Abstract
Although a relationship between hypertension and the development of renal cancer and other types of cancer have been proposed for decades, the results of epidemiologic studies remain inconclusive. This study was conducted to evaluate the association between hypertension and genitourinary and gynecologic cancers in Taiwan.
In this study, we conducted a populated-based retrospective cohort study by using data from the Taiwanese National Health Insurance program. The study period was from 2000 to 2011, and the cohort comprised 111,704 insurants: 57,961 patients with hypertension and 53,743 patients without hypertension. A Cox proportional hazard regression analysis was performed to estimate the effects of hypertension on genitourinary and gynecologic cancers risk.
Among the patients with hypertension, the risks of developing renal and uterine corpus cancers were significantly higher in the hypertension group than they were in the nonhypertension group. Further stratified analyses by sex, age, and hypertension duration revealed distinct cancer-specific patterns. Higher cancer risk appears to be more obvious among younger hypertensive patients with longer follow-up time.
The results of this study indicate that Taiwanese patients with hypertension have higher risks for some types of cancer, and cancer-specific patterns vary by sex, age, and hypertension duration.
INTRODUCTION
Hypertension is a chronic disease that is frequently observed in middle-aged and older populations, and it places a great burden on public health. A previous analysis showed that, overall, 26.4% of the adult population worldwide had hypertension in 2000, and 29.2% of adults are projected to have this condition by 2025.1 The age-standardized rate of hypertension in Taiwan between 1993 and 1996 was 23.7%.2 It is one of the primary risk factor of cardiovascular disease and cerebrovascular stroke,3–5 which are the leading causes of death worldwide.6
In addition to these well-documented hypertension-related diseases, previous studies have examined the possible association between hypertension and cancer risk. Despite numerous studies either directly or indirectly examining the association between hypertension and cancer, the results have typically been conflicting and have not established causality.7 The most frequently reported cancer associated with hypertension is renal cancer, which has been investigated in numerous epidemiological studies that have indicated higher risks among hypertension patients.8–11 Other individual cancer sites that have been documented as a possible association with hypertension or high blood pressure include breast, uterine corpus, bladder, prostate, colorectum, lung, pancreas, liver, and head and neck cancer12–19; however, the results for these cancers remain inconclusive.
Based on a review of the literature, no nationwide population-based study has discussed the relationship between hypertension and genitourinary and gynecologic cancers risk. This study examined whether the suggested patterns of cancer risk among patients with hypertension is relevant in Taiwan. This retrospective cohort study was conducted to assess whether an increased risk of genitourinary and gynecologic cancers exists in patients with hypertension. The research database was derived from the National Health Insurance (NHI) program in Taiwan.
METHODS
Data Source
Taiwan launched the NHI program in March 1995. Approximately 23.74 million enrollees—accounting for 99% of the Taiwan's population—were covered by this program at the end of 2009.20 This retrospective cohort study analyzed data sets from the Longitudinal Health Insurance Database 2000 (LHID2000), which was released by the National Health Research Institutes (NHRI). The LHID2000 contains the data of 1,000,000 randomly sampled insurants from the 2000 Registry for Beneficiaries, which contains all medical records of insurants from 1996 to 2011.
According to the NHRI, no significant difference exists in sex, age, or health care costs between the cohorts in the LHID2000 and all enrolled insurants.21 Diseases were identified based on the International Classification of Diseases, Ninth Revision (ICD-9 Codes) in the LHID2000. The data files are deidentified and further scrambled before being released to researchers. This study was approved by the Institutional Review Board of China Medical University Hospital, Taichung, Taiwan.
Sampled Patients
From the LHID2000, we identified patients aged ≥20 years with diagnosis of hypertension (ICD-9-CM Codes 401–405) as hypertension cohort between January 1, 1998, and December 31, 2002. The date of first diagnosis for hypertension after 1995 was defined as index date. Patients whose cancer was diagnosed (ICD-9-CM Codes 140–208) before index date, those with incomplete medical information, or those who were <20 years were excluded. The comparison nonhypertension cohort was randomly selected (approximately 1 for every patient in the hypertension cohort) by frequency matched for age (every 5 years span) and index year under the same exclusion criteria. Finally, 57,961 patients were included as the hypertension cohort and 53,743 patients as the nonhypertension cohort. The participants were followed up until genitourinary and gynecologic cancers (ICD-9-CM Codes 174, 179, 180, 182, 185, 188, and 189) diagnosis, loss to follow-up, withdrawal from the NHI program, or until the end of 2011, whichever occurred first.
