Supplemental Digital Content is available in the text
Abstract
Neutrophilic urticarial dermatosis (NUD) resembles urticaria clinically but is a neutrophilic dermatosis histopathologically. The majority of patients with NUD have an underlying systemic condition, mainly, autoinflammatory disorders such as cryopyrin-associated periodic syndromes, Schnitzler syndrome, and adult-onset Still disease, but a few also have systemic lupus erythematosus (LE). Here, we confirm these data and we report relevant clinical and histopathological data of 7 patients with LE and NUD.
We retrospectively retrieved the medical records of all patients with LE in whom skin biopsy showed NUD in registers of Strasbourg and Montpellier University hospitals since 2000.
All were female and aged between 13 and 45 years. Skin lesions were typically rose or red macules or slightly elevated papules occurring in a wide distribution. Individual lesions resolved within 24 hours and were not or only slightly itchy. Every patient had associated signs, most of the time polyarthritis and/or fever. NUD was the presenting mode of LE in 2 patients. NUD was misdiagnosed as a classic lupus flare and led to therapeutic intensification with the introduction of immunosuppressive drugs in 4 patients. Histopathological findings consisted of intense neutrophilic interstitial and perivascular infiltrate with leukocytoclasia and without fibrinoid necrosis of vessel walls. Direct immunofluorescence testing showed a lupus band in 4 patients. Antinuclear antibodies were always positive, anti-dsDNA antibodies were positive in 5 patients, and anti-Ro/SSA antibodies in 6 patients. Immunosuppressive drugs such as prednisone, hydroxychloroquine, mycophenolate mofetil, and methotrexate were never effective to treat NUD. Antihistamines were effective in 1 patient and dapsone or colchicine was effective in 5 patients.
NUD is not exceptional in patients with systemic LE and is easily misdiagnosed as an acute LE flare. Furthermore, we show that conventional immunosuppressive LE treatments are not efficient and we underline the major interest of dapsone and colchicine, classic neutrophil migration inhibitors, in those patients.
INTRODUCTION
Neutrophilic urticarial dermatosis (NUD), the most recently delineated entity within the nosologic spectrum of the neutrophilic dermatoses (NDs), was first described in 2009 by Kieffer et al1 as an eruption consisting of rose or red macules or slightly elevated plaques vanishing within 24 hours. The histopathologic findings are a dense perivascular and interstitial infiltrate of neutrophils with leukocytoclasia but without vasculitis. In this initial study, 9 patients were reported and 7 had associated systemic diseases: adult-onset Still disease (3 patients), systemic lupus erythematosus (SLE) (3 patients), and Schnitzler syndrome (1 patient). It was not surprising to find patients with adult-onset Still disease and Schnitzler syndrome, entities that are considered as acquired autoinflammatory disorders with neutrophilic tissue infiltration, but the presence of patients with a connective disease such as lupus erythematosus (LE) was unexpected. This led us to review in detail the association between ND and LE.1,2
Several types of NDs have already been reported in patients with LE, such as pyoderma gangrenosum, Sweet syndrome, palisaded neutrophilic granulomatous dermatitis, amicrobial pustulosis of the folds, and recently NUD.3 Furthermore, bullous LE is a ND. The presence of neutrophilic infiltrate in early and evolving lesions of cutaneous LE is a well-known phenomenon4; therefore, including neutrophilic lesions in the classification of skin lesions in SLE has already been suggested.5
The great majority of patients with NUD have fever and joint pain. Therefore, the symptomatic set of rash, fever, and joint pain in a patient with known SLE is often mistaken for an exacerbation of LE leading to therapeutic intensification with immunosuppressors. However, the latter do usually not alleviate symptoms leading to an increase in immunosuppression, while dapsone and colchicine, classic neutrophil migration inhibitors, are generally effective to control NUD. This highlights the importance of correctly identifying this entity in lupus patients.
Here, we report 7 patients with NUD and SLE, and we have paid particular attention to the treatments undertaken and their effects.
