Abstract
Although hematogenous pyogenic spinal infections have been related to hemodialysis (HD), catheter-related sepsis, and sporadically, to other nosocomial infections or procedures, in most recent studies and reviews the impact of nosocomial infection as a risk factor for vertebral osteomyelitis (VO) is not well established. The aim of our study was to describe the risk factors, infectious source, etiology, clinical features, therapy, and outcome of health care associated VO (HCAVO), and compare them with community-acquired VO (CAVO) cases.
A retrospective cohort study of consecutive patients with hematogenous VO was conducted in our third-level hospital between 1987 and 2011. HCAVO was defined as onset of symptoms after 1 month of hospitalization or within 6 months after hospital discharge, or ambulatory manipulations in the 6 months before the diagnosis.
Over the 25-year study period, among 163 hematogenous pyogenic VO, 41 (25%) were health care associated, a percentage that increased from 15% (9/61) in the 1987–1999 period to 31% (32/102) in the 2000–2011 period (P < 0.01). The presumed source of infection was an intravenous catheter in 14 (34%), cutaneous foci in 8 (20%), urinary tract in 7 (17%), gastrointestinal in 3 (7%), other foci in 3 (7%), and unknown in 6 (15%). Staphylococcus aureus was the most frequently isolated microorganism (14 cases, 34%), followed by coagulase-negative Staphylococci (CoNS) in 6 (15%), and Enterobacteriaceae in 6 (15%) cases.
Compared with CAVO cases, patients with HCAVO were older (mean 66.0 SD 13.0 years vs 60.5 SD 15.5 years), had more underlying conditions (73% vs 50%, P < 0.05), neoplasm/immunosuppression (39% vs 7%, P < 0.005), chronic renal failure (19% vs 4%, P < 0.001), a known source of infection (85% vs 54% P < 0.05), Candida spp (7% vs 0%, P < 0.01) or CoNS infections (15% vs 2%, P < 0.05), higher mortality (15% vs 6%, P = 0.069), and a higher relapse rate in survivors (9% vs 1%, P < 0.05).
Presently, in our setting, one-third of hematogenous pyogenic VO infections are health care associated, and a third of these are potentially preventable catheter-related infections. Compared with CAVO, in health care associated hematogenous VO, mortality and relapse rates are higher; hence, further prevention measures should be assessed.
INTRODUCTION
Spontaneous hematogenous pyogenic vertebral osteomyelitis (VO) is an uncommon disease with an estimated incidence of 0.2 to 2.4 cases per 100,000 population/y.1–20 Previously described risk factors for VO are older age (incidence 6.5–9.8/100,000 population/y), diabetes mellitus, malignancy, immunosuppression, liver disease, end-stage renal failure, liver cirrhosis, intravenous drug use, and previous infection, particularly bloodstream infection.1–10,21–24 Recently, spinal infections have also been related to hemodialysis (HD),14,20,25 catheter-related sepsis,1,2,7,10,16,20,26 and sporadically, other nosocomial infections or procedures.3,13–16,20,26
Most recent studies and reviews have not mentioned nosocomial infection as a risk factor for VO. Nonetheless, in a small series of 20 VO cases published 18 years ago, Torda et al26 found that the infection was of nosocomial acquisition in 60% of patients, and a recent review has suggested that nosocomial infection may have contributed to the increase in spinal infections3. In our previous study10 and 1 by other authors,14 VO was considered nosocomial-acquired in around 19% of cases. Available data on the impact, source of infection, clinical findings, and outcome of health care associated hematogenous spinal infections, however, are limited.
The aim of this study was to describe the risk factors, infectious source, etiology, clinical features, therapy, and outcome of health care associated hematogenous pyogenic VO (HCAVO), and compare the findings with those of community-acquired VO (CAVO) cases.
PATIENTS AND METHODS
Study Design, Patients, and Settings
In this retrospective review of all consecutive VO cases diagnosed at Vall d’Hebron Hospital, a tertiary referral center for complex vertebral surgery patients, patients with previous spinal instrumentation or surgery, tuberculosis (n = 53), brucellosis (n = 13), or no definitive bacteriological diagnosis (n = 16) were excluded. All consecutive adult patients (≥18 years) diagnosed with VO from January 1987 to December 2011 were enrolled. Patients were identified from our infectious diseases department VO registry, and most of them were followed-up by one of the authors (C.P.).
Data Collection
Demographic, clinical, diagnostic, treatment, and follow-up data were obtained by chart abstraction and entered in a database created specifically for the study. To assess the effectiveness of antibiotic and surgical therapy, only survivor patients followed-up for at least 1.5 years to detect relapses after therapy completion were included.
