Supplemental Digital Content is available in the text
Abstract
Obstructive sleep apnea (OSA) is seen in approximately 60% to 70% of patients with stroke or ischemic heart disease (IHD). The relationship between OSA and recurrent vascular events and all-cause mortality remains inconclusive. We aimed to systematically evaluate the associations between OSA and serious adverse outcomes following stroke or IHD by a meta-analysis of prospective cohort studies.
We searched MEDLINE and EMBASE in August 2014 for studies that evaluated the prospective associations between OSA and the risk of stroke, IHD, and death among stroke or IHD patients. Outcome data were pooled using random effects meta-analysis. Subgroup, heterogeneity, and sensitivity analyses and publication bias were performed.
Of 13 hospital-based cohort studies included, 5 reported results on stroke (n = 860), 6 reported on IHD (n = 1083), and 11 reported on all-cause death or cardiovascular death (n = 1930). OSA was significantly associated with the risk of stroke, IHD, and all-cause mortality after stroke or IHD. The pooled relative risks were 1.94 (95% confidence interval [CI], 1.29–2.92) for stroke, 1.83 (95% CI, 1.15–2.93) for IHD, and 1.59 (95% CI, 1.33–1.89) for all-cause mortality. There was no evidence of significant between-study heterogeneity. The results did not materially change in the sensitivity analyses for the outcomes of stroke and all-cause mortality.
OSA may be a significant predictor of serious adverse outcomes following stroke or IHD. More high-quality studies are needed to determine if better treatment of OSA leads to fewer recurrent vascular events, especially a large-scale, multicenter randomized-controlled trial.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive interruption of ventilation during sleep caused by collapse of the pharyngeal airway and affects approximately 18% of middle-aged adults.1,2 A recent meta-analysis that combined the results from 9 prospective cohort studies reported that OSA was significantly associated with stroke in participants without previous cardiovascular disease.3 Furthermore, previous observational studies suggested that long-term continuous positive airway pressure (CPAP) treatment improved cardiovascular risk in patients with OSA.4,5 Compared with the general population, patients with stroke or ischemic heart disease (IHD) have a 3- to 4-fold higher prevalence of OSA, approximately 60% to 70%.6,7 It is possible that treating OSA could improve clinical recovery in those patients.8 Therefore, OSA has been considered as a potential new treatment target for patients with stroke or IHD.1,8,9 However, whether OSA increases risk of recurrent cardiovascular events remains debated.
So far, multiple studies have sought to demonstrate whether OSA increases the risk of recurrence and death after cardiovascular disease, but the results were inconsistent and the sample sizes of most studies were relatively small. Moreover, as far as we know, there is no study available that evaluates the associations between OSA and serious adverse outcomes in patients with stroke or IHD through meta-analysis. Considering the high prevalence of OSA in those patients and its treatability, we aimed to perform a systematic review and meta-analysis to identify whether OSA increases the risk of recurrence and death after stroke or IHD.
METHODS
The methodology for this study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10 Ethical approval was not necessary for this meta-analysis as the results for publication only involved deidentified pooled data from individual studies that have received ethics approval.
Study Eligibility Criteria
Studies were eligible for inclusion if they met the following criteria: English language articles; prospective studies that recruited patients who had been diagnosed with stroke or IHD; the diagnosis of OSA was based on apnea-hypopnea index (AHI), which was obtained by polysomnography (AHI is defined as the frequency of apneas and hypopneas/hour of sleep)1; follow-up duration of ≥6 months after stroke or IHD; reporting the results of our interest outcomes, including stroke, IHD, and all-cause or cardiovascular mortality.
Search Strategy
A systematic search was performed on August 25, 2014 by searching MEDLINE and EMBASE (OvidSP). Details of the search strategy are provided in Table 1. Initially, titles alone were reviewed for suitability. The abstracts of suitable titles were obtained, and these were then reviewed for suitability for full-text retrieval. Data were then extracted as described in the following text from suitable full-text reports. Additional appropriate reports were added when discovered by citation tracking.
TABLE 1.
Search Strategy: Searching MEDLINE and EMBASE (Ovid SP) on August 25, 2014

Data Extraction
References were screened and data were extracted independently by 2 authors (FZ and XS) using a predetermined data collection template. To resolve discrepancies about inclusion of studies and interpretation of data, a third investigator (WX) was consulted, and consensus was reached by discussion. The following data were recorded: first author's last name, year of publication, location of study, inclusion and exclusion criteria, sample size, demographic details of patients, follow-up period, and outcomes.
