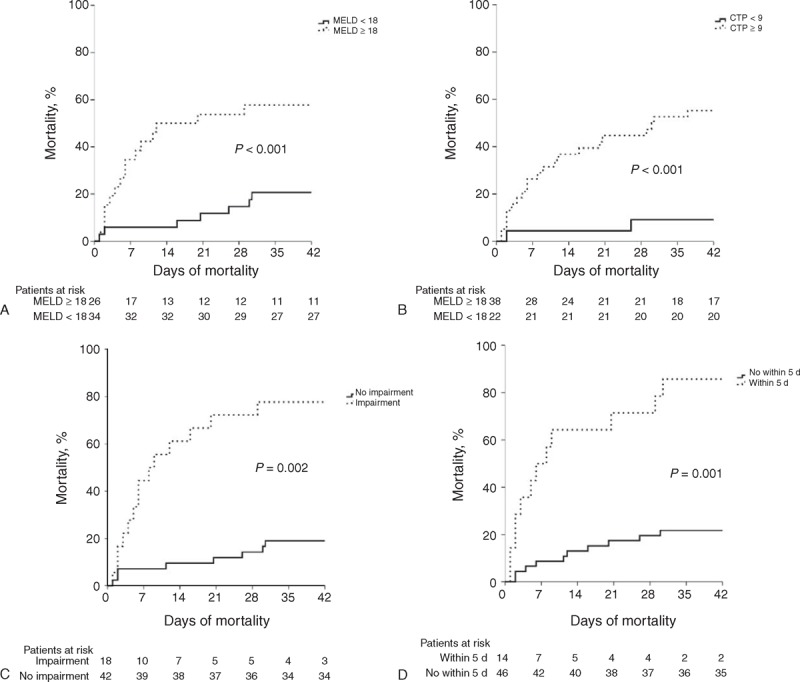
Kaplan–Meier estimates of 6-wk mortality rate after acute gastric variceal bleeding in cirrhotic patients stratified by: (A) model for end-stage liver disease (MELD) score, (P < 0.001); (B) Child–Turcotte–Pugh (CTP) score (P < 0.001); (C) renal function impairment (P = 0.002); and (D) early rebleeding within 5 d (P = 0.001).
The deterioration of renal function is a major factor influencing survival in cirrhotic patients.34 In previous study,20 the diagnosis of AKI defined as a rise in serum creatinine of over 0.3 mg/dL or 50% increase in serum creatinine from baseline within 48 hours. It required previous baseline renal function and was compared within 48 hours; however, this was not always available in this tertiary hospital cohort because most of the patients were transferred from local community hospital. Here, We defined renal function impairment as a creatinine level > 1.5 mg/dL in those without preexisting renal disease and an increase of > 50% in those with preexisting renal disease. By logistic regression analysis, the renal function impairment is a critical predictor of mortality after acute GVB (63.6% vs 7.3%, P = 0.002) (Figure 3C). Previous studies35,36 showed that patients with AD (acute development of ascites, encephalopathy, and gastrointestinal hemorrhage) and organ failure(s) are at high risk for short-term death. Moreau et al22 defined these people as ACLF and found that the prevalence of ACLF in patients with AD was 30% and associated with a higher short-term mortality rate 15 times higher than that in patients with AD alone. In our study, we also found that ACLF played an important role in determining mortality within 6 weeks (OR: 4.67, 95% CI: 1.62–13.33, P = 0.004) compatible with Moreau conclusion.