Definitions
The diagnosis of hematogenous pyogenic VO was established on previously published criteria,10 which include absence of prior surgery, spinal instrumentation or contiguous VO, and presence of 3 criteria: compatible clinical picture; consistent imaging findings on computed tomography or magnetic resonance imaging, or increased uptake on technetium bone scan; isolation of pyogenic microorganisms in blood culture or samples obtained by open surgery or percutaneous biopsy of bone or adjacent tissues. Coagulase-negative Staphylococci (CoNS) and other skin contaminants were considered true pathogens only when isolated from a sterile bone biopsy in at least 2 samples or in 2 or more blood cultures drawn on separate occasions.
VO was considered health care associated according to published criteria with some modifications.27 First, onset of symptoms >1 month after hospitalization with no evidence of VO at the time of admission; there is not a previously established cutoff period for health care VO-associated designation; all health care cases took at least a month for symptoms to develop, or symptom onset within 6 months after discharge (designated nosocomial VO). Second, ambulatory diagnostic or therapeutic manipulations within 6 months before symptom onset (designated nosohusial VO), including long-term central venous catheter use, autologous or prosthetic arteriovenous fistula for HD, invasive intravascular techniques (cardiac catheterization, pacemaker insertion, other intravascular devices, intravenous chemotherapy, or contrast administration), urological, gynecological, or digestive procedures, and cutaneous manipulations (eg, acupuncture, intramuscular injections).
Catheter-related bloodstream infection is defined elsewhere.28 As to the presence of infective endocarditis (IE), only patients with definite IE according to the modified Duke criteria were included.
Renal failure was defined as serum creatinine ≥2 mg/dL. Neurological complication was established on the presence of motor deficits or meningitis.
Outcome
Patients were clinically followed-up during hospitalization and in the outpatient clinic. In-hospital mortality was defined as death by any cause during hospitalization. Related mortality was defined as death secondary to sepsis, VO complications, or IE in cases associated with this disease.
Treatment failure was defined based on the following: infection-related death during hospitalization; persistent infection (clinical signs or persistently high acute-phase reactants with no other inflammatory causes and isolation of the same microorganism); or relapse, established on reappearance or worsening of clinical signs and analyses after a period of improvement, and isolation of the same microorganism. To evaluate relapse, only patients followed-up for at least 1.5 years after antibiotic therapy were included.
Statistical Analysis
Normally distributed quantitative variables are expressed as the mean and standard deviation (SD) and those with skewed distribution as median and interquartile range (IQR). Data for discrete variables are expressed as percentages. Continuous variables were compared using the unpaired Student t or Mann–Whitney U test, and proportions using the χ2 or Fisher exact test, where appropriate. All statistical tests were 2-tailed, and statistical significance was set at a P value of <0.05. Statistical analyses were performed with Microsoft SPSS-PC+, 15.0 (SPSS, Chicago, IL).