PATIENTS AND METHODS
We performed a retrospective study and retrieved the medical records of all patients with LE whose skin biopsy showed NUD in registers of Strasbourg and Montpellier University Hospitals (France) since 2000. Under French law, this type of study, which does not involve any invasive investigation but relies on a retrospective analysis of patient files, does not need the approval of the institutional review board.
Patients were included if they met the following criteria:
Diagnosis of NUD defined as recurrent or chronic cutaneous eruption consisting of macules, papules, or plaques resolving within 48 hours, pruritic or not, and histopathologic findings consisting of a diffuse neutrophilic infiltrate in the dermis with interstitial involvement with leukocytoclasia but without fibrinoid necrosis of vessel walls and without significant dermal edema.
Diagnosis of SLE according to American College of Rheumatology (ACR) and/or Systemic Lupus International Collaborating Clinics (SLICC) criteria or cutaneous LE based on classic clinical criteria and/or histological ascertainment of LE.
For all patients, relevant clinical data including age, gender, duration, distribution and morphology of skin lesions, history of LE, serologic data, medications at the time of diagnosis, and response to treatment were reviewed. Three patients were already described in the study by Kieffer et al1 (patients 5–7).
RESULTS
A total of 7 patients fulfilled the criteria. Their characteristics are summarized in Table 1 . A brief summary of 2 typical cases follows to illustrate the difficulty of the diagnosis and how it can be misdiagnosed as a classic lupus flare.
Table 1.
Summary of the 7 Patients Included in the Study

Patient 3
A 21-year-old female was admitted for a rash (Figure 1). She had a medical history of SLE for 4 years with glomerulonephritis type III, pancytopenia, photosensibility, polyarthritis, and cutaneous vasculitis. Antinuclear factors were positive with anti-dsDNA, anti-Ro/SSA, anti-ribonucleoprotein (RNP), and antiribosomal antibodies. She also had low complement: C3 = 0.23 g/L (0.75 < N < 1.40) and C4 = 0.02 g/L (0.10 < N < 0.34). She had no treatment for 1 year when she developed the rash. Lesions consisted of slightly erythematous migratory annular nonitching macules and papules on the face, the trunk, and the hands (Figure 1). She also complained of joint pain and chest pain. She reported similar episodes for 2 years. Laboratory tests showed an elevated erythrocyte sedimentation rate (100 mm/h) and polyclonal hypergammaglobulinemia (15.6 g/L). She was first treated with 0.75 mg/kg of prednisone without any improvement. The treatment was strengthened successively by hydroxychloroquine, antihistaminic, and mycophenolate mofetil. Finally, a dermatological advice was required. A skin biopsy was performed that showed an interstitial dermal neutrophilic infiltrate and leukocytoclasia. Direct immunofluorescence (DIF) revealed a lupus band. A diagnosis of NUD was made. Therefore, she was first treated with colchicine without improvement and then dapsone was introduced; as a result, skin lesions disappeared within 48 hours.