Furthermore, the occurrence of SIRS at arrival ER (63.7% vs 36.3%, P < 0.001) is a main predictive factor for the development of renal function impairment and associated with poor survival. There are several possible mechanisms that may explain this finding. First, the increase of systemic and intrahepatic chemokine levels could impair the circulatory dysfunction that commonly is found in cirrhotic patients, leading to further activation of vasoconstrictor systems and favoring renal hypoperfusion and parenchymal injury.37 Second, the existence of SIRS could promote endothelial dysfunction and hepatocellular damage and disturb the intrahepatic circulation, thus favoring the development of clinical complications.38 Third, the presence of SIRS could be indicative of a possible bacterial infection, either occult or clinically evident, which is a known precipitating factor of renal failure in patients with chronic liver diseases.39 Fourth, the cytokine “overspill” that characterized bacterial infections and SIRS could have a direct deleterious role in the genesis of renal dysfunction by stimulating apoptosis and necrosis of tubular cells.40 The above hypothesis may explain the correlation between SIRS and renal function impairment in cirrhotic patients.41
Among the 132 patients recruited, total 35 patients (26.5%) occurred rebleeding after initial successful treatment and most rebleeding episode occurred within 5 days (n = 21, 60%). The overall early rebleeding rate is 15.9% similar to Prachayakul et al6 and Jairath et al3 reports. Besides, more than half of patients with early rebleeding had mortality within 6 weeks (n = 12, 57.1%). Rebleeding within 5 days plays a critical role in determining the predictor of mortality within 6 weeks after acute GVB (54.5% vs 8.2%, P = 0.001) (Figure 3D). It may be related to temporary increasing portal pressure after endoscopic hemostasis. Avgerinos et al42 performed repeated HVPG measurements before and immediately after endoscopic treatment and every 24 hours for a 5-day period. They found that endoscopic injection sclerotherapy (EIS) causes a sustained increase in HVPG than ligation group related to leaving a less number of portal-systemic collaterals that account in part for the differences in HVPG after treatment. Although the mean portal pressure in patients with GVs is lower than in those with EVs, the portal pressure is an important determinant of rebleeding or death in patients with GVs if their portal pressure is >12 mm Hg.43 These results also clearly justify the importance of early administration of a vasoactive drug for 120 hours after EIS.42,44 In our study, we also found SIRS when ER arrival and active bleeding during EGD are associated with the predictors of rebleeding within 5 days. The size and classification of GV has limited impact on the mortality prediction after acute GV bleeding (Table 3). In high risk of early rebleeding patients, we can consider further aggressive intervention such as TIPS or balloon-occluded retrograde transvenous obliteration, which is effective and has the potential benefit of increasing portohepatic blood flow and therefore may be an alternative for patients that may not tolerate TIPS45–47 if early rebleeding occurred to prevent mortality.
Our study aimed at only predicting mortality in acute GVB. However, most recruited patients (n = 117, 88.6%) had EV concurrently and more than one third (n = 43, 36.8%) of them had EV bleeding history before. There were several studies mentioned about predictors of mortality after acute EV bleeding. Bambha et al33 analyzed 256 cirrhotic patients and found MELD score >18, requiring >4 units of PRBCs transfusion within the first 24 hours or with active bleeding at endoscopy were at increased risk of mortality within 6 weeks. Han et al48 analyzed 102 cirrhotic patients with HCC and found that the poor hepatic function, active bleeding at endoscopy, failure to control bleeding, and impaired renal function were predictors of in-hospital mortality after acute variceal bleeding. In our experience, Chen et al24 analyzed 128 cirrhotic patients after acute EV bleeding between January 2007 and December 2008 in our hospital, we found that MELD score ≥21.5, hypovolemic shock, and nonselective β-blocker use after rebleeding were predictors of 6 weeks mortality. In conclusion, poor liver function plays an important role in determining mortality both after acute GV and EV bleeding.
There are still some limitations in our study. First, it was conducted in a retrospective cohort and some patients were lack of serum creatinine values at baseline that could be compared when bleeding event occurred. Second, not all of the patients receive prophylactic antibiotics during the acute bleeding stage. Thus, the impact of prophylactic antibiotics use on the post-GV bleeding survival remained unclarified.
In summary, our study clearly documented that mortality rate within 6 weeks after acute GVB post-Histoacryl injection treatment is as high as 16.7%. It is crucial to identify those who were at high risks of mortality after GV bleeding that intensive monitoring is required. Those who have impaired renal function, deteriorated liver function with CTP score ≥9 as well as MELD score ≥18, rebleeding within 5 days, and ACLF are independent predictors of mortality within 6 weeks. Based on these findings, we recommend intense monitoring of liver and renal function as well as sign of early rebleeding in the high-risk patients. Further assessment of liver transplantation may be indicated in the higher MELD score patients encountering acute GV bleeding.