Ethical Considerations
Because data collection began in 1987 and no direct patient contact was planned, the requirement for informed consent was waived.
RESULTS
Over the 25-year study period, hematogenous pyogenic VO was diagnosed in 163 patients, and 41 cases (25%) were health care associated. Percentage of HCAVO increased from 15% (9/61) in 1987–1999 to 31% (32/102) in 2000–2011 (P < 0.01).
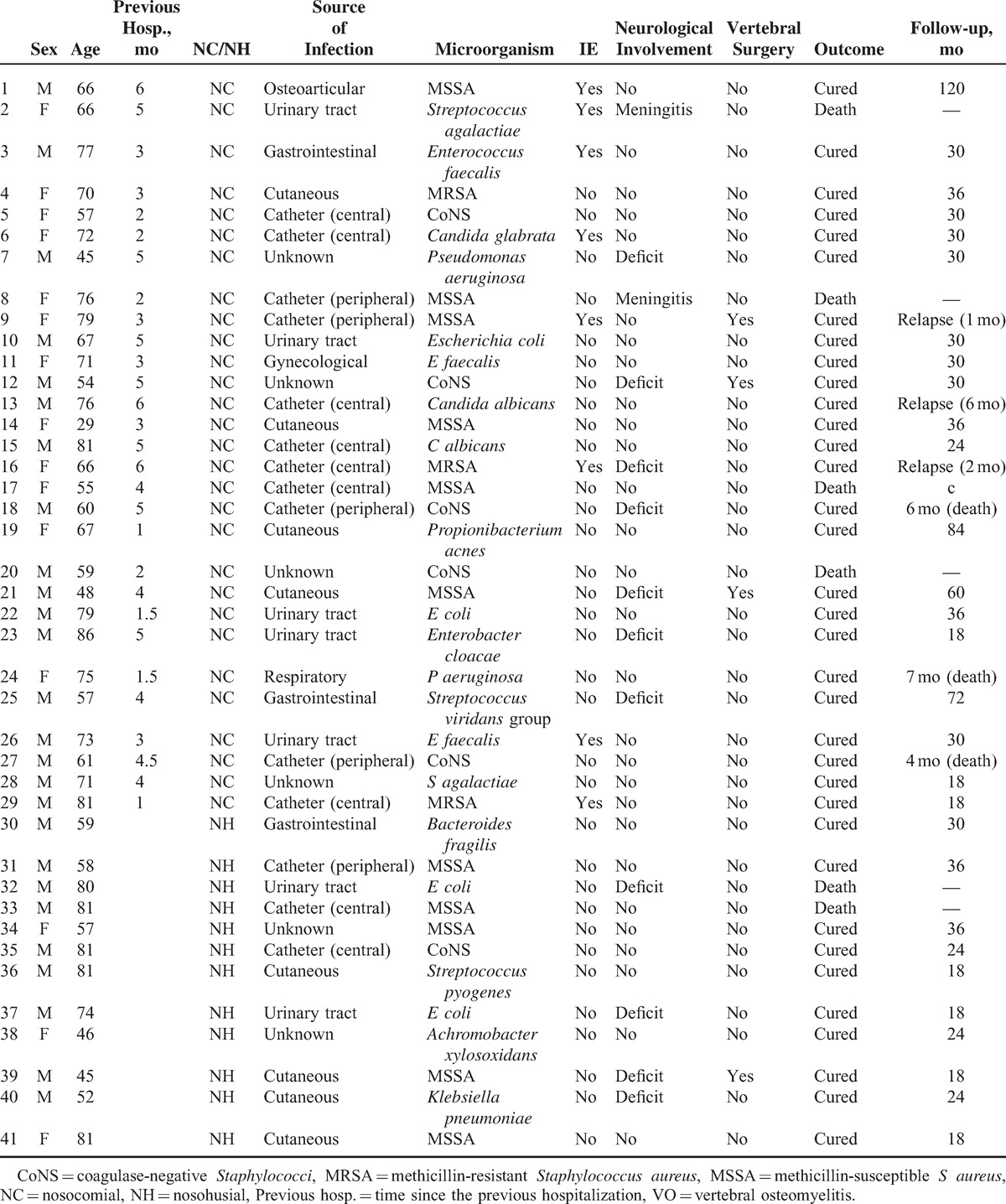
Twenty-six (63%) HCAVO patients were men, with a mean age of 66.3 years (SD 13.0). Mean age was similar in the 2 periods (61.9 years [SD 14.8] vs 62.1 years [SD 15.3], P = non significant). Twenty-nine of the 41 cases (71%) were considered nosocomial (in all symptoms of VO started at least 1 month after hospital discharge) and 12 (29%) nosohusial. Demographic data, clinical features, and laboratory results in HCAVO cases are shown in Table 1.
TABLE 1.
Description of the 41 Cases of Health Care Associated Hematogenous Pyogenic VO
Source of Infection
The infectious source was established in 35 (85%) HCAVO cases (Table 2). The most important was catheter-related infection in 14 (34%), 9 due to central venous catheters and 5 due to peripheral venous catheters. Seven of these patients had a previous documented catheter-related bloodstream infection due to the same microorganism isolated in VO, 4 patients had previous phlebitis, 2 cases of Candida albicans infection occurred in patients with previous parenteral nutrition and no other Candida foci, and 1 renal transplant patient had well-documented plasmocoagulase-negative VO, with no other source of infection. Microbiological etiologies of catheter-related VO are outlined in Table 2.
TABLE 2.
Presumed Source of Infection in 41 Health Care Associated Hematogenous Pyogenic VO Cases

Overall, HCAVO was associated with an HD in 5/41 cases (12%), all due to long-term catheter-related infection and none secondary to an arteriovenous fistula.
Among the cutaneous foci, 1 had a previous streptococcal cellulitis, 4 infected skin ulcers (2 Klebsiella spp, 1 methicillin-susceptible Staphylococcus aureus [MSSA], and 1 methicillin-resistant S aureus [MRSA]), and 4 skin abscesses (1 developed a gluteus abscess after an intramuscular injection, and 12 weeks later was diagnosed with S aureus HCAVO).
Among 7 cases related with previous urological foci, 5 were associated with urinary catheterization, 1 secondary to urological surgery with a complicated postoperative urinary tract infection (UTI), and 1 patient had recurrent UTI after prostatic cancer surgery. Previous bacteriemic UTI was documented in only 1 urinary-related case.
There were 3 digestive-associated cases, 2 related with esophageal manipulation, and 1 with a previous endoscopic retrograde cholangiopancreatography study.
The osteoarticular-related case developed concomitant bacteriemic S aureus HCAVO and prosthetic knee joint infection 6 months after the initial prosthetic procedure. The respiratory-related case was a Pseudomonas aeruginosa colonized patient who developed P aeruginosa VO 1.5 months after tracheal prosthesis manipulation.
Among the 12 nosohusial cases, 3 (25%) were considered catheter-related, all of staphylococcal etiology.
Etiology
Blood cultures were positive in 29/40 (72.5%) patients (Table 3). Bone samples in 22 patients isolated a microorganism in 18 cases (diagnostic yield 82%), all concordant with the blood culture isolate.
TABLE 3.