FIGURE 1.
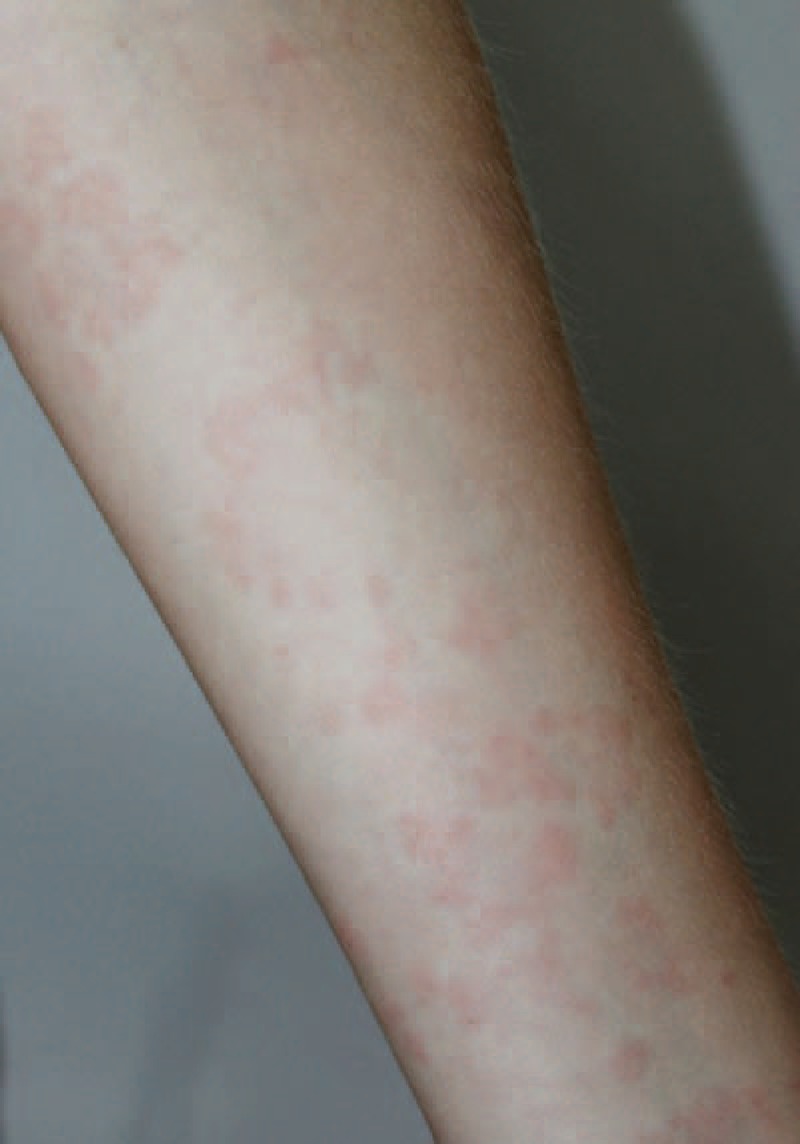
Red pale macules of the arm (patient 3).
Patient 6
A 22-year-old female was referred for a rash. She suffered from SLE for 2 years, with polyarthritis, pericarditis, and hemolytic anemia treated with prednisone and hydroxychloroquine. Antinuclear factors were positive with anti-Smith, anti-RNP, and anti-Ro/SSA antibodies. Physical examination revealed slightly erythematous papules and macules of the trunk and the upper limbs. The lesions were nonpruriginous. They were associated with joint pain. She reported similar episodes twice before. The skin biopsy showed an interstitial dermal neutrophilic infiltrate with leukocytoclasia and altered collagen bundles. Direct immunofluoresence testing was negative. She was successfully treated with colchicine. The skin lesions recurred 1 year later, a few days after colchicine was stopped.
NUD Features
Clinical Findings
All patients were female aged between 13 and 45 years. The skin lesions were typically rose or red macules or slightly elevated papules occurring in a wide distribution. The lesions were annular in 1 patient. The face and the extremities were involved in 1 patient. The trunk was involved in 6 patients, upper limbs in 5 patients, and lower limbs or buttocks in 2 patients. The lesions were nonitching in 6 patients and slightly itching in 1 patient. One patient had a Koebner phenomenon. The skin lesions were never photodistributed. Associated signs were joint pain (5 patients), fever (1 patient), abdominal pain (2 patients), chest pain (1 patient), episcleritis (1 patient), vespertilio rash (3 patients), pharyngeal pain (2 patients), and paresthesia of fingers (2 patients). NUD was the first manifestation of LE in 2 cases (patients 1 and 4). The time of evolution of NUD before diagnosis ranged from 1 to 12 years. For patient 5, it occurred in the context of nephrotic syndrome with kidney failure with membranous lupus nephritis (type V according to the International Society of Nephrology/Renal Pathology Society 2003 classification of lupus nephritis).
Histopathological Findings
We observed in all patients an intense neutrophilic interstitial and perivascular infiltrate associated with leukocytoclasia but without significant edema or fibrinoid necrosis of vessel walls. Isolated necrobiotic collagen bundles were found in 3 patients and mild vacuolar change of the basal layer was observed in 1 patient (patient 4). DIF testing performed in 6 patients detected a lupus band in 4 patients and was negative in 2 patients.
