Supplemental Digital Content is available in the text
Abstract
Malignant transformation in fibrous dysplasia (FD) is uncommon. The purpose of this study was to investigate clinical and imaging features, and outcomes of malignant transformation in monostotic FD.
Data for 10 pathologically confirmed malignant transformations in monostotic FD from January 2005 to December 2013 were retrospectively reviewed. Patient data were recorded, and radiographs (n = 10), computed tomography (CT) (n = 5), magnetic resonance (MR) (n = 4), and bone scintigrams (n = 10) were evaluated for lesion location, margin, cortical destruction, marrow involvement, periosteal reaction, and soft tissue mass by 2 musculoskeletal radiologists with agreement by consensus. Clinical features, management, and prognosis were also analyzed for each of the 10 cases.
There were 8 male and 2 female patients (mean age 46.5 ± 15.9 years). The affected sites were the femur (n = 4), humerus (n = 2), tibia (n = 3), and ilium (n = 1). Five cases had received previous surgery and 5 cases had no history of surgery. No patients had been given prior irradiation treatment. For the 5 cases with surgery, radiographs and CT showed purely osteolytic lesions with poor margination in the curettage area (n = 5), cortical destruction (n = 5), obvious soft tissue mass (n = 1), and mineralization (n = 2). For the 5 cases without surgery, radiographs and CT identified poorly marginated, osteolytic lesions within or near the area with “ground-glass” opacity (n = 4), cortical erosion (n = 4), and mineralization (n = 2). Magnetic resonance imaging (MRI) also identified lesions with heterogeneous signal intensity and pronounced enhancement. Bone scintigraphy revealed eccentric increased uptake of radionuclide in monostotic lesion (n = 10). Pathology reports revealed osteosarcoma (n = 7), fibrosarcoma (n = 2), and malignant fibrous histiocytoma (MFH) (n = 1). At the end of the study, 1 patient died from tumors, 1 patient was alive with lung metastasis, 1 patient experienced recurrence, and 7 patients were alive without recurrence.
Patients with FD and a history of surgery should be followed up, for the osteolytic lesions in the operative areas strongly indicate the malignant transformation. The radiographic feature of FD-related malignancies is poorly marginated, mineralized, and osteolytic lesions with cortical destruction. Further investigations are needed to explore the pathogenesis of malignancies in FD and to establish optimal therapeutic strategies.
INTRODUCTION
Fibrous dysplasia (FD) is an uncommon bone disease1 that is characterized by the replacement of normal bone structure with abnormal fibro-osseous connective tissue. FD generally has typical imaging findings. Radiographically, the healthy bone is replaced with a more radiolucent, “ground-glass”–appearing pattern, with no visible trabecular pattern. There may be endosteal scalloping of the inner cortex, but the periosteal surface is smooth and nonreactive.1 FD is generally considered a benign, pediatric disease and usually becomes dormant by adulthood. However, FD lesions have the potential to become malignant over decades. Coley and Stewart described the first identified case of sarcoma arising in FD in 1945.2 Malignancies in FD can occur in monostotic and polyostotic FD. The frequency of malignant change is increased in the polyostotic form of FD, especially in patients with McCune–Albright syndrome or Mazabraud syndrome.1,3–5 Malignant transformation in monostotic FD has rarely been reported. Therefore, in this study, we report 10 unpublished cases of spontaneous transformation in monostotic FD. We present their characteristic clinical features; radiographic, MRI, and computed tomography (CT) features; treatment modalities; and prognoses. The purpose of this study was to investigate the clinical and imaging features of 10 cases of malignant transformation in monostotic FD.
MATERIALS AND METHODS
Patients
Records for all FD in the pathologic archives (comprising records for the years 2005–2013) of our institution (Department of Pathology, Shanghai No.6 Municipal Hospital) were retrospectively reviewed by 2 experienced pathologists. Criteria for inclusion in the study were as follows: malignant transformation was recognized pathologically; and imaging studies (radiography, bone scintigraphy, and/or CT, and/or magnetic resonance [MR] imaging) were available for review. Five hundred forty-two patients were identified with histopathologically proved FD who had undergone percutaneous biopsy (n = 315) and surgical treatment (n = 227). Among them, 15 patients were proved to have malignant transformation while only 14 patients had radiological image data. Four patients suffering from polyostotic lesions were ruled out based on the bone scintigraphy. So the study ultimately involved a retrospective review of 10 cases with malignant transformation in monostotic FD, who had been surgically treated and followed up at the Department of Orthopedic Oncology. No case presented cutaneous pigmentation, endocrine disturbances, or precocious puberty. This retrospective study was approved by our hospital's institutional review board, and the requirement for informed consent was waived. The flowchart is listed in appendix, http://links.lww.com/MD/A129.
Clinical Information and Image Evaluation
Clinical characteristics recorded included patient sex, age, and presenting symptoms (eg, pain, swelling, presence of a soft tissue mass), with anatomic site involved. Two musculoskeletal radiologists (20 and 18 years of experience) with complete knowledge of the image diagnosis reviewed the images, and agreement was reached by consensus. Images reviewed included radiographs (n = 10), CT (n = 5), MR (n = 4), and bone scintigraphy (n = 10).