Microorganisms Causing Health Care Associated Pyogenic VO
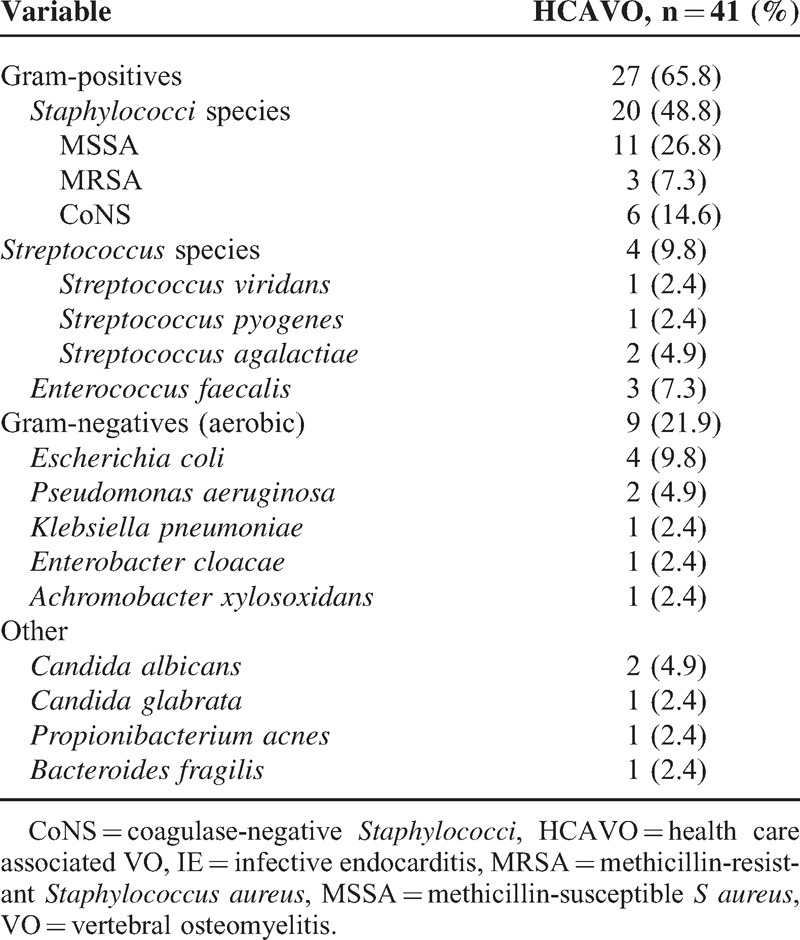
S aureus was the most frequently isolated microorganism (14 cases, 34%), followed by CoNS and Enterobacteriaceae (Table 3). There were 2 cases of anaerobic HCAVO. In 1, caused by Bacteroides spp, the patient had an esophageal fistula and developed VO after esophageal manipulation, whereas the other patient had well-documented Propionibacterium acnes VO (3 positive biopsy samples) 1 month after cardiac catheterization.
Catheter-associated HCAVO cases were due to staphylococci (n = 11) or Candida spp (n = 3), and urinary tract related cases were mainly due to gram-negative microorganisms (n = 5/7).
Comparison Between HCAVO and CAVO
The characteristics of patients with HCAVO and CAVO are presented in Table 4. Patients with HCAVO were older and underlying diseases were more frequent than in the CAVO group, particularly malignancy/immunosuppressive treatment and chronic renal failure, whereas intravenous drug use was associated with CAVO. Of note, malignant disease and immunosuppressor treatment were significantly higher in patients diagnosed after 1999 (23 [22.5%] vs 2 [3.3%], P = 0.001). Among these 25 patients, 20 had an active neoplasm and 5 received immunosuppressors for a solid transplant or a systemic disease.
TABLE 4.