Validity Assessment
The quality of all included studies was assessed using a modified scoring system, which allowed a total score of 0 to 6 points (6 refers to the highest quality) on the basis of the PRISMA statement.10 The system allocated 1 point for each of the following: appropriate inclusion and exclusion criteria; diagnosis of stroke, IHD, and all-cause mortality based on accepted clinical criteria; OSA assessed with a standard polysomnography; sample size >150; follow-up duration >3 years; adjustments made for age, sex, body mass index, diabetes, hypertension, and smoking.
Statistical Analyses
Relative risk (RR) was used as the estimate effect of OSA on the risk of stroke, IHD, and all-cause mortality across studies. Hazard ratio (HR) was directly considered as RR. When a study did not report HR or RR of the outcomes of interest, we calculated the unadjusted RR based on the original data of events by ourselves.
For meta-analysis of stroke, the outcomes included were stroke and transient ischemic attack. For IHD, we included outcomes that were reported as myocardial infarction, angina, revascularization, and acute coronary syndrome. For mortality, outcomes included all-cause mortality and cardiovascular death. In addition, we performed a separate meta-analysis to evaluate the effect of CPAP treatment on adverse outcomes following stroke or IHD in OSA patients.
Data were analyzed within a random-effect framework that takes study heterogeneity into account to generate the pooled RR and 95% confidence interval (CI). The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic. Potential publication bias was assessed with the Egger test and represented graphically with funnel plots of the natural log of the RR versus its standard error.11 A 2-sided P value of <0.05 was judged significant for all analyses. All statistical meta-analyses were done with STATA (version 11; Stata Corp, College Station, TX).
RESULTS
Study Selection
From a total of 2906 reports in the initial search, 46 articles met the inclusion criteria and were selected for detailed assessment. Thirty-three studies were further excluded because of studies that did not use AHI to diagnose OSA, studies that investigated in-hospital outcomes, duplicated cohorts, community-based cohort studies, or cross-sectional studies. Finally, a total of 13 studies12–24 were included in this meta-analysis. Figure 1 shows the study selection process according to the PRISMA statement.10
FIGURE 1.
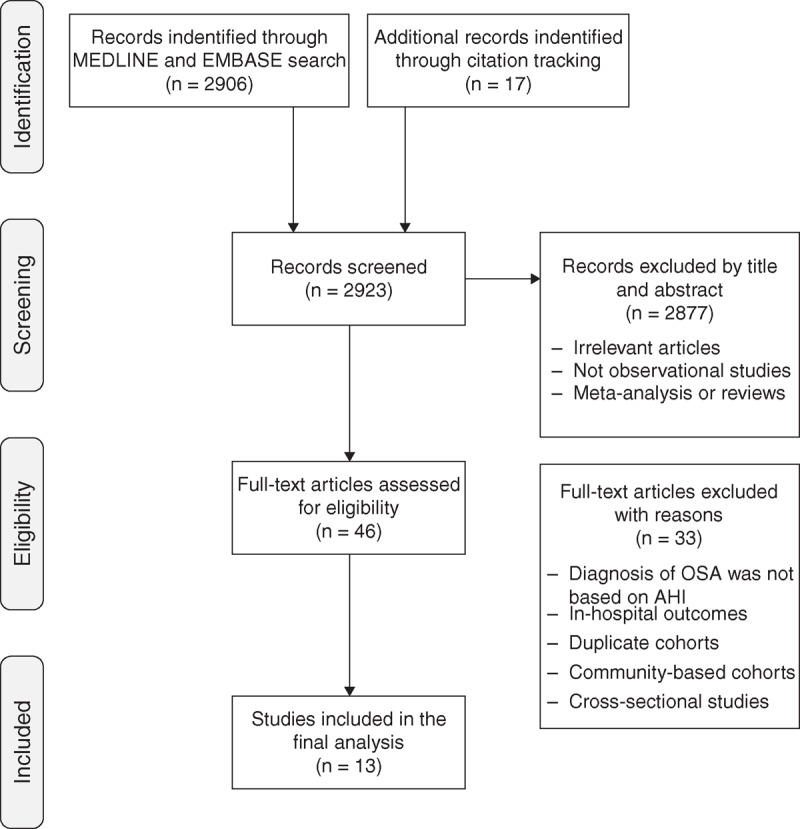
Study selection flow diagram adapted from the PRISMA statement. AHI = apnea-hypopnea index, OSA = obstructive sleep apnea, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study Characteristics
Characteristics of the 13 included studies are summarized in Table 2. Of the 13 hospital-based cohort studies, participants of 6 studies12,14,17,19–21 were stroke patients and the other 7 studies13,15,16,18,22–24 included IHD patients. In total, 5 studies12,14,18,19,22 (n = 860) reported the risk of stroke, 6 studies13,14,16,18,22,24 (n = 1083) reported IHD risk, and 11 studies (n = 1930) reported all-cause mortality12,14–17,19–23 or cardiovascular death.18 Six studies scored ≥5 (relatively high quality), 3 studies scored equal to 4, and 4 studies scored equal to 3 (relatively low quality).