Footnotes
Abbreviations: EVs = esophageal varices, GVs = gastric varices.
W-JJ and C-YL equally contributed in the writing of this manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46:922–938. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010; 362:823–832. [DOI] [PubMed] [Google Scholar]
- 3.Jairath V, Rehal S, Logan R, et al. Acute variceal haemorrhage in the United Kingdom: Patient characteristics, management and outcomes in a nationwide audit. Dig Liver Dis 2014; 46:419–426. [DOI] [PubMed] [Google Scholar]
- 4.Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology 1997; 25:307–312. [DOI] [PubMed] [Google Scholar]
- 5.Chang YJ, Park JJ, Joo MK, et al. Long-term outcomes of prophylactic endoscopic histoacryl injection for gastric varices with a high risk of bleeding. Digest Dis Sci 2010; 55:2391–2397. [DOI] [PubMed] [Google Scholar]
- 6.Prachayakul V, Aswakul P, Chantarojanasiri T, et al. Factors influencing clinical outcomes of Histoacryl(R) glue injection-treated gastric variceal hemorrhage. World J Gastroenterol 2013; 19:2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarin SK, Lahoti D, Saxena SP, et al. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology 1992; 16:1343–1349. [DOI] [PubMed] [Google Scholar]
- 8.Hou MC, Lin HC, Lee HS, et al. A randomized trial of endoscopic cyanoacrylate injection for acute gastric variceal bleeding: 0.5 mL versus 10 mL. Gastrointest Endosc 2009; 70:668–675. [DOI] [PubMed] [Google Scholar]
- 9.Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999; 117:626–631. [DOI] [PubMed] [Google Scholar]
- 10.Moitinho E, Planas R, Banares R, et al. Multicenter randomized controlled trial comparing different schedules of somatostatin in the treatment of acute variceal bleeding. J Hepatol 2001; 35:712–718. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico G, De Franchis R, Cooperative Study G. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003; 38:599–612. [DOI] [PubMed] [Google Scholar]
- 12.Abraldes JG, Villanueva C, Banares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008; 48:229–236. [DOI] [PubMed] [Google Scholar]
- 13.Augustin S, Muntaner L, Altamirano JT, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol 2009; 7:1347–1354. [DOI] [PubMed] [Google Scholar]
- 14.Augustin S, Altamirano J, Gonzalez A, et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol 2011; 106:1787–1795. [DOI] [PubMed] [Google Scholar]
- 15.Tan PC, Hou MC, Lin HC, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology 2006; 43:690–697. [DOI] [PubMed] [Google Scholar]
- 16.de Franchis R, Baveno VF. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010; 53:762–768. [DOI] [PubMed] [Google Scholar]
- 17.Hashizume M, Kitano S, Yamaga H, et al. Endoscopic classification of gastric varices. Gastrointest Endosc 1990; 36:276–280. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Kumar A, Angus PW, et al. Diagnosis and management of acute variceal bleeding: Asian Pacific Association for Study of the Liver recommendations. Hepatol Int 2011; 5:607–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terg R, Gadano A, Cartier M, et al. Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: a retrospective study. Liver Int 2009; 29:415–419. [DOI] [PubMed] [Google Scholar]
- 20.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut 2013; 62:131–137. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest J 1992; 101:1644. [DOI] [PubMed] [Google Scholar]
- 22.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–1437. [DOI] [PubMed] [Google Scholar]
- 23.Rerknimitr R, Chanyaswad J, Kongkam P, et al. Risk of bacteremia in bleeding and nonbleeding gastric varices after endoscopic injection of cyanoacrylate. Endoscopy 2008; 40:644–649. [DOI] [PubMed] [Google Scholar]
- 24.Chen WT, Lin CY, Sheen IS, et al. MELD score can predict early mortality in patients with rebleeding after band ligation for variceal bleeding. World J Gastroenterol 2011; 17:2120–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368:11–21. [DOI] [PubMed] [Google Scholar]