Univariate Comparison Between 41 Patients With Health Care Associated Hematogenous Pyogenic VO (HCAVO) and 122 Patients With CAVO
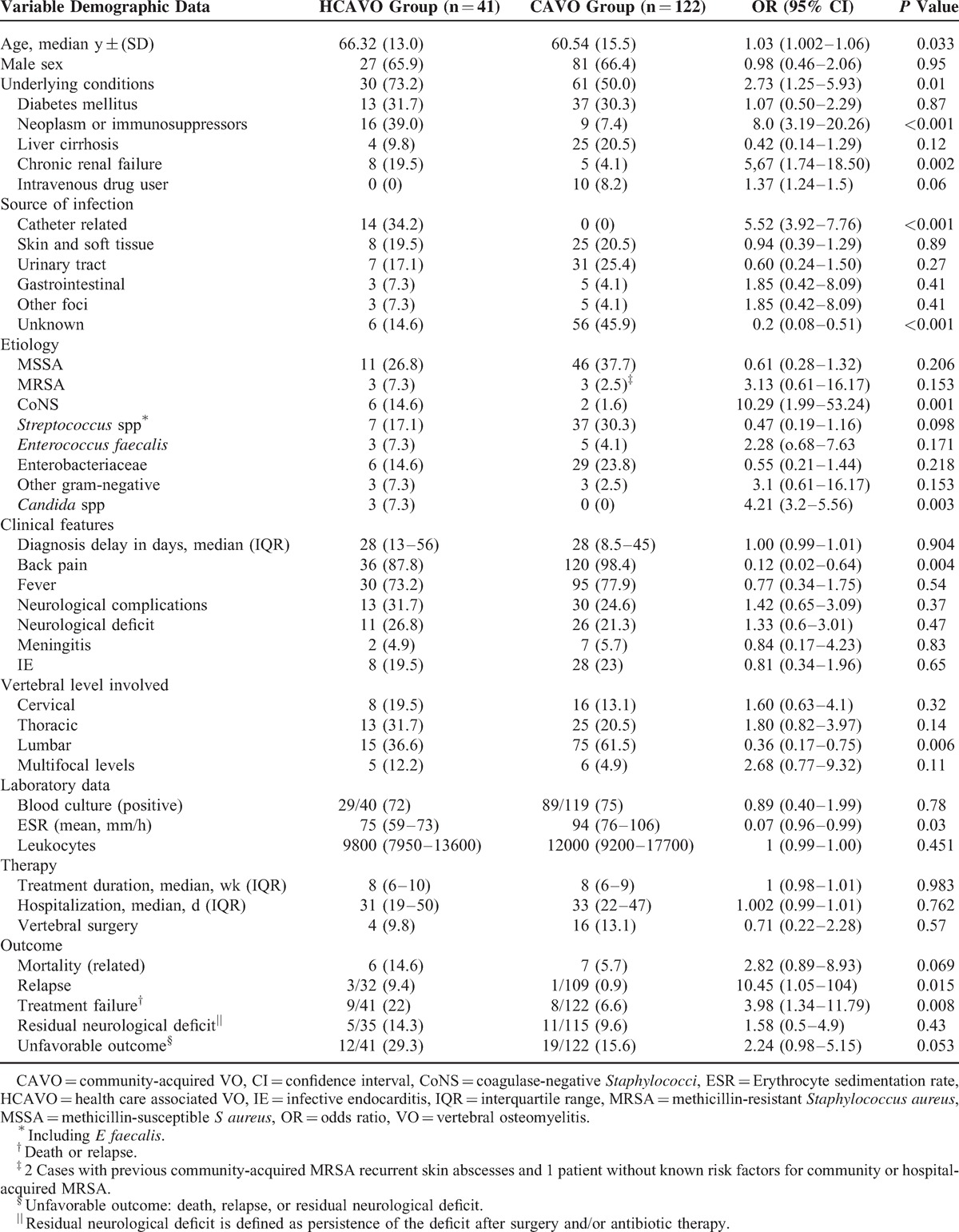
Median diagnostic delay was similar in the 2 groups. The source of infection was established in 85% of HCAVO, whereas in CAVO, the source was unknown in nearly half the cases. Catheter-related infection was the most frequent source in HCAVO, whereas UTI was the most common origin in CAVO patients. As was expected, there were a higher percentage of infections due to CoNS and Candida spp in the HCAVO group, mainly associated with well-documented catheter-related infections.
Back pain and fever were the most common symptoms, with back pain being less frequent in HCAVO. Among 5 HCAVO patients who did not present back pain, the clinical presentation was persistent fever in 4 (2 persistent bloodstream infection), and the diagnosis of VO was established after excluding IE and other infectious foci.
Overall, IE was diagnosed in 8/41 (20%) patients, and 8/27 (30%) gram-positive HCAVO; none of the gram-negative HCAVO had IE. Four of the 14 patients (29%) with catheter-related HCAVO had IE. Nevertheless, the percentage of IE was similar in HCAVO and CAVO cases.
Neurological complications were observed in nearly one-third of HCAVO, a percentage slightly higher than in CAVO cases. In HCAVO, 27% of patients had a neurological deficit and 2 had meningitis (5%). Three patients required spinal surgery because of paraplegia. The incidence of neurological complications in S aureus HCAVO was similar to non-S aureus infections, 4/14 (29%) versus 9/27 (33%).
As to treatment, empirical antibiotics were started only in patients with suspected bloodstream infection, with adjustment according to the susceptibility pattern of isolated bacteria. HCAVO cases were treated for a median of 8 weeks (IQR 6–9 weeks). IE patients received 4 to 8 weeks of intravenous therapy according to the isolated microorganism; 2 patients required further oral therapy due to paravertebral abscesses. Non-IE cases were treated for a median of 8 weeks (IQR 6–10 weeks), within which there were 4 weeks of oral therapy (IQR 2–6 weeks). Five patients, with gram-negative VO, were treated orally alone.
Spinal surgery was performed in 4/41 (10%) cases: in 3 patients because of neurological complications (paraplegia) and in 1 with persistent fever to drain a large paravertebral abscess.