Radiographs (n = 10) were evaluated for lesion location, any associated abnormality in the medullary canal (“ground-glass appearance” lesion, osteolytic lesion), margin (either complete/incomplete sclerotic marginated or poorly marginated), the presence and extent (mild, moderate, marked) of mineralization, cortical destruction (erodent, thin), periosteal reaction, and soft tissue mass.
CT images (n = 5) examinations were performed on 16-slice CT scanner (Siemens Medical Solutions, Erlangen, Germany) (n = 2) and 64-slice CT scanner (LightSpeed VCT; GE Healthcare, Waukesha, WI) (n = 3) with both bone and soft tissue windows at variable thicknesses (n = 3 at 2 mm, n = 2 at 10 mm) and sagittal and coronal reformatted images (n = 3). CT images were evaluated for the presence of lesion location, any associated abnormality in the medullary canal (eg, replacement of the yellow marrow by “ground-glass appearance” lesion or soft tissue attenuation), margin (either complete/incomplete sclerotic marginated or poorly marginated), the presence and extent of mineralization, cortical destruction, periosteal reaction, and soft tissue mass. CT images were further assessed for the presence and extent (mild, moderate, or marked) of mineralization in the lesion, cortical destruction, and soft tissue mass. CT images were also assessed for the predominant attenuation (lower than that of muscle, similar to that of muscle, or higher than that of muscle) and homogeneity (whether homogeneous or heterogeneous) of the nonmineralized component of the soft tissue mass.
MR imaging (n = 4) was performed on Philips Achieva 3.0T superconducting MR scanner (Koninklijke Philips NV, Amsterdam, the Netherlands). MR imaging sequences used to obtain the images available for review included a standard (spin echo) T1-weighted sequence (repetition time [TR]/echo time [TE], 400–560/10–20 milliseconds), an intermediate-weighted/T2-weighted sequence (TR/TE = 1500–3800/70–100 milliseconds) with or without fat suppression, and short-tau inversion recovery (STIR) (TR/TE = 3000–4000/41.3–48 milliseconds; inversion time [TI], 150 milliseconds). STIR is a special kind of fat suppression sequence, which is highly sensitive for detection of pathologic lesions and bone marrow edema. T1-weighted sequences were performed before and after injection of a gadolinium chelate. Imaging planes included the transverse plane and at least 1 long axis in all patients. MR images were evaluated for the presence of any associated abnormality in the medullary canal (eg, replacement of the normal yellow marrow signal intensity on T1- and/or T2-weighted MR images), soft tissue edema, soft tissue mass, and pattern of contrast enhancements. MR images were also assessed for the predominant signal intensity and homogeneity (homogeneous or heterogeneous) of the nonmineralized component of the soft tissue mass on T1-weighted images (the signal intensity of the mass was considered to be low when it was lower than that of muscle, intermediate when it was similar to that of muscle, and high when it was similar to that of fat) and T2-weighted images (the signal intensity of the mass was considered to be low when it was similar to that of muscle, intermediate when it was similar to that of fat, and high when it was greater than that of fat). The pattern of enhancement at the solid portions of the lesion was categorized as homogeneous or heterogeneous. The degree of enhancement was subjectively assessed as being mild, moderate, and marked.
Bone scintigraphy (n = 10) was performed on the SPECT system (Siemens Medical Solutions USA, Inc. Valley Stream Parkway, Malvern, PA) before surgery. During the period of follow-up, 7 patients had gone through this examination again. Tc-99m methylene diphosphonate was intravenously administered to patients immediately after preparation. Bone scintigrams were evaluated for monostotic or polyostotic lesion, ruling out metastasis of secondary sarcoma, and recurrence after therapy.
Statistical Analysis
Quantitative variables are expressed as the means ± standard deviations (SDs).
RESULTS
Clinical Features
The summary of the clinical data for the 10 patients is shown in Table 1. The frequency of malignant transformation was 2.77% (15/542). In our study, only monostotic FD with malignant transformation were discussed. The affected sites were the femur (4 cases), humerus (2 cases), tibia (3 cases), and ilium (1 case). The patient ages (8 males [80%] and 2 females [20%]) at the time of initial presentation of FD ranged from 14 to 67 years (mean age 42.6 ± 16.2 years); their ages at the time of malignant transformation ranged from 21 to 70 years (mean age 46.5 ± 15.9 years); 80% (8/10) of patients were past the fourth decade of life. The symptoms of FD in the remaining patients were variable and generally of long duration, including asymptomatic (3 cases), swelling and pain (1 case), pain (1 case), deformities (1 case), and pathologic fractures (4 cases). The symptoms of sarcoma were generally pain and swelling and usually developed rapidly.
TABLE 1.