TABLE 2.
Characteristics of Included Cohort Studies
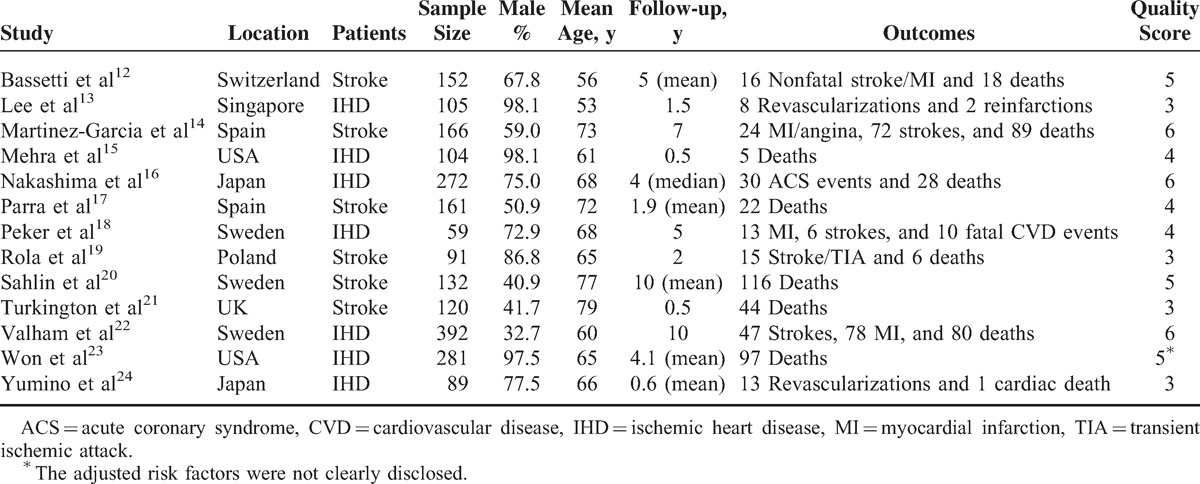
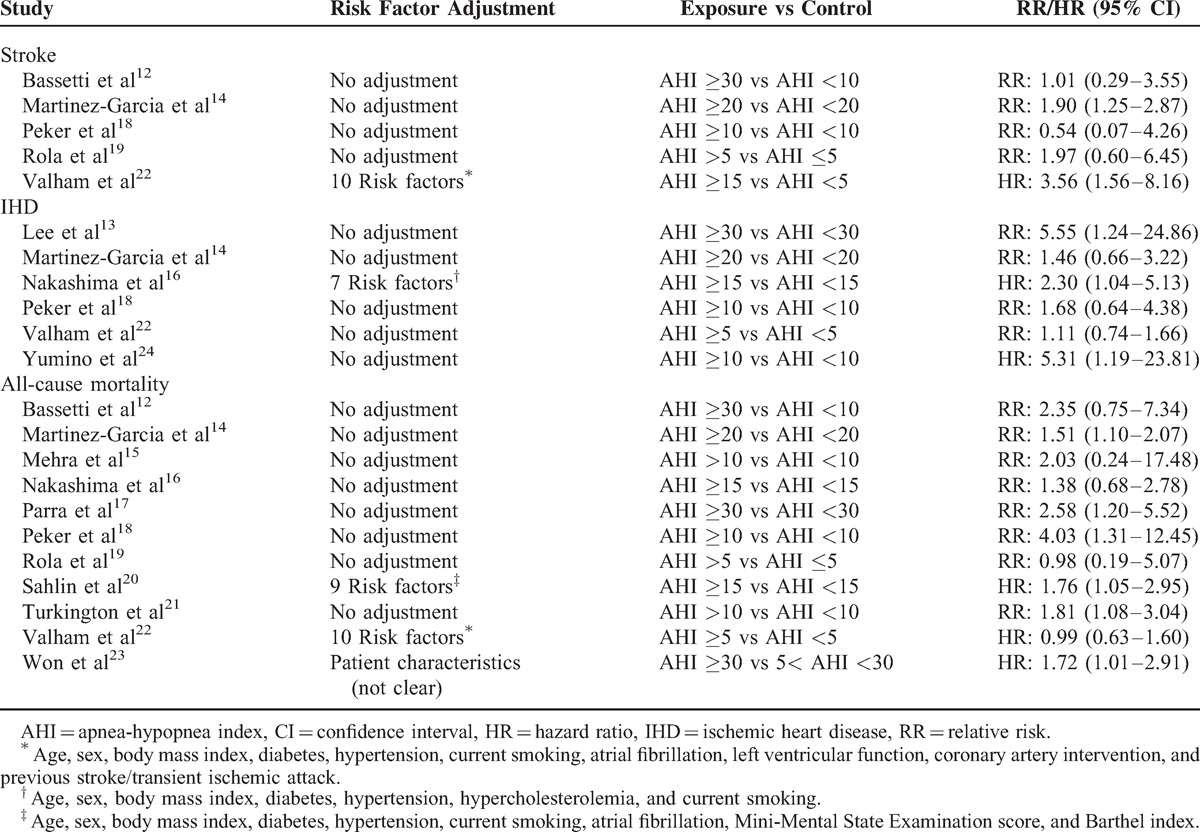
Table 3 presents the results of the associations between OSA and 3 different outcomes from the 13 studies. Most of the studies did not report the adjusted HRs needed for our meta-analyses. In this scenario, we calculated the unadjusted RR based on the original data. The key inclusion and exclusion criteria of the 13 studies are described in Supplementary Table S1, http://links.lww.com/MD/A116. Outcomes were assessed mainly from interviews, hospital discharge registries, death certificates, and medical records.
TABLE 3.
Adjusted Risk Factors and Data Extraction of Included Cohort Studies
OSA and the Risk of Stroke
Five studies were included in the meta-analysis for the associations of OSA with the risk of stroke. Of these, 3 studies12,14,19 were conducted in stroke patients and 2 studies18,22 were conducted in IHD patients. The RRs for the association varied from 0.54 to 3.57 across studies. Overall, OSA was significantly associated with the