Variables of Exposure
The variables considered in this study were age (≤34, 35–49, 50–64, and ≥65 years) and sex (male/female). Area of residence was divided into 4 levels of urbanization according to several criteria, including population density (people/km2), population ratio of various educational levels, population ratio of elderly people, population ratio of agriculture workers, and the number of physicians per 100,000 people. Level 1 was considered the highest degree of urbanization and Level 4 was the lowest. Urbanization Levels 1 and 2 were defined as urban areas, whereas Levels 3 and 4 were classified as rural areas. The categories used for occupations in this study included public servants, laborers (farmer, fishermen, and industry workers), businessman, low-income earners, and “others.” Participants were classified as low-income earners if their insured income was lower than the level required for a premium to be payable. The category for insurance-premium-based income was grouped into 3 levels: ≤NT$15,840, NT$15,841–25,000, and >NT$25,000 per month (US$1 = approximately NT$30).
Baseline comorbidities and medications included diabetes (ICD-9-CM Code 250), hyperlipidemia (ICD-9-CM Code 272), stroke (ICD-9-CM Codes 430–438), ischemic heart disease (ICD-9-CM Codes 410–414), asthma and chronic obstructive pulmonary disease (ICD-9-CM Codes 490–496), alcoholism (ICD-9-CM Codes 291, 303, 305.00–305.03, 790.3, and V11.3), alcoholic liver damage (ICD-9-CM Codes 571.0, 571.1, and 571.3), and antihypertensive agents. Antihypertensive agents included angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, α-blocker, β-blocker, calcium channel blockers, thiazides, loop diuretics, potassium-sparing diuretics, and others.
Statistical Analysis
The continuous variables were summarized by mean and standard deviation, whereas the categorical variables were summarized by frequency and percentage. The categorical variables were analyzed using a χ2 test,22,23 and the continuous variables of the baseline characteristics of the hypertension and nonhypertension patients were analyzed using a Student t test. Univariate and multivariate Cox proportional hazard regression analyses were performed to estimate the hazard ratio (HR) with a 95% confidence interval (CI) for cancer. Significant variables