Clinical Features of Sarcoma in FD
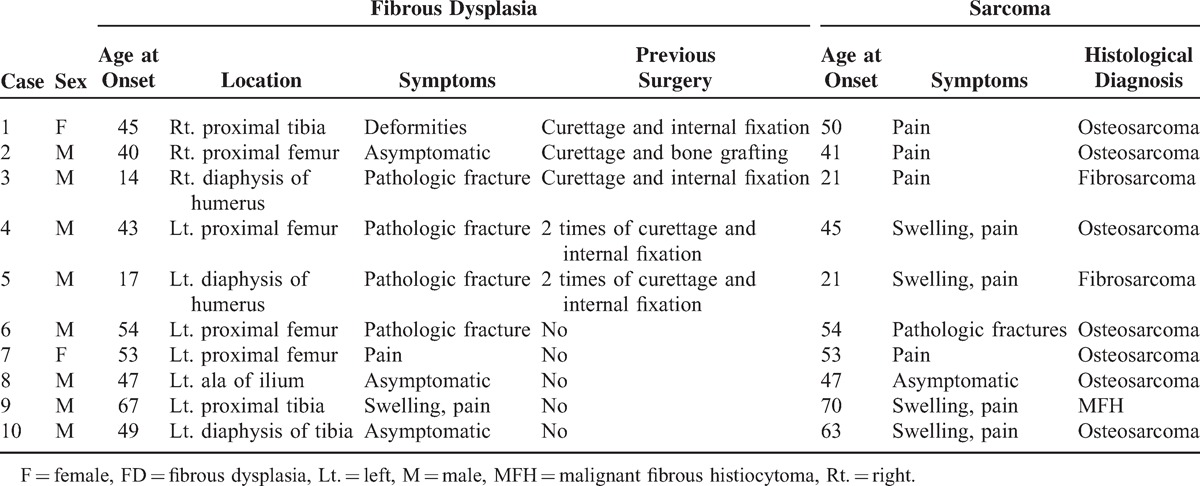
No patient had been given prior irradiation treatment. Five cases had received surgical therapy (curettage and internal fixation, n = 4; curettage and bone grafting, n = 1). Two cases had received curettage and internal fixation twice because of FD recurrence. Five other cases had not received any surgical therapy.
Imaging Features
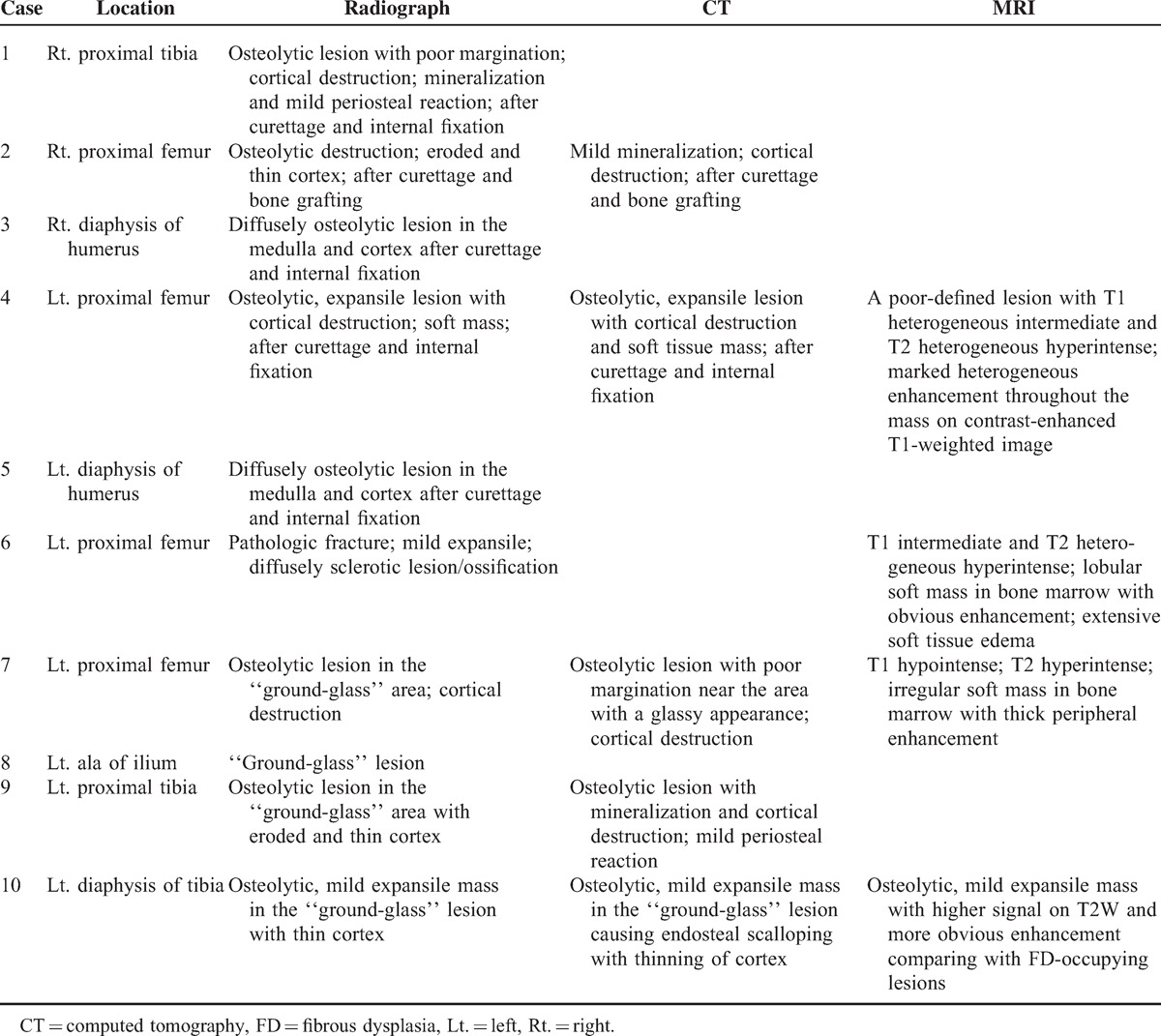
Table 2 summarizes the image findings of 10 FD patients with sarcomatous transformation.