risk of stroke in patients with stroke or IHD (RR = 1.94; 95% CI, 1.29–2.92; P = .001). There was no evidence of significant between-study heterogeneity (I2 = 13.2%, P = .33, Figure 2).
FIGURE 2.
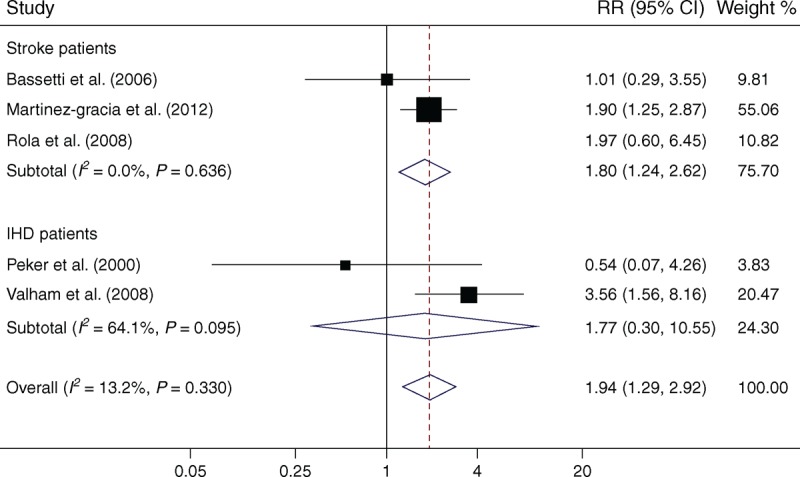
Meta-analysis of OSA and associated risk of stroke in patients with stroke or IHD. Squares represent point estimates for effect size expressed as a RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, IHD = ischemic heart disease, OSA = obstructive sleep apnea, RR = relative risk.
Subgroup analysis by patient type found a significant RR, 1.80 (95% CI, 1.24–2.62; P = .002) in stroke patients, with no evidence of statistical heterogeneity (I2 = 0.0%, P = .64, Figure 2).
OSA and the Risk of IHD
Five studies13,16,18,22,24 in IHD patients and 1 study14 in stroke patients were included in the pooled analysis. OSA was also found to be a significant risk factor for IHD in patients with IHD or stroke (RR = 1.83; 95% CI, 1.15–2.93; P = .011). No evidence of significant between-study heterogeneity was found (I2 = 44.2%, P = .11, Figure 3).
FIGURE 3.