HCAVO patients required lengthy hospitalization (around 1 month), but duration was not longer than in CAVO cases. Six HCAVO patients died during admission (15%). Death was infection-related in all, and 3 patients had S aureus sepsis.
Among 35 patients who survived hospitalization and underwent outpatient follow-up, 3 died of their underlying disease in the first 7 months, and the remaining 32 had long-term follow-up (median 30 months, IQR 20–42 months). In this group, 3 (9%) relapsed, and 2 of them ultimately died due to the infection: 1 with C albicans HCAVO treated with 8 weeks of fluconazole who relapsed 6 months later and 1 with MSSA VO and concomitant IE treated with cloxacillin for 6 weeks who relapsed 1 month later. The third patient, a catheter-related MRSA HCAVO with IE and a paravertebral abscess, that could not be drained because of surgical contraindications, was treated with vancomycin for 8 weeks and orally with cotrimoxazole/rifampin for 8 weeks, and relapsed 2 months after completing therapy. She was treated with linezolid and relapsed again, and finally cured after 6-month therapy with teicoplanin and rifampin (follow-up 5 years).
Treatment duration and hospital stay were similar in the 2 groups, but HCAVO had a higher percentage of treatment failures and a trend to higher mortality (15% vs 6%, P = 0.069) and relapses (9% vs 1%, P = 0.015).
DISCUSSION
The results of our study show that nosocomial infection is now a common source of hematogenous pyogenic VO in our setting. One-third of these infections were catheter-related and potentially preventable. Compared with CAVO, health care related hematogenous VO was associated with higher mortality and relapse rates. In our earlier experience (1987–1999), 15% of VO cases were HCAVO, whereas our more recent data (2000–2011) show that nearly one-third of spinal infections are health care associated. The high incidence of HCAVO contrasts with findings from previous studies, with reported rates of 19%.10,14 Several factors could contribute to this higher incidence: first, our hospital is a referral center for complicated spinal infections, and these are more common in patients with underlying diseases, as was seen in our HCAVO cases; second, the use of diagnostic and therapeutic techniques (outpatient parenteral nutrition and HD) has considerably increased; third, there is a high index of suspicion in our attending physicians, based on a detailed history of previous admissions and health care procedures, particularly those with a risk of bloodstream infection.
Although an increase in the aging of the population and immunosuppression may have had an influence on the higher incidence of VO in recent years, age was similar (61.9 vs 62.1 years) in the 2 study periods in our series, but the incidence of underlying immunosuppressive diseases was higher in the second period. Thus, in our opinion, health care associated infections and malignancy/immunosuppression are now the most important factors that contribute to an increase in the overall incidence of VO in the population.
The most common infectious source was a previous catheter-related infection (one-third of cases), a percentage similar to the 40% (33/83) we found in patients with health care associated IE27 and lower than the 46% (148/319) of health care associated bloodstream infections.28 Although our group follows the guidelines for catheter-related bloodstream infections, new VO cases still occur. These data indicate the need to improve the care in all steps provided to patients with intravascular devices and to strictly follow the therapeutic guidelines for catheter-related bloodstream infection, especially regarding septic complications in S aureus infection.29,30
In other recent studies, VO has also been associated with HD therapy.14,20,25 In our experience, this was the source of infection in 12% of HCAVO and 3% of all VO cases, similar to the 4% recently reported14 and <18% reported by Bhavan et al.20 The percentage of VO in HD patients is not known, although it is estimated that 1.3% of HD patients with bloodstream infection will develop this complication31 and an incidence of 1 episode every 215 patient-years has been reported.32 In our patients, no VO cases were associated with arteriovenous fistula infection, in accordance with previous findings of a 3-fold higher incidence in patients dialyzed with tunneled central venous catheters than in those with arteriovenous fistulae.32 Mortality in this subset of patients is high, at 11% to 46%.25,32 Thus, it is crucial to reinforce preventive measures and provide prompt diagnosis and treatment.
S aureus was the most commonly isolated microorganism, especially in catheter-related cases. In 2 large series, VO was a complication of S aureus bloodstream infection in 1.7% (146/8739 cases)33 and 3% (22/724 cases)34 of patients. In our previous study of S aureus bloodstream infection, 2/146 (1.2%) hospital-acquired cases had an osteoarticular source of infection and in both patients, it was VO.35 The risk of VO in patients with intravascular device related S aureus bloodstream infection (IDRSAB) is similar, around 2.2% (7/324 cases),36 although the study did not establish whether VO was a direct consequence of IDRSAB or secondary to concomitant IE. In our study, only 21.4% of catheter-related HCAVO had IE, thus, VO was likely a direct consequence of catheter infection in most cases. The high risk of this complication in patients with nosocomial IDRSAB has practical implications. We believe that radiological imaging should be performed to exclude VO in all patients with a previous IDRSAB and new-onset or increased back pain.