TABLE 2.
Imaging Features of Sarcoma in FD
Sarcomatous Transformation in FD Patients With History of Surgery
Half of the patients (5/10) had a history of surgery (curettage and internal fixation or curettage and bone grafting). Radiographs were available for all 5 patients. It was difficult to identify the typical “ground-glass” opacities in their radiographs (Figure 1). Radiographically, all 5 cases showed purely osteolytic lesions with poor margination in the curettage area. The 5 sarcomas involved the medulla and cortex; the cortex was very thin and disrupted, and obvious soft tissue mass was observed in only 1 case. There was slight periosteal reaction and mineralization associated with only 1 of the 5 sarcomas (Figure 2). Radiographically visible mineralization was not prominent in the other 4 cases.
FIGURE 1.

A 45-year-old man with malignancies in FD on the left femur (case 4). Anteroposterior plain film (A), lateral plain film (B), and axial CT image (C) show expansile, osteolytic lesion in the operative area, with cortical destruction and a large soft tissue swelling. The mass reveals low signal intensity on the coronal T1-weighted MR image (D) and heterogeneous high signal intensity on the coronal short-tau inversion recovery (STIR, a special kind of fat suppression sequence) image (E). Contrast-enhanced fat-suppressed T1-weighted image (F) shows marked heterogeneous enhancement throughout the mass. CT = computed tomography, FD = fibrous dysplasia, MR = magnetic resonance.
FIGURE 2.
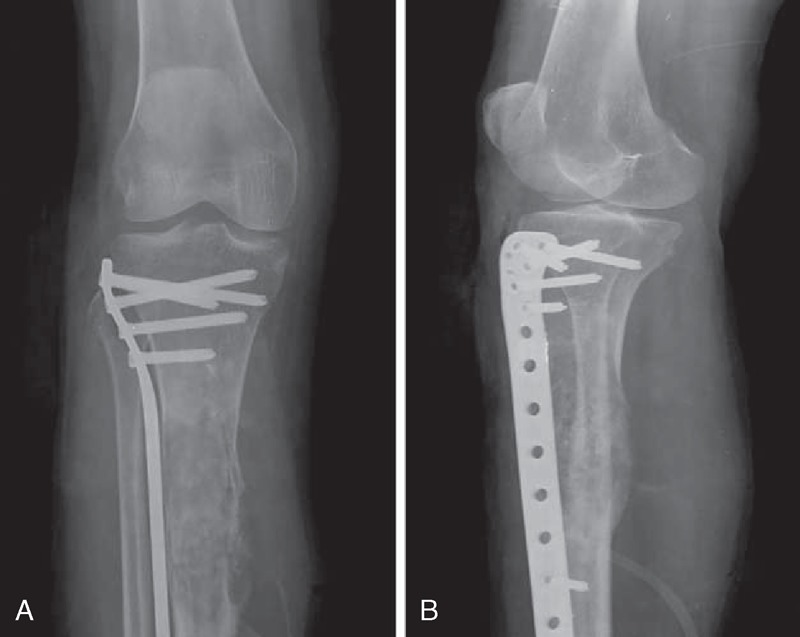
A 50-year-old female with malignancies in FD on the right proximal tibia with a history of operation (case 1). Anteroposterior plain film (A) and lateral plain film (B) show ill-defined osteolytic lesion with cortical destruction, periosteal reaction, and ossification. No “ground-glass” opacity can be found. FD = fibrous dysplasia.
Two cases underwent CT, providing the clearest depiction of cortical destruction and mineralization in sarcoma. Cortical erosion was observed in both cases (Figure 1); in 1 case, obvious mineralization could not be identified in the radiographs, and soft tissue mass was observed in the other case.