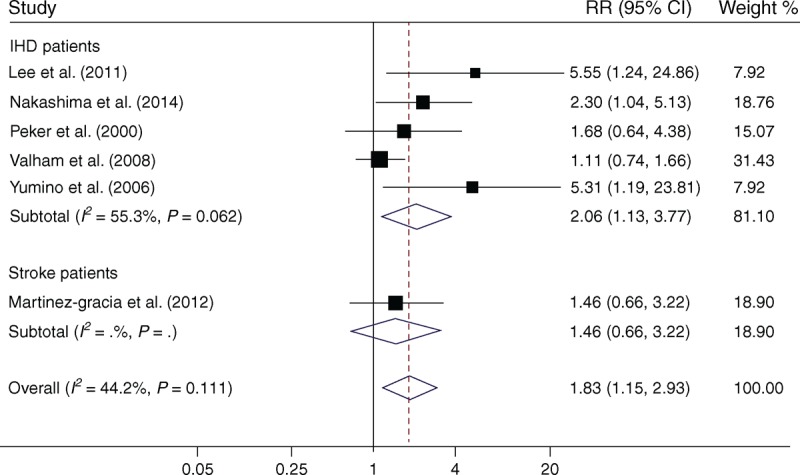
Meta-analysis of OSA and associated risk of IHD in patients with IHD or stroke. Squares represent point estimates for effect size expressed as a RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, IHD = ischemic heart disease, OSA = obstructive sleep apnea, RR = relative risk.
When only samples from IHD patients were included, there was positive evidence for association between OSA and recurrent risk of IHD (RR = 2.06; 95% CI, 1.13–3.77; P = .02), with moderate but nonsignificant heterogeneity (I2 = 55.3%, P = .062, Figure 3).
OSA and All-Cause Mortality
Eleven studies were included in the meta-analysis, 6 were12,14,17,19–21 in stroke patients and 5 were15,16,18,22,23 in IHD patients. The total number of the patients was 1930 with 515 deaths. Overall, OSA significantly increased the all-cause mortality (RR = 1.59; 95% CI, 1.33–1.89; P < .001). There was no evidence of heterogeneity across studies (I2 = 0.0%, P = .47, Figure 4).
FIGURE 4.
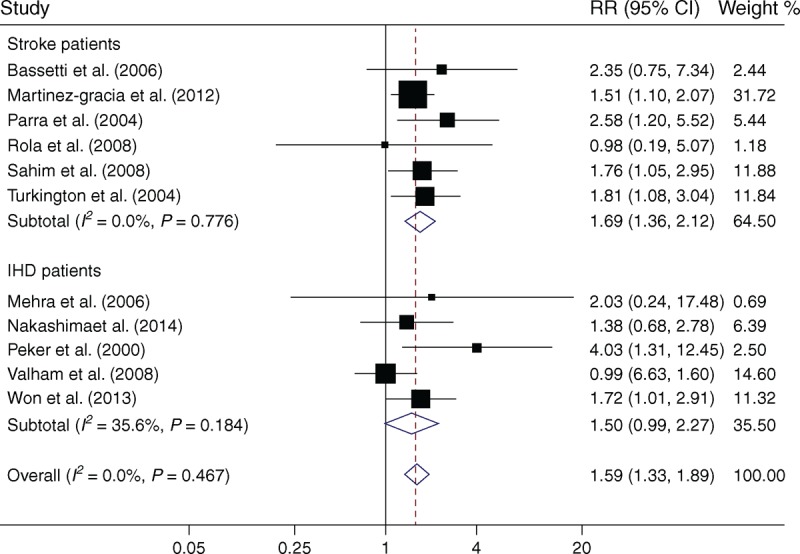
Meta-analysis of OSA and all-cause mortality in patients with stroke or IHD. Squares represent point estimates for effect size expressed as a RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, IHD = ischemic heart disease, OSA = obstructive sleep apnea, RR = relative risk.
Subgroup analysis showed that the association was significant in stroke patients (RR = 1.69; 95% CI, 1.36–2.12; P < .001), with no evidence of heterogeneity (I2 = 0.0%, P = .78). However, the association did not achieve significance in IHD patients (RR = 1.50; 95% CI, 0.99–2.27; P = .053, Figure 4).
Effect of CPAP Treatment on Adverse Outcomes
Three studies14,16,23 reported the specified results of outcomes by CPAP treatment in OSA patients and included in the meta-analysis. Supplementary Table S2, http://links.lww.com/MD/A116 shows the extracted data from the 3 studies. As shown in Figure 5, the treatment of OSA by CPAP in patients with stroke or IHD would significantly reduce the risk of adverse outcomes (RR = 0.41; 95% CI, 0.25–0.66; P < .001), without heterogeneity (I2 = 0.0%, P = .93).