identified by the univariate Cox analysis were included in a multivariate Cox proportional hazard model to identify the independent predictors of genitourinary and gynecologic cancers. We divided cancers into the following 6 groups: female breast cancer (ICD-9-CM Code 174), corpus uteri cancer (ICD-9-CM Codes 179 and 182), cervix uteri cancer (ICD-9-CM Code 180), prostate cancer (ICD-9-CM Code 185), bladder cancer (ICD-9-CM Code 188), and renal cancer (ICD-9-CM Code 189). All data processing and statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc., Cary, NC). A 2-tailed P value of <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Study Patients
The research sample of 111,704 patients comprised 57,961 patients with hypertension and 53,743 participants without hypertension. Table 1 shows the distribution of characteristics among the study patients. The mean age of the hypertension group was 56.3 years and that of the nonhypertension group was 53.4 years, with nearly 64.2% of hypertension patients aged ≥50 years. Patients with hypertension were more likely to be men, living in urban areas, and laborers. Compared with the nonhypertension group, the hypertension group was more likely to have other comorbidities, including diabetes, hyperlipidemia, stroke, ischemic heart disease, asthma and chronic obstructive pulmonary disease, alcoholism, and alcoholic liver damage, and antihypertensive agents (P < 0.001).
TABLE 1.
Characteristics of Patients in LHID2000
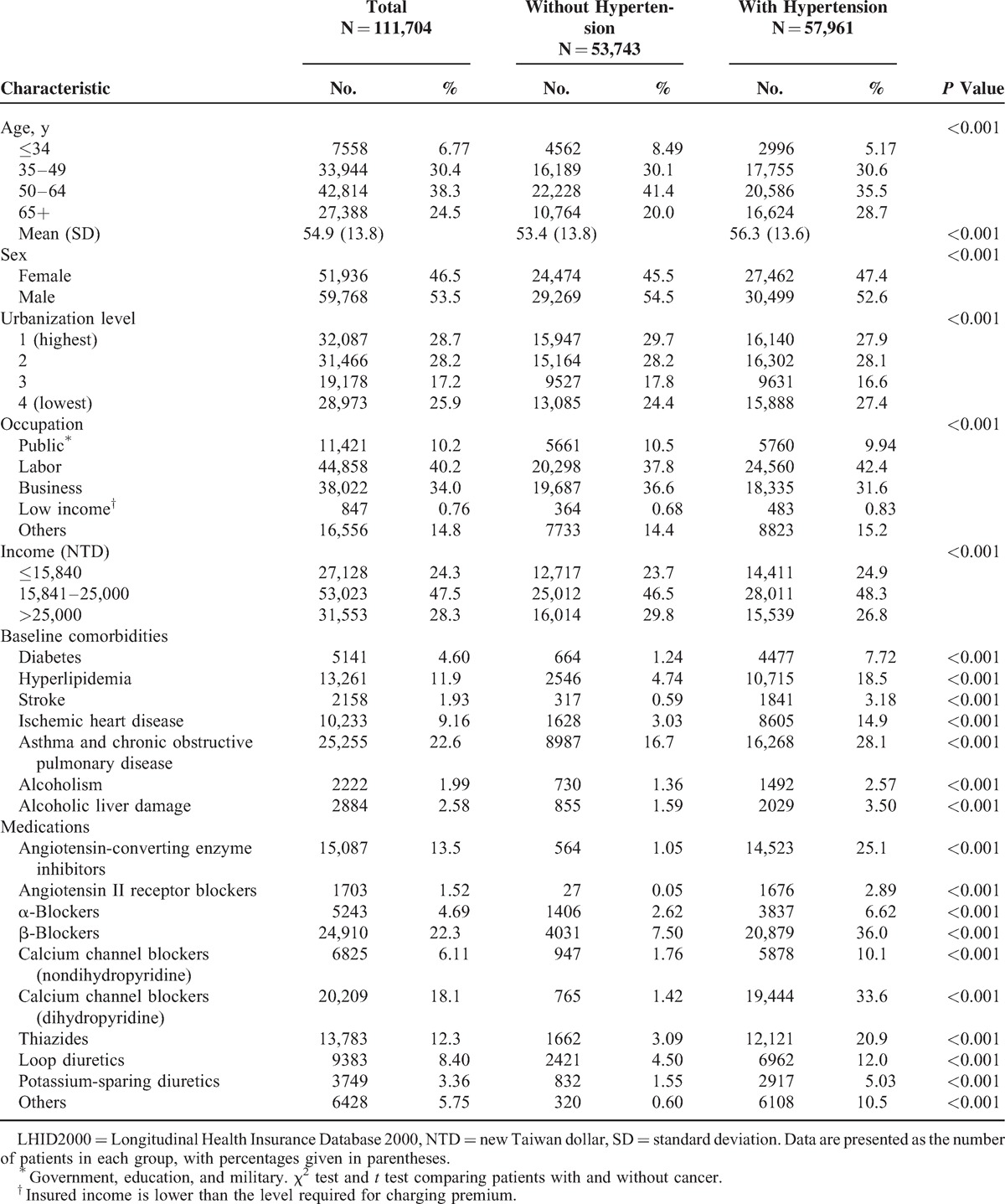
Cox Model for Analysis of Risk Factors of Hypertension Affecting Cancer Development
After adjusting for age, sex, urbanization level, occupation, income, and other comorbidities and antihypertensive agents, the HRs for uterine corpus and renal cancers were 1.88 and 1.56-fold higher for patients with hypertension, respectively (Table 2). The men with hypertension exhibited a significantly higher risk of genitourinary cancers compared with the nonhypertension group (Table 3). Female with hypertension had an 88% and 85% increased risk of uterine corpus cancer and renal cancer risk, respectively, compared with the nonhypertension group.
TABLE 2.
Cox Model With HRs and 95% CIs of Subdivision Cancer Associated With Hypertension in Patients
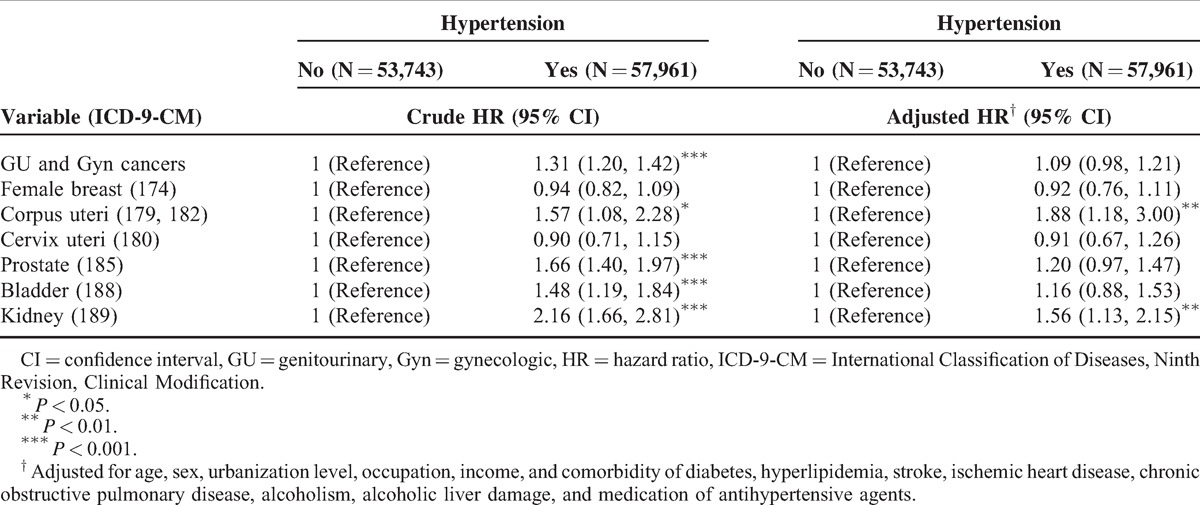
TABLE 3.