Only 1 case underwent MRI, which showed a poorly defined lesion with T1 heterogeneous intermediate signal and T2 heterogeneous hyperintense signal on the site of a previous curettage and internal fixation (Figure 1). Gadolinium-enhanced T1-weighted images showed marked heterogeneous enhancement throughout the mass (Figure 1).
By going through the prior films, malignant transformation of FD seemed to be a course of the disease, shown in Figure 3 (case 5).
FIGURE 3.

A 17-year-old male suffered pathologic fracture on the left shaft of humerus (case 5). The anteroposterior plain film (A) shows the “ground-glass” opacities in the humeral shaft and incomplete cortex. He was treated with internal fixation and was proved to have suffered FD. The anteroposterior plain film (B) shows internal fixation in the humerus shaft with the heterogenesis in the medulla and cortex 3 years later, during his second operation with the recurrence of FD. The anteroposterior plain film (C) shows further osteolytic destruction in the medulla and cortex after 6 months. He underwent the third operation and the final pathologic findings indicated the malignant change. FD = fibrous dysplasia.
Sarcomatous Transformation in FD Without Surgical Therapy
In the other 5 cases without a history of surgery, 4 cases had lesions with “ground-glass” opacity; in 3 of the 4 cases, radiographs showed poor margination or incomplete sclerotic margination and osteolytic destruction in the “ground-glass” opacity lesions with eroded and thin cortex (Figure 4); and only 1 case showed a homogeneous “ground-glass” opacity lesion with cortical integrity in the ilium. One case presented predominantly sclerotic lesions in the medulla with pathologic fracture, and “ground-glass” opacity was not prominent (n = 1). In the 5 cases, soft tissue masses and periosteal reactions were not prominent. Only 1 case was associated with visible mineralization in the lesion, and mineralization was not prominent in the 4 other cases.
FIGURE 4.

A 63-year-old man with malignancies in FD on left diaphysis of tibia (case10). Anteroposterior plain film (A) shows osteolytic lesion within the area of “ground-glass” appearance. Axial CT image (B) demonstrates the destruction of cortex. The coronal T1-weighted MR image (C) and the coronal fat-suppressed T2-weighted MR image (D) show the mass in bone marrow with low signal intensity on T1 (C) and higher signal compared with FD-occupying lesions on T2-weighted with fat suppression (D). The contrast-enhanced coronal fat-suppressed T1-weighted MR image (E) demonstrates marked enhancement throughout the mass. Microscopic findings of osteosarcoma (H&E, original magnification ×100) (F) showed anaplastic malignant tumor cells and neoplastic osteoid tissue, suggesting the diagnosis of the malignant change. CT = computed tomography, FD = fibrous dysplasia, MR = magnetic resonance.
Three patients without a history of surgery underwent CT. Cortical erosion was observed in all 3 cases (Figure 4); 1 case showed mineralization and mild periosteal reaction, which could not be identified in the radiographs.
Three patients underwent MRI, including 1 patient with pathologic fracture. TI-weighted MR images showed heterogeneous intermediate or hypointense lesions; T2-weighted MR images showed heterogeneous hyperintense lesions (Figure 4), and the T2-weighted signals of the sarcomatous lesions were higher than for the FD-occupying lesions (Figure 4).
Gadolinium-enhanced T1-weighted images showed different and more pronounced enhancements within sarcomatous lesions than within FD-occupying lesions (Figure 4). Among the 3 cases with gadolinium infusion, 2 cases showed central enhancement, and 1 case showed thick peripheral enhancement. Extraosseous soft tissue mass was observed in 2 cases, and soft tissue edema was observed in 1 case with pathologic fracture.
Bone scintigraphy (n = 10) revealed eccentric increased uptake of radionuclide in monostotic lesion of all patients.
Histopathology, Management, and Prognosis
Eight patients underwent resection, and 2 patients underwent osteotomy and knee replacement (Table 3). Pathology revealed 7 cases of FD with osteosarcoma (7/10) (Figure 4), 2 cases of FD with fibrosarcoma (2/10), and 1 case of FD with malignant fibrous histiocytoma (MFH) (1/10). Chemotherapy, mainly adriamycin–cisplatin (AP), was given as an adjuvant treatment for patients 2 and 4.
TABLE 3.