FIGURE 5.
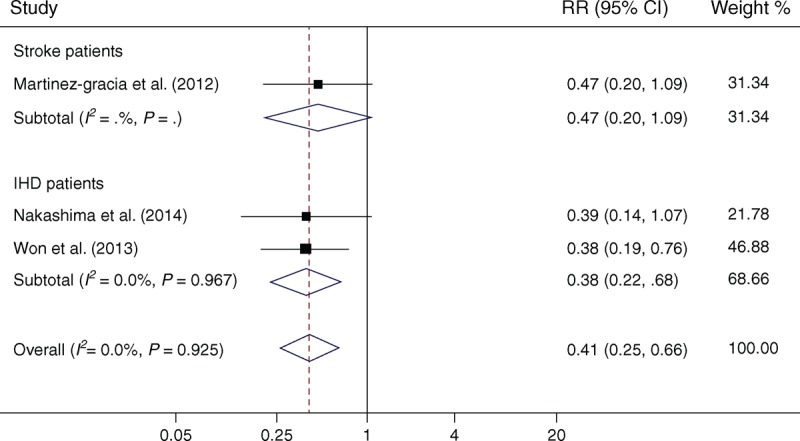
Meta-analysis of the effect of CPAP treatment on adverse outcomes following stroke or IHD in OSA patients. Squares represent point estimates for effect size expressed as a RR with the size proportional to the inverse variance of the estimate. Diamond represents pooled estimate. Lines represent 95% CIs. CI = confidence interval, CPAP = continuous positive airway pressure, IHD = ischemic heart disease, OSA = obstructive sleep apnea, RR = relative risk.
Sensitivity Analyses
To assess the effect of variations in quality, as well as possible sources of heterogeneity, a number of sensitivity analyses were conducted. Table 4 presents the results of sensitivity analyses according to various inclusion criteria. Overall, the pooled risk estimates did not materially change in the sensitive analyses for the outcomes of stroke and all-cause mortality. The pooled RRs for IHD were no longer significant in the analyses restricting to studies that have a sample size >150, follow-up duration >3 years, and quality score ≥4.
TABLE 4.
Results of Sensitivity Analyses According to Various Inclusion Criteria
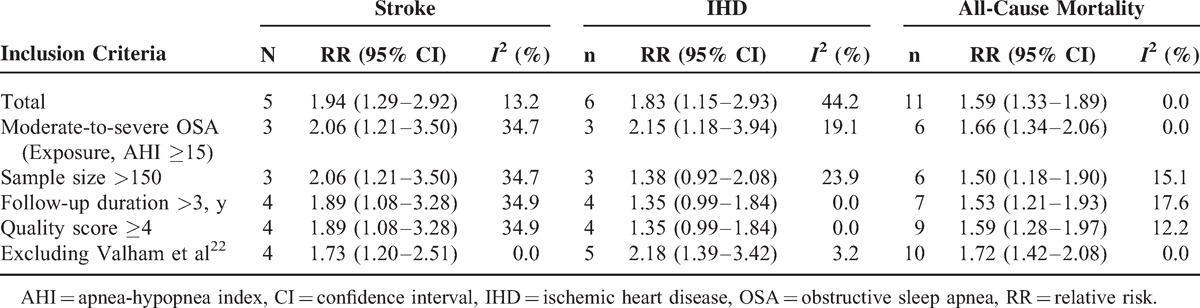
When excluding the study conducted by Valham et al22 from the analyses, the between-study heterogeneities were significantly reduced for the outcomes of IHD (from 44.2% to 3.2%) and stroke (from 13.2% to 0.0%, Table 4).
Publication Bias
There was no evidence of publication bias on the basis of either visual inspection of the funnel plots (Supplementary Figures S1 and S2, http://links.lww.com/MD/A116) or Egger test for the outcomes of stroke and all-cause mortality (P = 0.51 and P = 0.26, respectively). However, funnel plot (Supplementary Figure S3, http://links.lww.com/MD/A116) and Egger test suggested the presence of publication bias for the outcome of IHD (P = .006). Contour-enhanced funnel plot indicated that 3 supposed missing studies were in areas of statistical nonsignificance (P > 0.05) and relative risk <1 (Supplementary Figure S4, http://links.lww.com/MD/A116). This suggests that the asymmetry is likely because of publication bias.