From the clinical viewpoint, it is remarkable that back pain, which is almost always present in VO, was absent in 12% of HCAVO cases in our experience10 and in 14% in a large review.2 These patients had persistent fever or bloodstream infection after adequate antibiotic therapy of the underlying infection, and the diagnosis of VO was ultimately established after excluding other infectious foci (eg, IE and spleen abscess).
The percentage of IE in spontaneous pyogenic HCAVO was high (19%) and similar to CAVO cases. As we reported previously, this high incidence is attributable to our hospital being a referral center for IE and a high index of clinical suspicion.10 The high rate has practical implications and suggests that conversion to early oral therapy should be avoided until IE has been excluded, at least when it is clinically suspected and in gram-positive infections.1,3,10
As our group observed in health care associated IE,27 HCAVO also affects elderly people with underlying conditions. This comorbid status leads to close contact with the health system and a higher risk of acquiring bloodstream infection, IE, and VO, and it could have an influence on the prognosis of the disease.
In comparison with CAVO, HCAVO cases had higher mortality (15% vs 6%) and surviving patients a higher relapse rate (9% vs 1%) in our study. Although, to a certain extent, these findings may be related to the higher incidence of underlying conditions in HCAVO, difficult to treat microorganisms, such as Candida spp and MRSA, and in 1 case, the fact that surgical drainage of a large abscess was unfeasible due to the patient's poor general condition may have influenced the higher mortality rate.
As in all retrospective studies, there is a potential for bias and statistical imprecision. It is possible that in the earlier period, contact with the health care system was overlooked in some patients and they were misclassified as CAVO, although this would have increased the incidence of health care associated cases. Our study has the referral bias of a large tertiary teaching center with an IE and spinal surgery unit, which may have had an impact on the rate of IE cases and neurological complications. Echocardiography was not mandatory in enterobacterial infections, but the uncommon implication of this microorganism in IE leads us to believe that IE rates would not have changed substantially if it had been performed. Extrapolation of the results to other community hospitals should be done with caution. Detection of VO in pluripathological-hospitalized patients requires a high index of suspicion, and some HAVO cases may have been missed in severely ill patients. Nonetheless, our infectious diseases department is highly aware of this disease, and most cases are seen by the same staff member, a fact that would help minimize elements of bias.
In conclusion, in our institution, one-third of current hematogenous pyogenic VO infections are health care associated cases and a third of these are catheter-related infections, and therefore, potentially preventable. HCAVO affects elderly people with underlying conditions and is associated with higher mortality and relapse rates than CAVO. Maximizing aseptic measures before and during any invasive procedure (especially those related to intravascular catheters) could reduce the incidence of this condition.
Acknowledgment
We thank Celine Cavallo for English language support.
Footnotes
Abbreviations: CAVO = community-acquired vertebral osteomyelitis, CoNS = coagulase-negative staphylococci, HCAVO = health care associated vertebral osteomyelitis, HD = hemodialysis, IDRSAB = intravascular device related Staphylococcus aureus bloodstream infection, IE = infective endocarditis, IQR = interquartile range, MRSA = methicillin-resistant S aureus, MSSA = methicillin-susceptible S aureus, SD = standard deviation, UTI = urinary tract infection, VO = vertebral osteomyelitis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Zimmerli W. Vertebral osteomyelitis. New Eng J Med 2010; 362:1022–1029. [DOI] [PubMed] [Google Scholar]
- 2.Mylona E, Samarkos M, Kakalou E, et al. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009; 39:10–17. [DOI] [PubMed] [Google Scholar]
- 3.Gouliouris T, Aliyu S, Brown N. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010; 65 suppl 3:11–24. [DOI] [PubMed] [Google Scholar]
- 4.Skaf GS, Domloj NT, Fehlings MG, et al. Pyogenic spondylodiscitis: an overview. J Infect Public Health 2010; 3:5–16. [DOI] [PubMed] [Google Scholar]
- 5.Cheung WY, Luk K. Pyogenic spondylitis. Int Orthop 2012; 36:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Martino A, Papapietro N, Lanotte A, et al. Spondylodiscitis: standards of current treatment. Curr Med Res Opin 2012; 28:689–699. [DOI] [PubMed] [Google Scholar]
- 7.McHenry MC, Easly KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002; 34:1342–1350. [DOI] [PubMed] [Google Scholar]