Treatment and Outcome of Sarcoma in FD
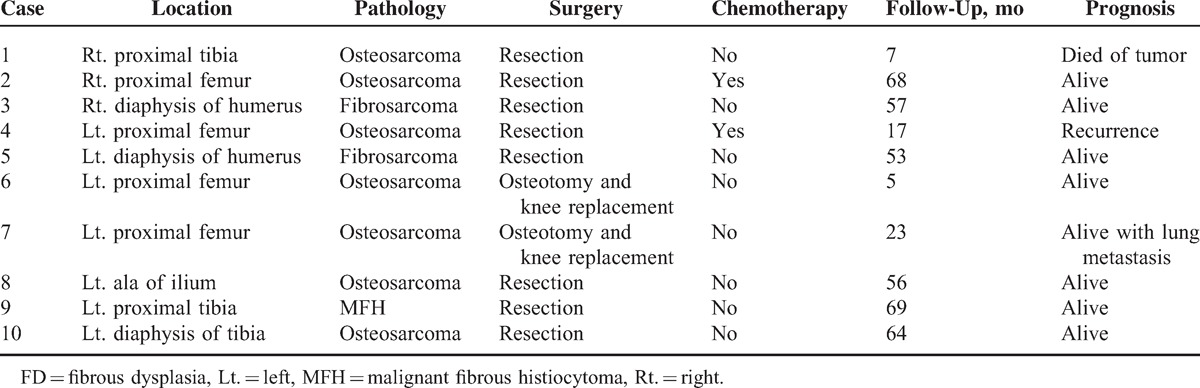
Of the 10 patients, at the end of the study, 1 patient died from tumors, 1 patient was alive with lung metastasis, 1 patient experienced recurrence, and 7 patients were alive without recurrence or additional lesions, proved by the radionuclide bone scan.
DISCUSSION
FD is a benign fibro-osseous lesion disease that has a rare but clear potential for malignant transformation. It has frequencies of 2.5% for all bone lesions and 7% for benign bone tumors.6–9 The monostotic form of the disease occurs most frequently and represents approximately 75% of all FD cases.1 However, the frequency of malignant change is increased with the polyostotic form of FD.1,3–5 Malignancies in monostotic FD are very rare.1 To the best of our knowledge, this study provides the comprehensive report of the imaging features of malignancies in monostotic FD using an evidence-based approach.
Clinical Features of Malignancies in FD
The frequency of malignancy in FD as reported in the literature ranges from 0.4% to 6.7%,1,6,10 with a higher frequency in polyostotic FD, especially in patients with Albright syndrome or Mazabraud syndrome.5,11,12 However, it is accepted that the actual frequency is less than 1% in most of the pertinent literature.4,6,13–15 In our study, the 2.77% (15/542) frequency of malignancy in FD was slightly higher than the frequencies reported in other studies, which may be explained by the referral of intractable cases to our hospitals. What is more, based on the principle of management of FD—lesions for which a definite diagnosis could be made by imaging appearance and clinic should not warrant diagnostic biopsy, especially in asymptomatic patients—many FD cases were not included in this study. It is generally accepted that individuals with polyostotic FD are at an increased risk of malignant change compared with monostotic FD cases.6,16 However, the higher number of malignancies in monostotic FD is likely explained by the significantly higher number of monostotic than polyostotic FD cases, and all cases in our study exhibited monostotic FD-related malignancies.
Of our 10 patients, 2 were females and 8 were males. Our data indicated that sarcoma in FD seemed to predominately occur in males. However, other literature on malignancy in FD reported no obvious gender predominance.6,17 Regarding age, the mean age at the time of sarcoma occurrence was 46.5 ± 15.9 years, and 80% of patients were past the fourth decade of life. This age distribution is consistent with the data previously reported in the literature.6,15
Our results further revealed that the diaphyses of long bones are the primary sites of malignant transformation in monostotic FD. The malignant sites found in this study are as follows, listed in decreasing order of frequency: femur (4 cases), tibia (3 cases), humerus (2 cases), and ilium (1 case). This finding is also consistent with the main predicted sites of monostotic FD in the skeleton.
Regarding the histotypes of sarcoma associated with FD, osteosarcoma (7/10) was the most common type, followed by fibrosarcoma (2/10) and MFH (1/10). This trend is similar to what has been previously reported in the literature.6 Chondrosarcoma could also be one of the histotypes of sarcoma associated with FD.6
Clinically, monostotic FD usually presents asymptomatically until pathologic fracture or incidental discovery. The symptoms of sarcoma were generally pain and swelling and usually developed rapidly. Rapid mass growth and swelling or pain are signs that could indicate the initiation of sarcomatosis in FD cases. Raised serum alkaline phosphatase (ALP) levels indicate bone metabolic hyperactivity, which should be considered in patients with suspicious malignant transformations.15
The exact pathogenesis of transformation of sarcoma in FD cases remains largely elusive. Many authors have emphasized the importance of radiation therapy in the development of sarcoma in FD cases.7,8 However, in our study, no patient had received previous radiation treatment. Moreover, radiotherapy is no longer recommended for the treatment of FD,1,8 indicating that radiotherapy is not the main trigger of sarcoma in FD. In our study, half of the patients had a history of surgery (curettage and internal fixation or curettage and bone grafting). Piero et al18 and Ramasamy et al19 considered that areas of surgical irritation, including irritation resulting from bone grafts and internal fixation, could be the niches in which mesenchymal stem cells develop tumors. There was not enough evidence to prove that surgical irritation caused the development of secondary sarcomas in the 5 patients investigated. The current knowledge about the pathogenesis of transformation of FD remains limited, and further investigation is warranted.