DISCUSSION
The results of the present meta-analysis demonstrated that OSA is associated with a significant increase in the risk of stroke, IHD, and all-cause mortality among patients with stroke or IHD. As OSA affects approximately 60% to 70% of the cardiovascular patients,6,7 its impact on cardiovascular burden is likely to be substantial. Our results suggest that OSA should be considered as a potential treatment target that might improve outcome and survival following stroke or IHD.
Three previous meta-analyses were performed to evaluate the association between OSA and cardiovascular risk, and the results were consistent. In the general population, OSA was significantly associated with stroke but not with IHD.3,25,26 However, to the best of our knowledge, there is no meta-analysis that evaluates the associations between OSA and serious adverse outcomes following stroke or IHD in terms of recurrent vascular events and all-cause mortality. Although previous observational studies of CPAP in general OSA populations reported statistically significant reductions in cardiovascular risk associated with CPAP compared with no CPAP,4,5 screening all participants may not be cost-effective given the costs of polysomnography. Taking into account higher prevalence of OSA and higher risk of cardiovascular events, it is rational to believe that screening for OSA in cardiovascular patients is more cost-effective.27
The mechanisms behind the increased risk of cardiovascular events in patients with OSA are still uncertain. A scientific statement, published by the American Heart Association/American College of Cardiology, indicated following 9 potential mechanisms at play: sympathetic activation, cardiovascular variability, vasoactive substances, inflammation, oxidative stress, endothelial dysfunction, insulin resistance, thrombosis, and intrathoracic pressure changes.1 A recent review by Kohler and Stradling28 pointed out 3 biological mechanisms underlying the association of OSA with cardiovascular disease: intermittent hypoxia leading to increased oxidative stress, systemic inflammation, and sympathetic activity; intrathoracic pressure changes leading to excessive mechanical stress on the heart and large artery walls; and arousal-induced reflex sympathetic activation with resultant repetitive blood-pressure rises.
Based on this systematic review and meta-analysis, OSA could be identified as a risk factor for recurrence and mortality following vascular events, and treatment of OSA by CPAP would significantly reduce the risk of adverse outcomes. However, few randomized-controlled trials (RCTs) evaluated the effect of CPAP on recurrence and mortality among cardiovascular patients. Our literature review identified only 1 small RCT (n = 126) evaluating the effect of CPAP on cardiovascular risk in stroke patients. No significant improvement in cardiovascular mortality was reported at 24 months (0% in the CPAP group vs 4.3% in the control group, P = .161).29 More RCTs are needed to determine the effect of CPAP on the risk of recurrent cardiovascular events after stroke or IHD. Given the relatively low enrollment and completion rates, no single center is likely to recruit a large enough sample. Therefore, a large-scale, multicenter trial may be the only way to find out whether CPAP helps these patients.
The major advantages of this meta-analysis included the prospective cohort studies, enlarged sample sizes, the robust results from sensitivity analysis, and no significant between-study heterogeneity. However, there are some limitations that should be noted. First, most RRs for meta-analyses were calculated by us and not adjusted for traditional cardiovascular risk factors because the original studies did not report the adjusted estimates. Thus, we acknowledge the possibility that confounding could affect the results. Second, our systematic review was limited to English-language studies only, which can introduce selection bias. A relevant article in Chinese identified from the published Chinese literature was not included in this study.30 We also cannot rule out the possibility that there are other relevant articles published in languages other than English or Chinese. However, we consider that the possibility of selection bias is reduced by the relatively large number of studies available in English. In addition, previous studies have demonstrated that excluding studies published in languages other than English has generally little effect on summary effect estimates.31,32 Third, the ranges of AHI among both exposure and control groups are inconsistent across studies. Originally, we planned to conduct separate meta-analyses for the associations between OSA severity (ie, mild, moderate, and severe OSA) and outcomes. However, most studies did not report the specified results according to OSA categories because of small sample size. Similarly, the limitation has also been found in other meta-analyses.3,26,33 We perform sensitivity analyses to solve this limitation, at least to a certain degree. In the analyses restricting to studies that have AHI ≥15 in exposure groups, the pooled risk estimates did not materially change. Fourth, all of the included studies were hospital-based, which may lead to selection bias and limit the generalization of the findings. Finally, as with any meta-analyses, publication bias remains a threat to the validity of our results.
In summary, based on this systematic review and meta-analysis, we suggest that OSA may be a significant predictor of serious adverse outcomes following stroke or IHD in terms of recurrent vascular events and all-cause mortality. More high-quality studies are needed to determine if better treatment of OSA leads to fewer recurrent vascular events in stroke or IHD patients, especially a large-scale, multicenter RCT.