Cox Model With HRs and 95% CIs of Subdivision Cancer Associated With Hypertension in Patients
The age-specific HR showed that patients with hypertension were at higher risk of uterine corpus and renal cancers among patients aged ≤49 years, compared with those without hypertension (Table 4).
TABLE 4.
Cox Model With HRs and 95% CIs of Subdivision Cancer Associated With Hypertension in Patients

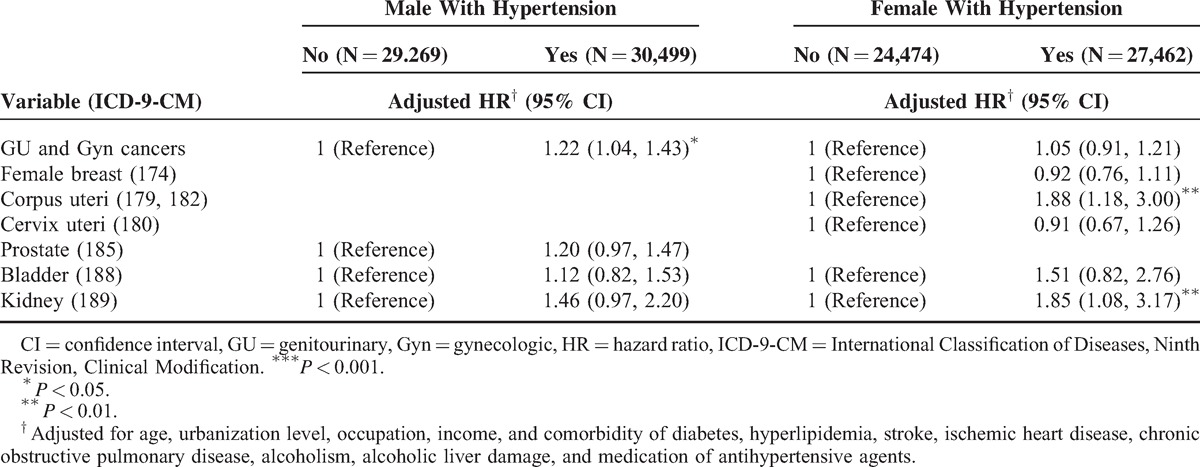
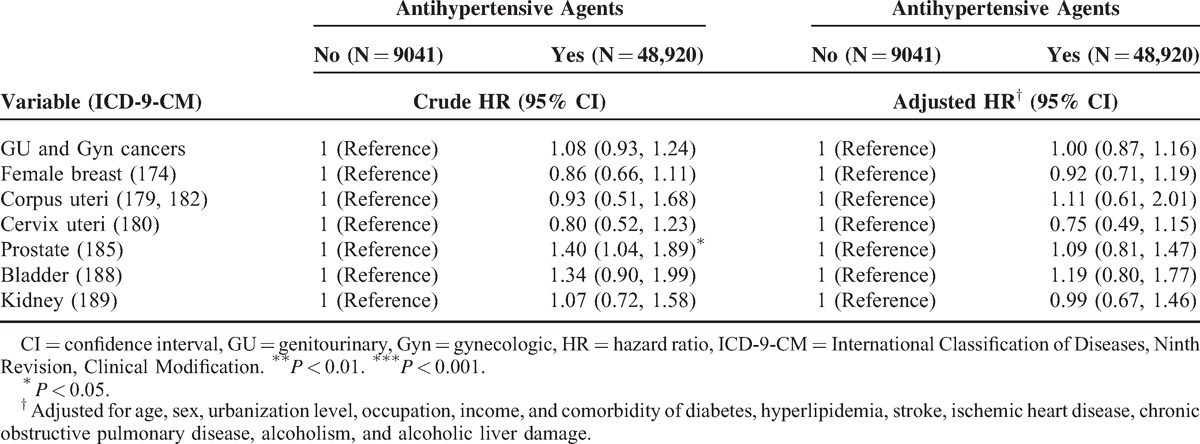
Furthermore, an analysis of risk factors for hypertension for developing various types of cancer was stratified by follow-up time (Table 5). Compared with the nonhypertension group, the patients with hypertension had a 2.69-fold hazard of uterine corpus cancer and 1.66-fold hazard of renal cancer in the >5 years follow-up. Table 6 demonstrated the results of subdivision cancer risk among hypertension patients by treatment. It revealed that whether using antihypertensive agents or not did not affect the subsequent cancer risk.