Biological Findings
Polyclonal hypergammaglobulinemia was observed in 5 patients. Erythrocytes sedimentation rate was elevated in 3 patients. C-reactive protein was elevated in 3 patients (mean value = 36 mg/L [extremes, 26–44]).
LE Features
Antinuclear antibodies were always positive; anti-dsDNA antibodies were present in 5 patients, and anti-Ro/SSA antibodies in 6 patients. The complement was low in 4 patients and noninterpretable in 1 patient because of a congenital C4 deficiency. Treatments at the time of diagnosis for the 5 patients with a known LE were hydroxychloroquine (but with poor observance in 1 patient), prednisone, and mycophenolate mofetil for 2 patients, and hydroxychloroquine and prednisone for 1 patient. Six patients had SLE according to the SLICC and ACR criteria. Three patients had kidney involvement.
Treatment
The symptomatology of NUD lead to a therapeutic intensification in 4 patients with the introduction of immunosuppressive drugs. Those treatments including prednisone, methotrexate, hydroxychloroquine, mycophenolate mofetil, and azathioprine were always ineffective on skin lesions but could be effective on joint pain (patient 2: methotrexate; patient 4: azathioprine and mycophenolate mofetil). Antihistamines were effective in 1 patient with a delay of 3 weeks. Dapsone or colchicine was effective in 5 patients with no major side effect.
DISCUSSION
We report 7 patients with NUD and SLE, showing that this association is not rare. NUD is characterized by rash, joint pain, and fever and thus was often initially misdiagnosed as a classic lupus flare, leading to introduction of immunosuppressive drugs, a measure that was potentially harmful and inefficient. However, when correct diagnosis was established and treatment with dapsone or colchicine was introduced, control of not only skin lesions but also associated signs was obtained in the majority of cases. Thus, recognition of NUD is important and relevant in clinical practice. Based on the lupus patients followed-up in our institutions, it can be estimated that a little <5% of lupus patients seen at a tertiary dermatology referral center have NUD.