Footnotes
Abbreviations: AHI = apnea-hypopnea index, CI = confidence interval, CPAP = continuous positive airway pressure, HR = hazard ratio, IHD = ischemic heart disease, OSA = obstructive sleep apnea, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized-controlled trial, RR = relative risk.
WX and FZ contributed equally to this work.
This study was funded by the National Natural Science Foundation of China (contract no. 81302503). The fund provider had no role in study design, data collection and analysis, decision to publish, and preparation of the manuscript. No additional external funding was received for this study.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008; 118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 3.Loke YK, Brown JW, Kwok CS, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012; 5:720–728. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 5.Doherty LS, Kiely JL, Swan V, et al. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005; 127:2076–2084. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010; 6:131–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Stern R. Discordance of individual risk estimates. J Am Coll Cardiol 2010; 56:743.Author reply 743–744. [DOI] [PubMed] [Google Scholar]
- 8.Tomfohr LM, Hemmen T, Natarajan L, et al. Continuous positive airway pressure for treatment of obstructive sleep apnea in stroke survivors: what do we really know? Stroke 2012; 43:3118–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minnerup J, Schabitz WR. Improving outcome after stroke: time to treat new targets. Stroke 2012; 43:295–296. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006; 37:967–972. [DOI] [PubMed] [Google Scholar]
- 13.Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med 2011; 7:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Garcia MA, Campos-Rodriguez F, Soler-Cataluna JJ, et al. Increased incidence of nonfatal cardiovascular events in stroke patients with sleep apnoea: effect of CPAP treatment. Eur Respir J 2012; 39:906–912. [DOI] [PubMed] [Google Scholar]
- 15.Mehra R, Principe-Rodriguez K, Kirchner HL, et al. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med 2006; 7:521–528. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima H, Kurobe M, Minami K, et al. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care 2014; [Published online ahead of print date: May 22, 2014]. http://acc.sagepub.com/content/early/2014/05/20/2048872614530865 Accessed August 26, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Parra O, Arboix A, Montserrat JM, et al. Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J 2004; 24:267–272. [DOI] [PubMed] [Google Scholar]
- 18.Peker Y, Hedner J, Kraiczi H, et al. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med 2000; 162:81–86. [DOI] [PubMed] [Google Scholar]
- 19.Rola R, Jarosz H, Wierzbicka A, et al. Sleep disorderd breathing and recurrence of cerebrovascular events, case-fatality, and functional outcome in patients with ischemic stroke or transient ischemic attack. J Physiol Pharmacol 2008; 59 suppl 6:615–621. [PubMed] [Google Scholar]
- 20.Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med 2008; 168:297–301. [DOI] [PubMed] [Google Scholar]
- 21.Turkington PM, Allgar V, Bamford J, et al. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax 2004; 59:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valham F, Mooe T, Rabben T, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation 2008; 118:955–960. [DOI] [PubMed] [Google Scholar]
- 23.Won CH, Chun HJ, Chandra SM, et al. Severe obstructive sleep apnea increases mortality in patients with ischemic heart disease and myocardial injury. Sleep Breath 2013; 17:85–91. [DOI] [PubMed] [Google Scholar]
- 24.Yumino D, Tsurumi Y, Takagi A, et al. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007; 99:26–30. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Ouyang Y, Wang Z, et al. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 169:207–214. [DOI] [PubMed] [Google Scholar]
- 26.Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta-analysis of prospective cohort studies. Atherosclerosis 2013; 229:489–495. [DOI] [PubMed] [Google Scholar]
- 27.Brown DL, Chervin RD, Hickenbottom SL, et al. Screening for obstructive sleep apnea in stroke patients: a cost-effectiveness analysis. Stroke 2005; 36:1291–1293. [DOI] [PubMed] [Google Scholar]
- 28.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 2010; 7:677–685. [DOI] [PubMed] [Google Scholar]
- 29.Parra O, Sanchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011; 37:1128–1136. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Y, Gao X. Study on the relationship between obstructive sleep apnea hypopnea syndrome and acute cerebral infarction prognosis. Chinese General Practice 2012; 15:2616–2618. [Google Scholar]
- 31.Juni P, Holenstein F, Sterne J, et al. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 2002; 31:115–123. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol 2000; 53:964–972. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Hou WS, Zhang XW, et al. Obstructive sleep apnea and risk of stroke: a meta-analysis of prospective studies. Int J Cardiol 2014; 172:466–469. [DOI] [PubMed] [Google Scholar]