TABLE 5.
Cox Model With HRs and 95% CIs of Subdivision Cancer Associated With Hypertension in Patients

TABLE 6.
Cox Model With HRs and 95% CIs of Subdivision Cancer Among Hypertension Patients by Treatment
DISCUSSION
In this nationwide population-based cohort study, we observed that overall genitourinary and gynecologic cancer risk was higher in hypertension group, and the hypertension patients were more likely to have renal and uterine corpus cancers. Further analyses stratified by sex and age indicated that only women of age ≤49 years with hypertension were at a higher risk of renal cancer. Women with hypertension were at a significantly higher risk of uterine corpus cancer, but only limited to those with age ≤49 years. Stratified analysis by follow-up time revealed that significantly higher risks of renal and uterine corpus cancers were limited to >5 years follow-up.
Cancer has been the leading cause of death in Taiwan for 3 decades. The age-adjusted incidence rate has increased steadily, reaching 295 new cases per 100,000 people in 2011.24 Cancer is becoming a more crucial public health problem in the context of population aging and changes in lifestyles,25 gaining the attention of the government, which has promoted several primary and secondary prevention programs for major cancers, such as avoidance of high-risk factors and periodical cancer screening, aimed at reducing cancer incidence and mortality rates.25 Therefore, additional population-based investigations regarding cancer-preventive epidemiology continue to develop. Considering the high prevalence of hypertension, a minor hazard of cancer development could have critical clinical implications from a public health perspective, and this study on cancer risk among hypertension patients was therefore conducted.
Our data demonstrated that overall genitourinary and gynecologic cancer risk was higher in the hypertension group. The plausible mechanism for the possible linkage between cancer and hypertension remains unclear, but it has been hypothesized that predisposition to cancer is increased by chronic inflammation,26 and also that vascular inflammation could be one of the reasons in the pathogenesis of hypertension.27
Cancer-specific site analysis revealed a significantly higher risk of renal cancer among the hypertension group, but subdivision analyses found that the significant difference was only observed in women, age ≤49 years or >5 years follow-up time. Hypertension has been documented as one of the risk factors for renal cancer in both men and women.8–11,13 A metaanalysis of 18 studies revealed a 1.6-fold increase in the risk of renal cancer among hypertension patients.28 In a large province-wide cohort study of hypertension patients in Finland, Lindgren et al13 reported that the incidence of renal cancer was significantly higher in hypertension patients than in the general population. This was more evident in women (SIR = 1.43, 95% CI = 1.12–1.80) than in men (SIR = 1.20, 95% CI = 0.86–1.63). In addition, the Hunt Study in Norway reported that high blood pressure levels in women (but not in men) were associated with a relative risk of developing renal cancer.10 It is consistent with our findings that only women had a significantly higher risk (adjusted HR = 1.85, 95% CI = 1.08–3.17). Hypertensive men had a marginally significantly higher risk for developing renal cancer (adjusted HR = 1.46, 95% CI = 0.97–2.20). Our findings are partially compatible with the study from Chow et al8 who investigated the hypertension and the risk of renal cancer in men and found that men initially <50 years old had the highest risks. The mechanism of the relationship between hypertension and renal cancer remains uncertain. Angiogenic and other growth factors were associated with higher blood pressure, and these may also be involved in renal carcinogenesis.8,18,29,30 Lipid peroxidation has also been proposed as the mechanism responsible for the increased risk of renal cancer risk associated with both hypertension and obesity.31
For other genitourinary and gynecologic cancer sites, previous studies have reported inconsistent findings.12 Several studies have shown an association between hypertension and the risk of uterine corpus cancer,13,15,32,33 but it is unclear whether this positive relationship is due to hypertension or the interaction with weight.34–36 In addition, other studies did not find the association.37,38 The positive association in our study was only observed in hypertensive women with ≤49 years of age. Some studies have indicated that hypertension is associated with breast cancer risk,14,15,39 whereas other studies have failed to identify a relationship.12,13,40,41 A recent case–control study in Uruguay reported that hypertension is a risk factor for premenopausal breast cancer.42 Breast cancer was observed in this study to pose no significant association with hypertension; however, a nonsignificant trend was seen toward decreased risk of breast cancer among older women (≥50 years). We found no factor explaining this possible preventive effect and assumed that lifestyle modification of hypertension patients (eg, smoking cessation, exercise, and avoidance of unhealthy food) may partially account for this. Hypertension, a component of the metabolic syndrome, was indicated to be associated with prostate cancer.17,43,44 However, not all epidemiological studies have supported this link.12,13 In this study, more prostate patients were observed in the hypertension group, but the difference did not reach the statistically significant level (adjusted HR = 1.20, 95% CI = 0.97–1.47). Häggström et al45 conducted a prospective cohort study to evaluate the metabolic syndrome and bladder cancer risk and found that high blood pressure was associated with bladder cancer risk in men, but not in women. Stocks et al12 also highlighted that men with hypertension are more likely to develop bladder cancer. Other studies did not find any relationship between hypertension and bladder cancer risk.13,46 Our study also failed to demonstrate this relationship.