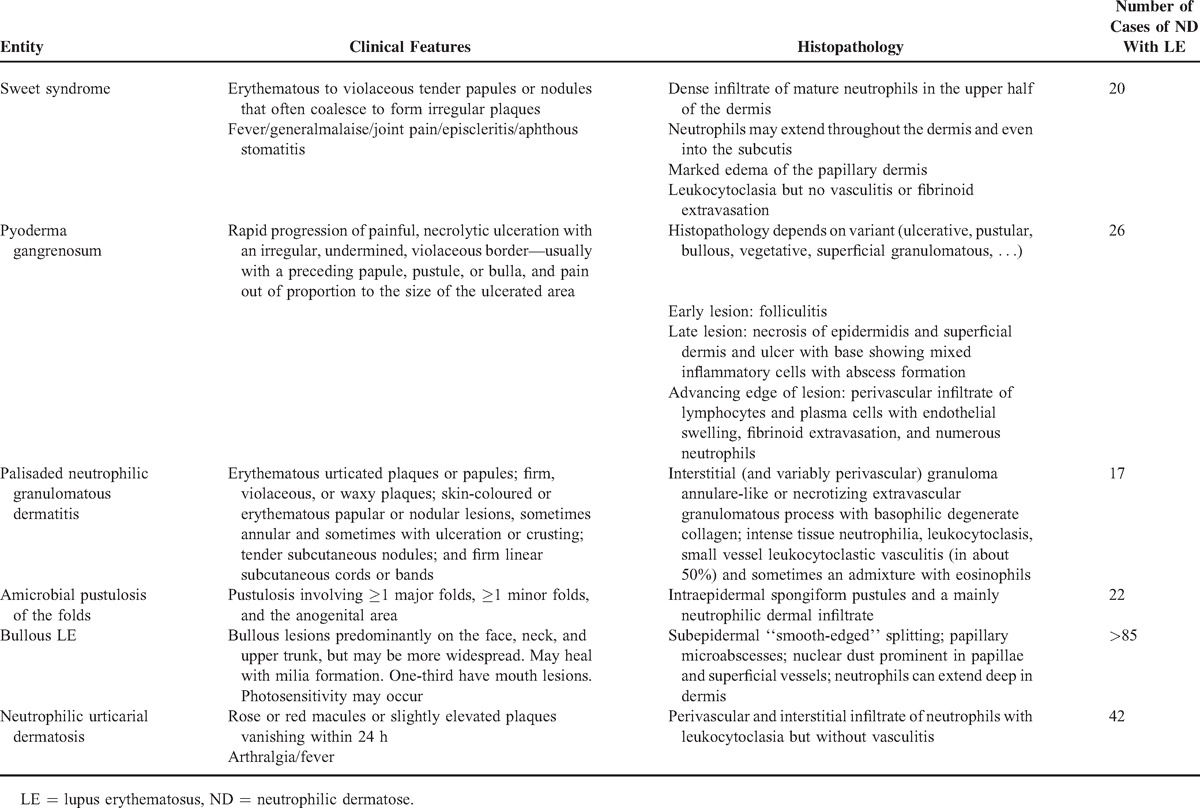
NDs in the setting of LE are frequently encountered. As illustrated in Table 2 and Appendix Table 2, http://links.lww.com/MD/A125, there are already many clinical observations reported in the literature, mainly as case reports: Sweet syndrome (N = 20), pyoderma gangrenosum (N = 26), palisaded neutrophilic granulomatous dermatitis (N = 17), amicrobial pustulosis of the folds (N = 22), and bullous LE (N > 85). More recently, Larson and Granter3 reviewed NDs associated with LE including Sweet-like syndrome, nonbullous neutrophilic LE, NUD, and autoimmunity-related ND: 38 patients were reported under these names including the 3 patients of the Kieffer et al1 study. We believe all those observations belong to the same nosologic spectrum, delineating a subset of lupus patients to which we previously referred as neutrophilic cutaneous LE.6 Those patients have exaggerated innate immune response and it is yet not known if this portends prognostic value for the SLE per se (see below). But the neutrophilic part of the disease needs a specific management, with drugs usually not prescribed for similar symptoms—rash and joint pain—occurring in patients with SLE. Thus, all of those observations exhibit the frequency of ND in the setting of LE and support the idea of including them in a classification of skin involvement of LE.5 As already stated, they are important to recognize because they can be the first manifestation of LE and their management is very peculiar.
Table 1 (Continued).
Summary of the 7 Patients Included in the Study
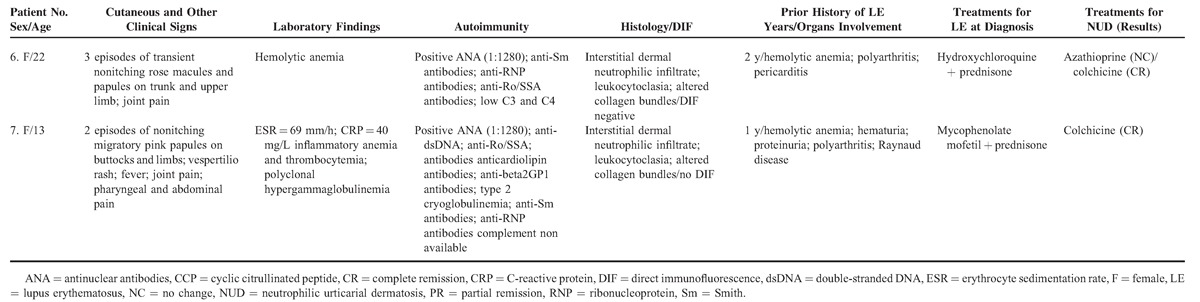
Table 2.