- 26.Seewald S, Ang TL, Imazu H, et al. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos). Gastrointest Endosc 2008; 68:447–454. [DOI] [PubMed] [Google Scholar]
- 27.Cheng LF, Wang ZQ, Li CZ, et al. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol 2010; 8:760–766. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia G, Morbin T, Patarnello E, et al. Visceral fistula as a complication of endoscopic treatment of esophageal and gastric varices using isobutyl-2-cyanoacrylate: report of two cases. Gastrointest Endosc 2000; 52:267–270. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald BD, Caldwell SH, Hespenheide EE, et al. N-2-butyl-cyanoacrylate for bleeding gastric varices: a United States pilot study and cost analysis. Am J Gastroenterol 2003; 98:1982–1988. [DOI] [PubMed] [Google Scholar]
- 30.Tan YM, Goh KL, Kamarulzaman A, et al. Multiple systemic embolisms with septicemia after gastric variceal obliteration with cyanoacrylate. Gastrointest Endosc 2002; 55:276–278. [DOI] [PubMed] [Google Scholar]
- 31.Chen YY, Shen TC, Soon MS, et al. Life-threatening pericarditis after N-butyl-2-cyanoacrylate injection for esophageal variceal bleeding: case report. Gastrointest Endosc 2005; 61:487–489. [DOI] [PubMed] [Google Scholar]
- 32.Chalasani N, Kahi C, Francois F, et al. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology 2002; 35:1282–1284. [DOI] [PubMed] [Google Scholar]
- 33.Bambha K, Kim WR, Pedersen R, et al. Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008; 57:814–820. [DOI] [PubMed] [Google Scholar]
- 34.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006; 44:217–231. [DOI] [PubMed] [Google Scholar]
- 35.Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol 2012; 57:1336–1348. [DOI] [PubMed] [Google Scholar]
- 36.Wlodzimirow K, Abu-Hanna A, Chamuleau RA. Acute-on-chronic liver failure – its definition remains unclear. J Hepatol 2013; 59:190–191. [DOI] [PubMed] [Google Scholar]
- 37.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2012; 10:65–71. [DOI] [PubMed] [Google Scholar]
- 38.Cazzaniga M, Dionigi E, Gobbo G, et al. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol 2009; 51:475–482. [DOI] [PubMed] [Google Scholar]
- 39.Terra C, Guevara M, Torre A, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology 2005; 129:1944–1953. [DOI] [PubMed] [Google Scholar]
- 40.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 2004; 65:1357–1365. [DOI] [PubMed] [Google Scholar]
- 41.Thabut D, Massard J, Gangloff A, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology 2007; 46:1872–1882. [DOI] [PubMed] [Google Scholar]
- 42.Avgerinos A, Armonis A, Stefanidis G, et al. Sustained rise of portal pressure after sclerotherapy, but not band ligation, in acute variceal bleeding in cirrhosis. Hepatology 2004; 39:1623–1630. [DOI] [PubMed] [Google Scholar]
- 43.Tripathi D, Therapondos G, Jackson E, et al. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut 2002; 51:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toyonaga A, Iwao T, Sumino M, et al. Portal pressure after prophylactic sclerotherapy in patients with high-risk varices. J Hepatol 1994; 21:515–520. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Pagan JC, Barrufet M, Cardenas A, et al. Management of gastric varices. Clin Gastroenterol Hepatol 2014; 12:919–928. [DOI] [PubMed] [Google Scholar]
- 46.Girotra M, Raghavapuram S, Abraham RR, et al. Management of gastric variceal bleeding: role of endoscopy and endoscopic ultrasound. World J Hepatol 2014; 6:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naeshiro N, Aikata H, Kakizawa H, et al. Long-term outcome of patients with gastric varices treated by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 2014; 29:1035–1042. [DOI] [PubMed] [Google Scholar]
- 48.Han ML, Chen CC, Kuo SH, et al. Predictors of in-hospital mortality after acute variceal bleeding in patients with hepatocellular carcinoma and concurrent main portal vein thrombosis. J Gastroenterol Hepatol 2014; 29:344–351. [DOI] [PubMed] [Google Scholar]