Imaging Features of Malignancies in FD
In our study, the imaging manifestations are distinctly different between the patient groups with and without surgery, discussed separately in the present article in order to facilitate discussion. Compared with the images of 5 patients without a history of surgery, the other 5 patients with a history of surgery showed osteolytic lesions in the operative areas, with immediate signs of aggressiveness (poorly defined margins, cortical destruction, and soft tissue masses) even on the plain film. The tumors in patients without a history of surgery were mostly classical osteolytic lesions with poorly delineated borders within or near the areas showing “ground-glass” opacities and cortical destruction,6,17 while soft tissue masses were not obvious; only 1 case without the previous operation showed no visible osteolytic lesion in the “ground-glass” area and no cortex degeneration (case 8). Mineralization was occasionally observed in 2 cases with operation and in 2 cases without operation. Periosteal reaction was observed in 1 case with operation and 1 case without operation.
CT provided the clearest depiction of cortical destruction and mineralization in sarcomas.1,17 Two cases (cases 2 and 9) exhibited mild mineralization and 1 case (case 9) exhibited a mild periosteal reaction, which could not be identified in the radiographs. MRI is well suited for demonstrating bone marrow involvement and accompanying soft tissue masses.17 Although MRI is very sensitive, the findings are nonspecific. MR images often show heterogeneous signal intensity on all pulse sequences. In some cases, the sarcomas are observed as masses that have a higher signal on T2-weighted images and more pronounced enhancement on contrast-enhanced T1-weighted images compared with FD-associated lesions,17 as in case 10. Cortical destruction, soft tissue masses, and peritumoral edema were also observed on MR images. The lesions with heterogeneous signal intensity, more pronounced enhancement, cortical destruction, and soft tissue masses associated with FD suggested malignant transformation in FD cases.
In general, the imaging characteristics of sarcoma in FD did not vary according to histological type (ie, osteosarcoma, fibrosarcoma, and MFH) in our study. Mineralization detected in primary osteosarcoma is more common than in MFH and fibrosarcoma. In our study, most cases of osteosarcoma associated with FD were characterized by lytic lesions without mineralization. One case of MFH associated with FD presented visible mineralization. Thus, a demonstration of mineralization is not a sign of osteosarcoma associated with FD, and the definitive diagnosis of histological types of malignant FD is based only on histology. Imaging studies greatly aided the discovery of signs of malignant transformation in FD, enabling diagnoses to be made as early as possible.
Treatment and Prognosis of Malignancies in FD
An accurate and timely diagnosis of sarcoma is essential for initiating adequate treatment. However, little information about the management of sarcoma associated with FD is available. Complete surgical resection remains the primary treatment option for sarcoma associated with FD. Adjuvant treatments, including prechemotherapy and/or postchemotherapy or radiotherapy, are recommended for improving local control and overall survival in patients with secondary sarcoma associated with FD.20,21 Postoperative radiotherapy or chemotherapy should be considered in selected patients, particularly in patients with positive or uncertain resection margins.15 The chemotherapy regimen depends on the histological types of the secondary sarcomas. In our study, 2 patients with secondary osteosarcoma underwent more than 6 courses of AP chemotherapy, and none of the other patients underwent chemotherapy or radiotherapy.
Regarding prognosis, Ruggieri et al6 reported that prognosis is typically poor, with a mortality rate of 53.6% in FD patients with malignant changes. Several authors have supported the claim that the outcomes for these patients are disappointing, citing lower overall survival rates compared with those of primary sarcomas.20,21 At the end of the study, 1 of the 10 patients had died from the tumor (1/10), 1 continued to live with lung metastasis (1/10), 1 experienced disease recurrence (1/10), and the other patients were alive without recurrence or additional lesions (7/10). It is difficult to compare the prognoses of patients with sarcomas associated with FD in our study with prognoses in other studies because it is unclear whether the patients in other studies received adequate surgical treatment and chemotherapy.
There are several limitations in our study. First, as the 542 patients with FD were proved by pathology, many cases diagnosed by imaging appearance and clinic were not included, leading to the high percentage of malignancies in FD compared with that in other reported series. Second, because of rare occurrence of sarcoma in FD, the number of cases was small. Third, since the 2 reviewers were aware that all of the study patients had a presumptive diagnosis of malignant transformation in monostotic FD to the data review and the use of consensus for evaluation, there was the possibility for interpretive bias. Fourth, not all patients received MR or CT and the inability to control imaging parameters. Furthermore, the cause of malignant transformation in FD is not clear; we cannot establish imaging or clinical criteria that predict malignant transformation in monostotic FD.
CONCLUSION
Rapidly increasing pain without apparent trauma or a significant rapid change in radiological appearance, especially in mineralization and cortical destruction, should alert the clinician to the malignant change. Although there was not enough evidence to prove that surgical irritation caused the development of secondary sarcomas, the patients with FD and a history of surgery should be followed up. While the osteolytic lesions in the operative areas appear with immediate signs of aggressiveness (poorly defined margins, cortical destruction, and soft tissue masses), it strongly indicates the malignant transformation. The most consistent radiographic feature of FD-related malignancies in patients without previous operation is poorly marginated, mineralized, osteolytic lesions within or near areas of “ground-glass” opacity with cortical destruction. Further investigations are needed to explore the pathogenesis of malignancies in FD and to establish optimal therapeutic strategies.