Neutrophilic Dermatosis and Lupus Erythematosus
The rash observed in patients with NUD is very characteristic and should be suspected in patients with pale red macules or only slightly raised nonitching papules vanishing within 24 hours. In this particular case, a dermatological look as well as skin biopsy is crucial, which shows the typical neutrophilic dermal infiltrate. This is all the more important since effective nonimmunosuppressive treatment exists, namely, dapsone or colchicine. Indeed, dapsone, a classic neutrophil migration inhibitor is often used in dermatology to treat other NDs such as dermatitis herpetiformis or pyoderma gangrenosum.7 It has already been reported effective in other LE-associated ND such as bullous LE8 or amicrobial pustulosis of the folds.9 Therapeutic dosages of dapsone range from 50 to 200 mg/d. The lowest effective dose should be given to minimize the possible side effects such as methemoglobinemia, hemolytic anemia, or hypersensitivity syndrome (also referred to as dapsone syndrome10). Before starting the treatment, glucose-6-phosphate dehydrogenase deficiency should be excluded. Regular controls of blood cell count, methemoglobinemia, and liver functions should be done. By analogy with autoinflammatory syndromes,11 pyoderma gangrenosum,12 Sweet syndrome,13 or leukocytoclastic vasculitis,14 colchicine had been used to treat NUD and other LE-associated ND with a good efficacity. It had been effective in 2 of the patients reported here. Therapeutic doses for NUD range from 0.5 to 1 mg/d.15 It may cause nausea, vomiting, diarrhea, and abdominal pain but is most of the time well tolerated.
In our study, dapsone or colchicine was associated with immunosuppressive drugs in 4 patients and colchicine was the only treatment in only 1 patient. Therefore, the real effect of neutrophil migration inhibitors alone is difficult to appreciate. Their use as monotherapy in patients with SLE having NUD needs further study to elucidate their efficacity as sole treatment.
Characteristics of LE in our patients with NUD are heterogeneous. Some patients had only LE-specific skin and/or joint involvement, while others had systemic involvement. Kidney was the most frequently involved organ. It is so far not known if NUD is a prognostic factor or an indicator of systemic disease activity of SLE. In our study, expression of LE varied considerably. In 1 case (patient 5), NUD seemed to be associated with systemic activity leading to renal failure. A kidney biopsy was performed at the time that showed no neutrophil infiltration but a typical lupus glomerulonephritis type V. NUD was the presenting mode of LE in 2 cases. Patient 1 had no systemic involvement to this day. Patient 4 developed a systemic lupus flare with rash, joint pain, and digestive involvement leading to introduction of prednisone. In the study of Pavlidakey et al,16 NUD was the presenting mode in 6 of 7 patients with LE.
Interestingly, the anti-Ro/SSA and/or anti-La/SSB antibodies were positive in 6 of our patients and status was unknown in 1. Regarding the literature, anti-Ro/SSA antibodies were positive in 8 patients, negative in 9 patients, and their status was not specified in 18 patients.16–20 This raises the question whether anti-Ro/SSA antibodies could be related in anyway with NUD and potentially link innate and acquired immunity.
Thus, it will be a challenge in the future to determine if NUD portends a prognostic importance for the specific manifestations of SLE. Meanwhile, its recognition and appropriate treatment to avoid immunosuppressive overtreatment and relieve patients with treatments targeting neutrophils is essential. The association of NUD and SLE is another example of the expanding spectrum of disorders combining autoinflammatory and autoimmune mechanisms and possibly also immune deficiencies although this was not apparent in this series of patients.
CONCLUSION
We describe 7 more new cases of NUD occurring in the setting of LE, a situation that is not exceptional and easily misdiagnosed as an acute lupus flare. We show that conventional immunosuppressive LE treatments are not efficient, at least not alone, and we underline the major interest of dapsone and colchicine.