Our data indicated that younger hypertensive patients (≤49 years) are typically more vulnerable to cancer development (Table 4). Although cancer is more frequently observed in elderly adults, this observation implies that younger hypertension patients may need to focus more on subsequent cancer risk. A complication in the assessment of hypertension as a risk factor is the possibility that short-term hypertension (<5 years) may procure a protective effect,47 whereas a positive association has been observed with prolonged exposure to hypertension.48,49 Our results also indicated that longer follow-up time is more likely to see the increased risk in renal and uterine corpus cancers (Table 5). To clarify whether the use of antihypertensive agents can reduce cancer risks, we further analyzed subdivision cancer risk among hypertension patients by treatment and found that antihypertensive agents did not decrease cancer risks (Table 6). It is understandable because the association is much more complex. Besides antihypertensive agents, hypertension patients may have alternative ways to control their blood pressure, such as food restriction, body weight control, and exercise. Patients who take antihypertensive agents may have more severe or symptomatic diseases than patients who do not take antihypertensive agents. In addition, using single antihypertensive agent or combined antihypertensive agents may represent different groups of patients and may have different effects. However, all of these are uncontrolled factors of our NHI database.
This study has the strength of being a nationwide population-based design with high generalizability. However, several limitations should be acknowledged. First, the association between hypertension and cancer may be “diluted” by the fact that there was no screening for blood pressure and the diagnosis of hypertension in this study is based only on a retrospective analysis of reported diagnosis of hypertension, as hypertension is largely an asymptomatic disease. Second, no information was available regarding the patients’ lifestyle or behavior; consequently, we could not account for health–behavior-related factors, such as smoking status and alcohol consumption. Specific personality traits and various life events are associated with certain health-related behaviors and lifestyle factors (eg, smoking or inadequate nutrition). Unhealthy habits can increase the risk of developing cancer50 and hypertension.51–53 In this study, we attempted to account for smoking and alcohol-related illnesses to minimize the effect of potentially confounding variables. Third, obesity is a well-known risk factor for both cancer and hypertension,54,55 but the NHI database does not provide relevant information, such as body weight or body mass index. Consequently, we could not conduct more sophisticated tests. Fourth, information regarding family history of breast cancer or other cancers and genetic predisposition of development of cancer is lacking, and hence we could not take into consideration these potentially confounding factors while evaluating the data. Finally, a surveillance bias may exist because hypertension patients typically have regular clinical follow-up; thus, detecting more cancer patients in this group is expected.
In summary, this population-based cohort study shows that renal and uterine corpus cancer risks among hypertension patients in Taiwan are significantly higher than that of the general population. Higher cancer risk appears to be more obvious among younger hypertensive patients with longer follow-up time. Our findings emphasize the potentially complex relationship between hypertension and genitourinary and gynecologic cancers, and that the underlying mechanisms require further comprehensive investigation.
Footnotes
Abbreviations: CIs = confidence intervals, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHID2000 = Longitudinal Health Insurance Database 2000, NHRI = National Health Research Institutes.
H-TK and L-MS contributed equally to this work.
All authors have contributed significantly and they are in agreement with the content of the manuscript. Conception/Design: L-MS, H-TK, C-HK; Provision of study materials: all authors; Collection and/or assembly of data: all authors; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval of manuscript: all authors.