Footnotes
Abbreviations: ALP = alkaline phosphatase, AP = adriamycin–cisplatin, CT = computed tomography, FD = fibrous dysplasia, MFH = malignant fibrous histiocytoma, MR = magnetic resonance, STIR = short-tau inversion recovery, TE = echo time, TI = inversion time, TR = repetition time.
This research was supported by the National Natural Science Foundation of China (81171312). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that they have no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Riddle ND, Bui MM. Fibrous dysplasia. Arch Pathol Lab Med 2013; 137:134–138. [DOI] [PubMed] [Google Scholar]
- 2.Coley BL, Stewart FW. Bone sarcoma in polyostotic fibrous dysplasia. Ann Surg 1945; 121:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright F, Butter AM, Smith P. Syndrome characterized by posterities fibrosa disseminate, areas of pigmentation and endocrine dysfunction, with precocious puberty in females: report of five cases. N Engl J Med 1937; 216:727–746. [Google Scholar]
- 4.Schwartz DT, Alpert M. The malignant transformation of fibrous dysplasia. Am J Med Sci 1964; 247:1–20. [DOI] [PubMed] [Google Scholar]
- 5.Jhala DN, Eltoum I, Carroll AJ, et al. Osteosarcoma in a patient with McCune–Albright syndrome and Mazabraud's syndrome: a case report emphasizing the cytological and cytogenetic findings. Hum Pathol 2003; 34:1354–1357. [DOI] [PubMed] [Google Scholar]
- 6.Ruggieri P, Sim FH, Bond JR, et al. Malignancies in fibrous dysplasia. Cancer 1994; 73:1411–1424. [DOI] [PubMed] [Google Scholar]
- 7.Tanner HC, Jr, Dahlin DC, Childs DS., Jr Sarcoma complicating fibrous dysplasia: probable role of radiation therapy. Oral Surg Oral Med Oral Pathol 1961; 14:837–846. [DOI] [PubMed] [Google Scholar]
- 8.Altay M, Bayrakci K, Yildiz Y, et al. The development of osteosarcoma following radiotherapy for fibrous dysplasia. Acta Orthop Traumatol Turc 2004; 38:353–356. [PubMed] [Google Scholar]
- 9.Greenspan A. Orthopedic Radiology: A Practical Approach. 2nd ed.1992; Philadelphia: Raven Press, 600–612. [Google Scholar]
- 10.Abelanet R, Forest M, Meary R. Sarcomas occurring on fibrous dysplasia of bone. Apropos of a complex hemimelic form, with a review of the literature. Rev Chir Orthop Reparatrice Appar Mot 1975; 61:179–190. [PubMed] [Google Scholar]
- 11.Hansen MR, Moffat JC. Osteosarcoma of the skull base after radiation therapy in a patient with McCune–Albright syndrome: case report. Skull Base 2003; 13:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healey JH, Buss D. Radiation and pagetic osteogenic sarcomas. Clin Orthop Relat Res 1991; 270:128–134. [PubMed] [Google Scholar]
- 13.Huvos AG. Bone Tumors: Diagnosis, Treatment and Prognosis. 1991; Philadelphia: WB Saunders, 41–48. [Google Scholar]
- 14.Witkin GB, Guilford WB, Siegal GP. Osteogenic sarcoma and soft tissue myxoma in a patient with fibrous dysplasia and hemoglobins JBaltimore and S. Clin Orthop Relat Res 1986; 204:245–252. [PubMed] [Google Scholar]
- 15.Cheng J, Yu H, Wang D, et al. Spontaneous malignant transformation in craniomaxillofacial fibrous dysplasia. J Craniofac Surg 2013; 24:141–145. [DOI] [PubMed] [Google Scholar]
- 16.Campanacci M, Bertoni R, Capanna F. Malignant degeneration in fibrous dysplasia (presentation of 6 cases and review of the literature). Ital J Orthop Traumatol 1979; 5:373–381. [PubMed] [Google Scholar]
- 17.Hoshi M, Matsumoto S, Manabe J, et al. Malignant change secondary to fibrous dysplasia. Int J Clin Oncol 2006; 11:229–235. [DOI] [PubMed] [Google Scholar]
- 18.Picci P, Sieberova G, Alberghini M, et al. Late sarcoma development after curettage and bone grafting of benign bone tumors. Eur J Radiol 2011; 77:19–25. [DOI] [PubMed] [Google Scholar]
- 19.Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 2007; 21:304–310. [DOI] [PubMed] [Google Scholar]
- 20.Guadagnolo BA, Zagars GK, Raymond AK, et al. Osteosarcoma of the jaw/craniofacial region: outcomes after multimodality treatment. Cancer 2009; 115:3262–3270. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes R, Nikitakis NG, Pazoki A, et al. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg 2007; 65:1286–1291. [DOI] [PubMed] [Google Scholar]