- 8.Colmenero JD, Jiménez-Mejias ME, Sánchez-Lora FJ, et al. Pyogenic, tuberculous, and brucellar vertebral osteomyelitis: a descriptive and comparative study of 219 cases. Ann Rheum Dis 1997; 56:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carragee E. Pyogenic vertebral osteomyelitis. J Bone Joint Surg (Am) 1997; 79A:874–880. [DOI] [PubMed] [Google Scholar]
- 10.Pigrau C, Almirante B, Flores X, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med 2005; 118:1287–1292. [DOI] [PubMed] [Google Scholar]
- 11.Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci 2005; 9:53–66. [PubMed] [Google Scholar]
- 12.Nolla JM, Ariza J, Gómez-Vaquero C, et al. Spontaneous pyogenic vertebral osteomyelitis in nondrug users. Semin Arthritis Rheum 2002; 31:271–278. [DOI] [PubMed] [Google Scholar]
- 13.Lora-Tamayo J, Euba G, Narvaez JA, et al. Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: the impact of cases with no microbiologic diagnosis. Semin Arthritis Rheum 2011; 41:247–255. [DOI] [PubMed] [Google Scholar]
- 14.Aagard T, Roed C, Dragsted C, et al. Microbiological and therapeutic challenges in infectious spondylodiscitis: a cohort study of 100 cases, 2006–2011. Scand J Infect Dis 2013; 45:417–424. [DOI] [PubMed] [Google Scholar]
- 15.Cebrian J, Saez-Arenillas A, Urda AL, et al. Management of infectious discitis. Outcome in one hundred and eight patients in a university hospital. Int Orthop 2012; 36:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roblot F, Besnier JM, Juhel L, et al. Optimal duration of antibiotic therapy in vertebral osteomyelitis. Sem Arthritis Rheum 2007; 36:269–277. [DOI] [PubMed] [Google Scholar]
- 17.Patzakis MJ, Rao S, Wilkins J, et al. Analysis of 61 cases of vertebral osteomyelitis. Clin Orthop Rel Res 1991; 264:178–183. [PubMed] [Google Scholar]
- 18.Hadjipavlou AG, Mader JT, Necessary JT, et al. Hematogenous pyogenic spinal infections and their surgical management. Spine 2000; 25:1668–1679. [DOI] [PubMed] [Google Scholar]
- 19.Kourbeti IS, Tsiodras S, Boumpas DT. Spinal infections, evolving concepts. Curr Opinion Rheumatol 2008; 20:471–479. [DOI] [PubMed] [Google Scholar]
- 20.Bhavan K, Marschal J, Olsen MA, et al. The epidemiology of haematogenous vertebral osteomyelitis: a cohort study in a tertiary care hospital. BMC Infect Dis 2010; 10:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grammatico L, Baron S, Rusch E, et al. Epidemiology of vertebral osteomyelitis in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect 2008; 136:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchison C, Hanger C, Wilkinson T, et al. Spontaneous spinal infections in older people. Intern Med J 2009; 39:845–848. [DOI] [PubMed] [Google Scholar]
- 23.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect 2008; 56:401–412. [DOI] [PubMed] [Google Scholar]
- 24.Legrand E, Flipo R, Guuggenbuhl P, et al. Management of nontuberculous infectious discitis. Treatments used in 110 patients admitted to 12 teaching hospitals in France. J Bone Spine 2001; 68:504–509. [DOI] [PubMed] [Google Scholar]
- 25.Cervan A, Colmenero JD, Arco A, et al. Spondylodiscitis in patients under haemodyalisis. Int Orthop 2012; 36:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torda E, Gottlieb T, Bradbury R. Pyogenic vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis 1995; 20:320–328. [DOI] [PubMed] [Google Scholar]
- 27.Fernández-Hidalgo N, Almirante B, Tornos MP, et al. Contemporary epidemiology and prognosis of healthcare-associated infective endocarditis. Clin Infect Dis 2008; 47:1287–1297. [DOI] [PubMed] [Google Scholar]
- 28.Friedman ND, Kaye KS, Stout J, et al. Healthcare-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–797. [DOI] [PubMed] [Google Scholar]
- 29.Raad I, Hanna H, Maki D. Intravascular catheter-related infections: advances in diagnosis, prevention, and management. Lancet Infect Dis 2000; 7:645–657. [DOI] [PubMed] [Google Scholar]
- 30.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Garcia P, Castillo N, Jarque A, et al. Infectious spondylodiscitis in hemodialysis. Sem Dial 2010; 23:619–626. [DOI] [PubMed] [Google Scholar]
- 32.Abid S, Silva DE, Warwicker P, et al. Infective spondylodiscitis in patients on high-flux hemodialysis and on-line hemodiafiltration. Hemodil Int 2008; 12:463–470. [DOI] [PubMed] [Google Scholar]
- 33.Jensen AG, Espersen F, Skinhof P, et al. Increasing frequency of vertebral osteomyelitis following Staphylococcus bacteremia in Denmark 1980–1990. J Infect 1997; 34:147–151. [DOI] [PubMed] [Google Scholar]
- 34.Fowler VG, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus bacteremia. Arch Intern Med 2003; 163:2066–2072. [DOI] [PubMed] [Google Scholar]
- 35.Pigrau C, Rodriguez D, Planes AM, et al. Catheter-related Staphylococcus aureus bacteremia. Is sonographic study always necessary in its management? Eur J Clin Microbiol 2003; 22:713–719. [DOI] [PubMed] [Google Scholar]
- 36.Fowler VG, Justice A, Moore C, et al. Risk factors for haematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703. [DOI] [PubMed] [Google Scholar]