This work was supported by the study projects in China Medical University (CMU102-BC-2), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002), Health, and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan), and International Research-Intensive Centers of Excellence in Taiwan (I-RiCE) (NSC101-2911-I-002-303). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005; 365:217–223. [DOI] [PubMed] [Google Scholar]
- 2.Pan WH, Chang HY, Yeh WT, et al. Prevalence, awareness, treatment and control of hypertension in Taiwan: results of Nutrition and Health Survey in Taiwan (NAHSIT) 1993–1996. J Hum Hypertens 2001; 15:793–798. [DOI] [PubMed] [Google Scholar]
- 3.Whelton PK. Epidemiology of hypertension. Lancet 1994; 344:101–106. [DOI] [PubMed] [Google Scholar]
- 4.Chockalingam A, Campbell NR, Fodor JG. Worldwide epidemic of hypertension. Can J Cardiol 2006; 22:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Whelton PK. Epidemiology and prevention of hypertension. Med Clin North Am 1997; 81:1077–1097. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. The Top 10 Causes of Death in 2012. http://www.who.int/mediacentre/factsheets/fs310/en/ Accessed October 23, 2014. [Google Scholar]
- 7.Goon PK, Stonelake PS, Lip GY. Hypertension, anti-hypertensive therapy and neoplasia. Curr Pharm Des 2007; 13:2539–2544. [DOI] [PubMed] [Google Scholar]
- 8.Chow WH, Gridley G, Fraumeni JF, Jr, et al. Obesity, hypertension, and the risk of kidney cancer in men. N Engl J Med 2000; 343:1305–1311. [DOI] [PubMed] [Google Scholar]
- 9.Sanfilippo KM, McTigue KM, Fidler CJ, et al. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014; 63:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vatten LJ, Trichopoulos D, Holmen J, et al. Blood pressure and renal cancer risk: the HUNT Study in Norway. Br J Cancer 2007; 97:112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin JK, Chow WH, Mandel JS, et al. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer 1995; 63:216–221. [DOI] [PubMed] [Google Scholar]
- 12.Stocks T, Van Hemelrijck M, Manjer J, et al. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension 2012; 59:802–810. [DOI] [PubMed] [Google Scholar]
- 13.Lindgren AM, Nissinen AM, Tuomilehto JO, et al. Cancer pattern among hypertensive patients in North Karelia, Finland. J Hum Hypertens 2005; 19:373–379. [DOI] [PubMed] [Google Scholar]
- 14.Pereira A, Garmendia ML, Alvarado ME, et al. Hypertension and the risk of breast cancer in Chilean women: a case-control study. Asian Pac J Cancer Prev 2012; 13:5829–5834. [DOI] [PubMed] [Google Scholar]
- 15.Soler M, Chatenoud L, Negri E, et al. Hypertension and hormone-related neoplasms in women. Hypertension 1999; 34:320–325. [DOI] [PubMed] [Google Scholar]
- 16.Forootan M, Tabatabaeefar M, Yahyaei M, et al. Metabolic syndrome and colorectal cancer: a cross-sectional survey. Asian Pac J Cancer Prev 2012; 13:4999–5002. [DOI] [PubMed] [Google Scholar]
- 17.Martin RM, Vatten L, Gunnell D, et al. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes Control 2009; 20:1181–1192. [DOI] [PubMed] [Google Scholar]
- 18.Lindgren A, Pukkala E, Nissinen A, et al. Blood pressure, smoking and the incidence of lung cancer in hypertensive men in North Karelia, Finland. Am J Epidemiol 2003; 158:442–447. [DOI] [PubMed] [Google Scholar]
- 19.Stolzenber-Solomon RZ, Pietinen P, Taylor PR, et al. A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control 2002; 13:417–426. [DOI] [PubMed] [Google Scholar]
- 20.Cheng TM. Okma KGH, Crivelli L. Taiwan's National Health Insurance system: high value for the dollar. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. New Jersey: World Scientific; 2009. 71–204. [Google Scholar]
- 21.Report of National Health Research Institutes in Taiwan. http://nhird.nhri.org.tw/date_cohort.htm Accessed October 23, 2014. [Google Scholar]
- 22.Hu YU, Wu L, Yan LF, et al. Predicting subtypes of thymic epithelial tumors using CT: new prospective based on a comprehensive analysis of 216 patients. Sci Rep 2014; 4:6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Zhang J, Fan W, et al. MAPKAP1 rs10118570 polymorphism is associated with anti-infection and anti-hepatic fibrogenesis in schistosomiasis japonica. PLoS One 2014; 9:e105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taiwan Cancer Registry. http://cph.ntu.edu.tw/main.php?Page=N2 Accessed October 23, 2014. [Google Scholar]
- 25.Chiang CJ, Chen YC, Chen CJ, et al. Cancer trends in Taiwan. Jpn J Clin Oncol 2010; 40:897–904. [DOI] [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int 2014; 2014:406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrao G, Scotti L, Bagnardi V, et al. Hypertension, antihypertensive therapy and renal-cell cancer: a meta-analysis. Curr Drug Saf 2007; 2:125–133. [DOI] [PubMed] [Google Scholar]