Footnotes
Abbreviations: ACR = American College of Rheumatology, DIF = direct immunofluorescence, dsDNA = double-stranded DNA, LE = lupus erythematosus, ND = neutrophilic dermatosis, NUD = neutrophilic urticarial dermatosis, RNP = ribonucleoprotein, SLE = systemic lupus erythematosus, SLICC = Systemic Lupus International Collaborating Clinics, Sm = Smith.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Kieffer C, Cribier B, Lipsker D. Neutrophilic urticarial dermatosis; a variant of neutrophilic urticaria strongly associated with systemic disease. Report of 9 new cases and review of the literature. Medicine (Baltimore) 2009; 88:23–31. [DOI] [PubMed] [Google Scholar]
- 2.Lipsker D. Classification of specific manifestations of cutaneous lupus erythematosus: a time for change? The concept of dermal lupus erythematosus. Dermatology 2006; 212:324–326. [DOI] [PubMed] [Google Scholar]
- 3.Larson AR, Granter SR. Systemic lupus erythematosus-associated neutrophilic dermatosis: a review and update. Adv Anat Pathol 2014; 21:248–253. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman AB. Histologic Diagnosis of Inflammatory Skin Diseases: An Algorithmic Method Based on Pattern Analysis. 2nd ed1997; Baltimore, MD: Williams & Wilkins, 525–543. [Google Scholar]
- 5.Lipsker D. The need to revisit the nosology of cutaneous lupus erythematosus: the current terminology and morphologic classification of cutaneous LE: difficult, incomplete and not always applicable. Lupus 2010; 19:1047–1049. [DOI] [PubMed] [Google Scholar]
- 6.Lipsker D, Saurat JH. Neutrophilic cutaneous lupus erythematosus. At the edge between innate and acquired immunity? Dermatology 2008; 216:283–286. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 2001; 45:420–434. [DOI] [PubMed] [Google Scholar]
- 8.Ludgate MW, Greig DE. Bullous systemic lupus erythematosus responding to dapsone. Australas J Dermatol 2008; 49:91–93. [DOI] [PubMed] [Google Scholar]
- 9.Oberlin P, Bagot M, Perrussel M, et al. Amicrobial pustulosis and systemic lupus erythematosus. Ann Dermatol Venereol 1991; 118:824–825. [PubMed] [Google Scholar]
- 10.Allday EJ, Barnes J. Toxic effects of diaminodiphenylsulphone in treatment of leprosy. Lancet 1951; 258:205–206. [DOI] [PubMed] [Google Scholar]
- 11.Kallinich T, Haffner D, Niehues T, et al. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics 2007; 119:474–483. [DOI] [PubMed] [Google Scholar]
- 12.Kontochristopoulos GJ, Stavropoulos PG, Gregoriou S, et al. Treatment of pyoderma gangrenosum with low-dose colchicine. Dermatology 2004; 209:233–236. [DOI] [PubMed] [Google Scholar]
- 13.Maillard H, Leclech C, Peria P, et al. Colchicine for Sweet's syndrome: a study of 20 cases. Br J Dermatol 1999; 140:565–566. [DOI] [PubMed] [Google Scholar]
- 14.Callen JP. Colchicine is effective in controlling chronic cutaneous leukocytoclastic vasculitis. J Am Acad Dermatol 1985; 13:193–200. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan TP, King LE, Jr, Boyd AS. Colchicine in dermatology. J Am Acad Dermatol 1998; 39:993–999. [DOI] [PubMed] [Google Scholar]
- 16.Pavlidakey P, Mills O, Bradley S, et al. Neutrophilic dermatosis revisited: an initial presentation of lupus? J Am Acad Dermatol 2012; 67:e29–e35. [DOI] [PubMed] [Google Scholar]
- 17.Gleason BC, Zembowicz A, Granter SR. Non-bullous neutrophilic dermatosis: an uncommon dermatologic manifestation in patients with lupus erythematosus. J Cutan Pathol 2006; 33:721–725. [DOI] [PubMed] [Google Scholar]
- 18.Larson AR, Granter SR. Systemic lupus erythematosus-associated neutrophilic dermatosis—an under-recognized neutrophilic dermatosis in patients with systemic lupus erythematosus. Hum Pathol 2014; 45:598–605. [DOI] [PubMed] [Google Scholar]
- 19.Brinster NK, Nunley J, Pariser R, et al. Nonbullous neutrophilic lupus erythematosus: a newly recognized variant of cutaneous lupus erythematosus. J Am Acad Dermatol 2012; 66:92–97. [DOI] [PubMed] [Google Scholar]
- 20.Saeb-Lima M, Charli-Joseph Y, Rodriguez-Acosta ED, et al. Autoimmunity-related neutrophilic dermatosis: a newly described entity that is not exclusive of systemic erythematosus. Am J Dermatopathol 2013; 35:655–660. [DOI] [PubMed] [Google Scholar]