- 29.Schena FP, Strippoli GF, Wankelmuth P. Renal growth factors: past, present, and future. Am J Nephrol 1999; 19:308–312. [DOI] [PubMed] [Google Scholar]
- 30.Choueiri TK, Bukowski RM, Rini BI. The current role of angiogenesis inhibitors in the treatment of renal cell carcinoma. Semin Oncol 2006; 33:596–606. [DOI] [PubMed] [Google Scholar]
- 31.Gago-Dominguez M, Castelao JE, Yuan JM, et al. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States). Cancer Causes Control 2002; 13:287–293. [DOI] [PubMed] [Google Scholar]
- 32.Weiderpass E, Persson I, Adami HO, et al. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes Control 2000; 11:185–192. [DOI] [PubMed] [Google Scholar]
- 33.Friedenreich CM, Biel RK, Lau DC, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev 2011; 20:2384–2395. [DOI] [PubMed] [Google Scholar]
- 34.Serin IS, Ozcelik B, Basbug M, et al. Effects of hypertension and obesity on endometrial thickness. Eur J Obstet Gynecol Reprod Biol 2003; 109:72–75. [DOI] [PubMed] [Google Scholar]
- 35.Ali AT. Risk factors for endometrial cancer. Ceska Gynekol 2013; 78:448–459. [PubMed] [Google Scholar]
- 36.Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol 1992; 167:1317–1325. [DOI] [PubMed] [Google Scholar]
- 37.Fortuny J, Sima C, Bayuga S, et al. Risk of endometrial cancer in relation to medical conditions and medication use. Cancer Epidemiol Biomarkers Prev 2009; 18:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iatrakis G, Zervoudis S, Saviolakis A, et al. Women younger than 50 years with endometrial cancer. Eur J Gynaecol Oncol 2006; 27:399–400. [PubMed] [Google Scholar]
- 39.Largent JA, McEligot AJ, Ziogas A, et al. Hypertension, diuretics and breast cancer risk. J Hum Hypertens 2006; 20:727–732. [DOI] [PubMed] [Google Scholar]
- 40.Manjer J, Kaaks R, Riboli E, et al. Risk of breast cancer in relation to anthropometry, blood pressure, blood lipids and glucose metabolism: a prospective study within the Malmo Preventive Project. Eur J Cancer Prev 2001; 10:33–42. [DOI] [PubMed] [Google Scholar]
- 41.Peeters PH, van Noord PA, Hoes AW, et al. Hypertension and breast cancer risk in a 19 year follow-up (DOM cohort). J Hypertens 2000; 18:249–254. [DOI] [PubMed] [Google Scholar]
- 42.Ronco AL, De Stefani E, Deneo-Pellegrini H. Risk factors for premenopausal breast cancer: a case-control study in Uruguay. Asian Pac J Cancer Prev 2012; 13:2879–2886. [DOI] [PubMed] [Google Scholar]
- 43.Esposito K, Chiodini P, Capuano A, et al. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest 2013; 36:132–139. [DOI] [PubMed] [Google Scholar]
- 44.Beebe-Dimmer JL, Dunn RL, Sarma AV, et al. Features of the metabolic syndrome and prostate cancer in African-American men. Cancer 2007; 109:875–881. [DOI] [PubMed] [Google Scholar]
- 45.Häggström C, Stocks T, Rapp K, et al. Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 2011; 128:1890–1898. [DOI] [PubMed] [Google Scholar]
- 46.Jiang X, Castelao JE, Yuan JM, et al. Hypertension, diuretics and antihypertensives in relation to bladder cancer. Carcinogenesis 2010; 31:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamet P. Cancer and hypertension. An unresolved issue. Hypertension 1996; 28:321–324. [DOI] [PubMed] [Google Scholar]
- 48.Wannamethee G, Shaper AG. Blood pressure and cancer in middle-aged British men. Int J Epidemiol 1996; 25:22–31. [DOI] [PubMed] [Google Scholar]
- 49.Taylor JO, Cornoni-Huntley J, Curb JD, et al. Blood pressure and mortality risk in the elderly. Am J Epidemiol 1991; 134:489–501. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz S, Messerschmidt H, Dören M. Psychosocial risk factors for cancer development. Med Klin (Munich) 2007; 102:967–979. [DOI] [PubMed] [Google Scholar]
- 51.Rzheshevsky AV. Fatal “triad”: lipotoxicity, oxidative stress, and phenoptosis. Biochemistry (Mosc) 2013; 78:991–1000. [DOI] [PubMed] [Google Scholar]
- 52.Pannarale G, Acconcia MC, Gianturco L, et al. Cigarette smoking and ambulatory blood pressure: a case-control study in normotensives. J Hum Hypertens 2008; 22:129–131. [DOI] [PubMed] [Google Scholar]
- 53.Razvodovsky YE. Contribution of alcohol to hypertension mortality in Russia. J Addict 2014; 2014:483910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan SY, Johnson KC, Ugnat AM, et al. Association of obesity and cancer in Canada. Am J Epidemiol 2014; 159:259–268. [DOI] [PubMed] [Google Scholar]
- 55.Rahmouni K, Correia ML, Haynes WG, et al. Obesity-associated hypertension: new insights into mechanism. Hypertension 2005; 45:9–14. [DOI] [PubMed] [Google Scholar]


